Abstract
ATP7B is a copper dependent P-type ATPase, required for copper homeostasis. Taking advantage of high yield heterologous expression of recombinant protein, we investigated charge transfer in ATP7B. We detected charge displacement within a single catalytic cycle upon ATP addition and formation of phosphoenzyme intermediate. We attribute this charge displacement to movement of bound copper within ATP7B. Based on specific mutations, we demonstrate that enzyme activation by copper requires occupancy of a site in the N-terminus extension which is not present in other transport ATPases, as well as of a transmembrane site corresponding to the cation binding site of other ATPases.
Keywords: charge transfer measurements, copper displacement, heterologous expression, phosphoenzyme intermediate, recombinant Cu+-ATPase, solid supported membrane
1. Introduction
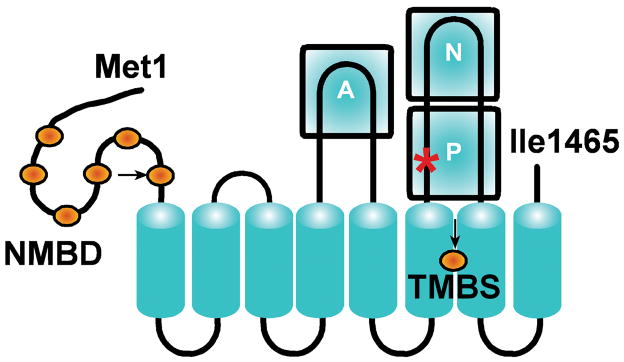
P-type ATPases are membrane-bound enzymes that utilize ATP for cation transport [1–3] using the catalytic mechanism of acid halogenases [4], which includes formation of a phosphoenzyme intermediate by covalent transfer of ATP γ-phosphate to an aspartyl residue within an invariant DKTG motif. In the P-type ATPases, ATP utilization is dependent on specific cation binding to an activating site, and phosphoenzyme formation is coupled to displacement/occlusion of the bound cation. Conformational isomerization of the phosphoenzyme intermediate is then followed by active transport of the occluded cation, and hydrolytic cleavage of Pi. The typical architecture of P-type ATPases, such as the well characterized sarcoplasmic reticulum (SR) Ca2+-ATPase [5] or Na+,K+-ATPase [6], comprises a transmembrane region with ten transmembrane segments including cation binding sites involved in catalytic activation and active transport. The headpiece of these proteins protrudes from the membrane, and includes the ATP binding site in the N domain, the aspartyl residue undergoing phosphorylation in the P domain, and a conserved TGE sequence in the A domain required for catalytic assistance of the final hydrolytic reaction. The ATP7B copper ATPase, involved in the etiology of Wilson disease [7], is included in the P1-type ATPase subfamily which is selective for soft and transition metals. Its function is to deliver copper to nascent metalloproteins and export excessive copper from the cell [8]. The ATP7B protein comprises a transmembrane region with eight membrane spanning segments, and a putative transmembrane metal binding site (TMBS) between the sixth and seventh transmembrane segments (Fig 1), corresponding to the specific cation binding/transport sites of other P-type ATPases. In addition to the A, N and P domains common to other P-type ATPases, the headpiece of ATP7B includes an N–metal binding domain (NMBD) with six additional copper binding sites (Fig 1). Furthermore, an important feature of ATP7B is the presence of serine residues that undergo phosphorylation attributed to protein kinase assistance, and may be involved in functionally relevant ATP7B interactions [9].
Fig 1.
Two-dimensional folding model of the ATP7B sequence. The diagram shows eight transmembrane segments including a copper (orange sphere) binding site (transmembrane copper binding site, TMBS). The extramembranous region includes: the nucleotide binding domain (N); the P domain, where D1027 (red asterisk) undergoes phosphorylation to form the catalytic phosphoenzyme intermediate; the actuator domain (A); the N-metal binding domain (NMBD) with additional six copper (orange spheres) binding sites; and a C-terminus chain. Mutations at the TMBS and NMBD copper sites are indicated by arrows.
Much progress in characterization of ATP7B and its isoform ATP7A (Menkes disease protein) has been obtained by heterologous expression in cultured cells [10,11]. We reported very recently high yield heterologous expression of ATP7B in COS-1 cells infected with adenovirus vector, and functional characterization of membrane-bound ATPase obtained with the microsomal fraction of infected cells [12]. In the present manuscript we show that the membrane-bound recombinant Cu+-ATPase obtained under the above-mentioned conditions is suited for adsorption on a solid supported membrane (SSM) and charge transfer measurements. The SSM method has been employed for detection of charge movements by primary and secondary active membrane transporters [13]. We demonstrate by pre-steady state charge measurements within a single catalytic cycle time frame, that the chemical potential of ATP is utilized for electrogenic movement of bound copper within the ATP7B protein. This charge movement is totally prevented by mutations of either a single NMBD site or the TMBS copper binding site. We also compare the time constants of cation displacement in the copper and in the calcium transport ATPases.
2. Materials and Methods
Recombinant adenovirus vector (rAdATP7Bmyc) containing CMV promoter driven WT human ATP7B cDNA, fused with 3′ c-myc tag, was constructed as explained previously [12]. Site directed mutations C983A and C985A (in the transmembrane copper binding site, TMBS) and C575A and C578A (in the 6th copper site of the N-metal binding domain, NMBD) were produced in pShuttleCMV-ATP7Bmyc plasmid using the QuickChange II Site-Directed Mutagenesis Kit (Stratagene, Cedar Creek, TX) as per the manufacturer’s instructions. The mutations were confirmed by sequencing, and related plasmids were used for construction of rAdATP7Bmyc mutants and infection of COS-1 cells. Microsomes were prepared from COS-1 cells and protein expression was evaluated by SDS gel electrophoresis or Western blotting as explained previously [12].
2.1. [32P]Phosphoenzyme formation by incubation of microsomes with [γ-32P]ATP
Microsomal protein (50 μg protein/ml) was incubated with 50 μM [γ-32P]ATP at 30 °C, in a reaction mixture containing 50 mM MES triethanolamine, pH 6.0, 300 mM KCl, 10 mM DTT, 3 mM MgCl2 and 5 μM CuCl2. Samples were quenched at serial times with 5% trichloroacetic acid. The quenched samples were pelleted by centrifugation at 5000 rpm for 5 min, washed with 0.125 N TCA, and finally resuspended in pH 8.3 (half sample) or 6.3 loading buffer (remaining half sample), and separated by Laemmli [14] (alkaline buffer) or Weber and Osborn [15] (acid buffer) gel electrophoresis. The gels were dried and exposed to a phosphor screen followed by scanning on a Typhoon scanner (Amersham, Piscataway Township, NJ) for stoichiometric determination of phosphoprotein relative to three [γ-32P]ATP standards placed on the gels. Comparative experiments were performed with microsomes derived from COS-1 cells infected with rAdATP7Bmyc (WT and mutants) and cells infected with rAdGFP (“sham”).
2.2. Measurement of charge movements
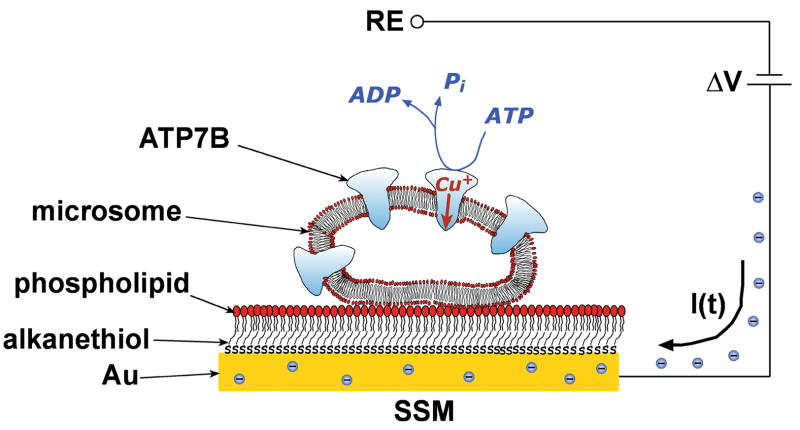
Charge movements were measured by adsorbing microsomes containing recombinant WT or mutant Cu+-ATPase on a SSM (Fig 2). The SSM consists of an alkanethiol monolayer covalently bound to a gold electrode via the sulfur atom, and a phospholipid monolayer on top of it [16]. After adsorption, the protein was activated by a concentration jump of a suitable substrate, i.e. ATP (Fig 2). If at least one electrogenic step is involved in the relaxation process, a current transient can be recorded along the external circuit [13]. Numerical integration of each transient is related to a net charge movement, which depends upon the particular electrogenic event. In addition, kinetic information can be obtained by fitting a sum of exponentially decaying terms to the current versus time curves.
Fig 2.
Schematic diagram of a microsome containing ATP7B adsorbed on a SSM (not drawn to scale). When charge displacement does occur, a compensating current I(t) flows along the external circuit (the blue spheres are electrons) if the potential difference, ΔV, applied across the whole system is kept constant. RE is the reference electrode. For simplicity, only four Cu+-ATPase molecules are shown within the microsome.
In ATP concentration-jump experiments, the non-activating solution contained 300mM KCl, 50 mM MES triethanolamine, pH 6.0, 5 mM MgCl2, 0.1 mM CaCl2, 10 mM DTT, and CuCl2 (5 μM) or BCS (1 and 5 mM); the activating solution contained, in addition, 100 μM ATP. The concentration jump experiments were carried out by employing the SURFE2ROne device (IonGate Biosciences, Frankfurt/M., Germany). The temperature was maintained at 22–23 °C for all the experiments.
To verify the reproducibility of the current transients generated within the same set of measurements on the same SSM, each single measurement of the set was repeated 6–8 times and then averaged to improve the signal to noise ratio. Standard deviations were usually found to be no >±5%. Moreover, each set of measurements was usually reproduced using three different SSM electrodes.
3. Results and Discussion
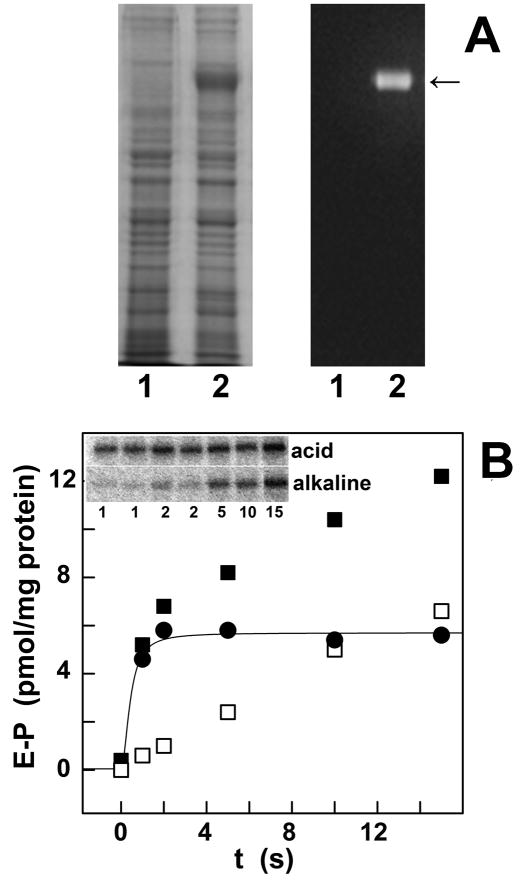
Taking advantage of high yield heterologous expression of ATP7B in COS-1 cells [12] and recovery of the expressed protein with the microsomal fraction of infected cells (Fig 3A), we clearly identified two distinct fractions of phosphoprotein formed by utilization of ATP (Fig 3B). An alkaline labile phosphoprotein fraction, corresponding to the catalytic ATPase intermediate (i.e., aspartyl phosphate), is formed rapidly and reaches steady state levels within one second at 30°C. Formation of the phosphoenzyme intermediate is prevented by mutation of the conserved Asp1027 or Cys residues within putative copper sites [12]. In addition, an alkaline stable fraction is formed within a more extended time scale (Fig 3B), due to phosphorylation of serine residues at least in part identified by mass spectrometry as Ser478, Ser481, Ser1121, and Ser1453 [12]. Phosphorylation of both aspartate and serine residues is copper dependent [12].
Fig 3.
Heterologous expression of ATP7B (A), and phosphorylation by ATP (B). (A): Electrophoretic analysis of the microsomal fraction of sham COS1 cells (left in each panel) and COS1 cells sustaining heterologous expression of ATP7B (right in each panel), followed by general staining of proteins with Coomassie Blue (left panel), and specific immunostaining of the expressed ATP7B protein using a monoclonal antibody for a c-myc tag (right panel). (B): Phosphorylation of ATP7B was obtained by addition of (γ-32P)ATP, followed by acid quenching at times specified in the figure (inset). The quenched protein was then dissolved in detergent, and subjected to electrophoretic analysis in acid or in alkaline media to detect total phosphoprotein (■), and distinguish alkaline labile (●, aspartyl phosphate) and alkaline stable (□, phosphorylated serines) fractions.
While detection of cation displacement can be demonstrated with radioactive isotopes for the SR Ca2+-ATPase and the Na+,K+-ATPase, analogous measurements with the copper ATPase are difficult, due to the low native abundance of the protein and unfavorable features (very short half life and high gamma emission) of the 64Cu radioactive isotope. Even though 64Cu has been used in steady state copper transport assay [10], its use in single cycle experiments would be very difficult. We recently reported detection of pre-steady state calcium movements by charge measurements with native or recombinant SR Ca2+-ATPase adsorbed on a SSM [17,18]. We have now applied this technique to studies of copper movements in the ATP7B protein. To this aim, we collected the microsomal fraction of COS-1 cells sustaining heterologous expression of recombinant ATP7B and allowed the microsomal membrane-bound protein to adsorb on the SSM electrode. We then activated ATP7B with saturating (100 μM) ATP delivered through a concentration jump, and detected the related current transient by the SSM method.
It is shown in Fig 4A that, in the presence of 5 μM CuCl2, the ATP concentration jump induces a current transient (Fig 4A, solid line), which is not observed in the presence of bathocuproine disulfonate (BCS), a specific copper chelator (Fig 4A, dotted line). To gain evidence for the functional relevance of the observed signal, we then repeated the measurements using specific mutants of ATP7B. To this aim, we produced mutations (see 2. Materials and Methods) at the TMBS or at the 6th NMBD copper sites, i.e. C983A/C985A and C575A/C578A respectively, obtaining expression of mutant protein as high as that of WT ATP7B (Fig 4, inset). Both mutants were found to be catalytically inactive, inasmuch as no phosphoenzyme intermediate was formed upon addition of ATP. When we attempted measurements of charge movements with these ATPase mutants, we observed no current transient following addition of ATP in the presence of 5μM CuCl2 (Figs 4B and 4C, solid lines) or in the presence of 1mM BCS (Figs 4B and 4C, dotted lines). Taken together, our electrical measurements indicate that the charge obtained by numerical integration of the ATP-induced current transient corresponds to an electrogenic event related to vectorial displacement of bound Cu+ upon utilization of ATP.
Fig 4.
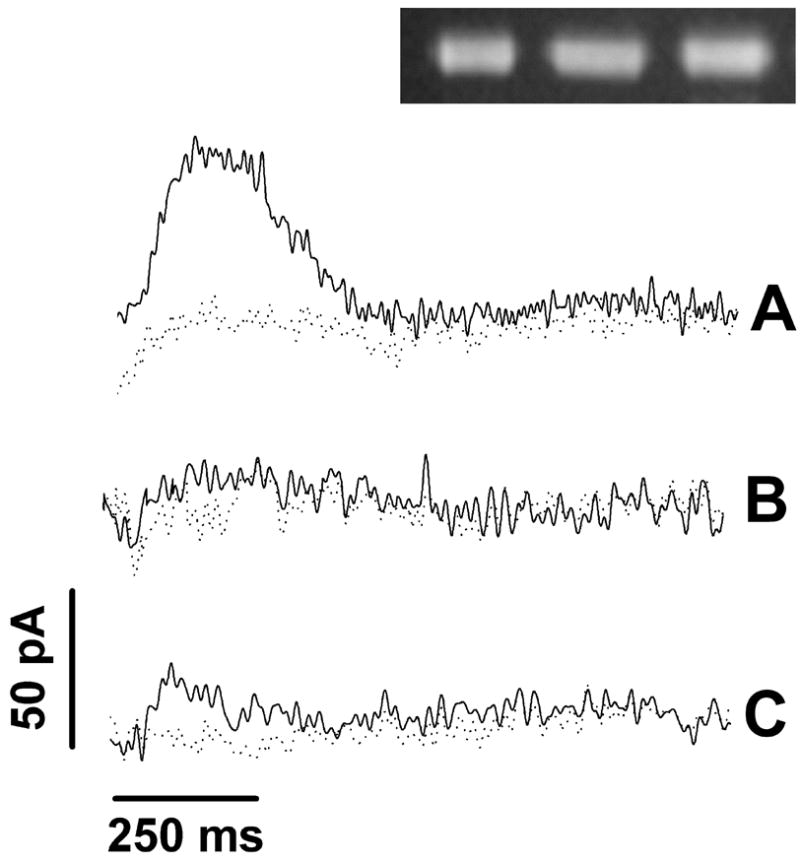
Recombinant WT and mutant Cu+-ATPase. Current transients induced by 100μM ATP concentration jumps in the presence of 5μM CuCl2 (solid lines) or 1mM BCS (dotted lines) on WT and mutant Cu+-ATPase: (A) WT Cu+-ATPase, (B) C983A/C985A mutant, and (C) C575A/C578A mutant. The right upper corner inset shows Western blots of the WT and two mutant proteins demonstrating nearly identical expression levels. The Western blots were obtained with an antibody reacting with the same c-Myc tag present in the three constructs.
It is noteworthy that the observed current transient is due to flow of electrons along the external circuit toward the electrode surface (Fig 2). Such current signal is required to compensate for the potential difference across the vesicular membrane produced by displacement of positive charge (i.e., bound cation) in the direction of the SSM electrode, if the applied voltage across the whole system is kept constant [18]. The current recorded by the SSM method is a measure of the rate of change of the transmembrane potential, and is not sensitive to stationary currents. Therefore, only electrogenic steps within the first catalytic cycle are detected, while steady-state events after the first cycle are not measured [18].
We have then compared (Fig 5) the different time frames of charge movements generated by addition of saturating ATP concentrations to the recombinant Cu+-ATPase (in the presence of 5 μM CuCl2, solid line), or to the recombinant SR Ca2+-ATPase (in the presence of 10 μM free Ca2+, dashed line). Analysis of the time frames then indicates that the charge transfer decay time constant for the recombinant WT Ca2+-ATPase signal (25 ± 0.3 ms) is in good agreement with values obtained with native SR microsomes [19]. On the other hand the charge transfer decay time constant obtained with ATP7B (140 ± 4 ms) is about one order of magnitude longer. These time constants are derived from first order exponential decays, and are therefore independent of the protein concentrations. Finally, it is worth noting that the decay time constant of the Cu+-ATPase current signal (140 ms) is within the time frame of aspartyl phosphate formation (Fig 3B), indicating its correspondence to completion of the first cycle of phosphorylation. This suggests a direct correlation between Cu+ displacement and phosphoenzyme formation, preceding phosphoenzyme hydrolytic cleavage.
Fig 5.
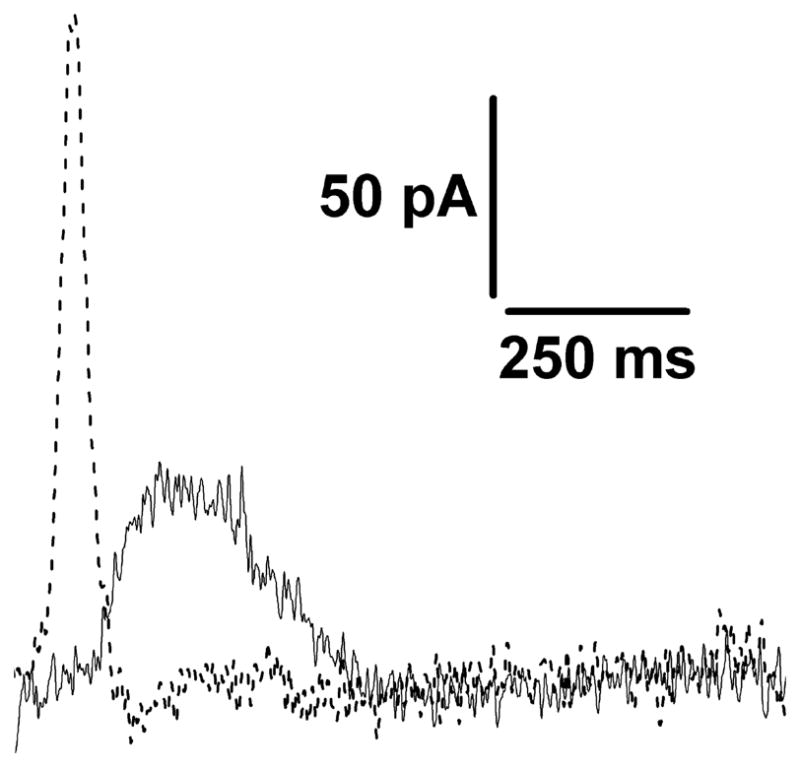
Recombinant Cu+-ATPase and SR Ca2+-ATPase. Current transients obtained after 100μM ATP concentration jumps on Cu+-ATPase (in the presence of 5μM CuCl2, solid line) and on SR Ca2+-ATPase (in the presence of 10μM free Ca2+, dashed line).
It should be pointed out that the observed decay time constants are pertinent to an initial partial reaction of the ATPase catalytic cycle. They are not equivalent to steady state turnover which is expected to be slower, due to rate limiting steps preceding the final hydrolytic cleavage of the phosphoenzyme intermediate.
In conclusion, we detected positive charge displacement within a single catalytic cycle of ATP7B upon addition of ATP and formation of phosphoenzyme intermediate. We attribute this positive charge displacement to movement of bound copper within the ATP7B protein, determining that a single cycle of the copper pump is electrogenic. We produced site directed mutations demonstrating that ATP7B activation requires occupancy of a copper binding site in the N-terminus extension which is not present in other transport ATPases, as well as of a transmembrane site corresponding to the cation binding site of other ATPases. We also found that the catalytic time constant for ATP7B is one order of magnitude longer than for the Ca2+-ATPase (SERCA).
Acknowledgments
This work was supported by the U.S. National Institutes of Health (RO301-69830 from the NHBLI), the Italian Ministry of Education, University and Research (PRIN 2008), the Ente Cassa di Risparmio di Firenze and the Italian Ministry of Foreign Affairs.
Abbreviations used
- BCS
bathocuproine disulfonate
- NMBD
N–metal binding domain
- SR
sarcoplasmic reticulum
- SSM
solid supported membrane
- TMBS
transmembrane metal binding site
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmgren MG, Axelsen KB. Evolution of P-type ATPases. Biochim Biophys Acta. 1998;1365:37–45. doi: 10.1016/s0005-2728(98)00041-3. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PL, Carafoli E. Ion motive ATPases. Ubiquity, properties and significance to cell function. Trends Biochem Sci. 1987;12:146–150. [Google Scholar]
- 3.Kühlbrandt W. Biology, structure and mechanism of P-type ATPases. Nat Rev Mol Cell Biol. 2004;5:282–295. doi: 10.1038/nrm1354. [DOI] [PubMed] [Google Scholar]
- 4.Burroughs AM, Allen KN, Dunaway-Mariano D, Aravind L. Evolutionary genomics of the HAD superfamily: understanding the structural adaptations and catalytic diversity in a superfamily of phosphoesterases and allied enzymes. J Mol Biol. 2006;361:1003–1034. doi: 10.1016/j.jmb.2006.06.049. [DOI] [PubMed] [Google Scholar]
- 5.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 angstrom resolution. Nature. 2000;405:647–655. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 6.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sørensen TLM, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1050. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 7.Forbes JR, Cox DW. Functional characterization of missense mutations in ATP7B: Wilson disease mutation or normal variant? Am J Hum Genet. 1998;63:1663–1674. doi: 10.1086/302163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. doi: 10.1152/physrev.00004.2006. [DOI] [PubMed] [Google Scholar]
- 9.Veldhuis NA, Valova VA, Gaeth AP, Palstra N, Hannan KM, Michell BJ, Kelly LE, Jennings I, Kemp BE, Pearson RB, Robinson PJ, Camakaris J. Phosphorylation regulates copper-responsive trafficking of the Menkes copper transporting P-type ATPase. Int J Biochem Cell Biol. 2009;41:2403–2412. doi: 10.1016/j.biocel.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Hung YH, Layton MJ, Voskoboinik I, Mercer JF, Camakaris J. Purification and membrane reconstitution of catalytically active Menkes copper-transporting P-type ATPase (MNK; ATP7A) Biochem J. 2007;401:569–579. doi: 10.1042/BJ20060924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsivkovskii R, Eisses JF, Kaplan JH, Lutsenko S. Functional properties of the copper-transporting ATPase ATP7B (the Wilson's disease protein) expressed in insect cells. J Biol Chem. 2002;277:976–983. doi: 10.1074/jbc.M109368200. [DOI] [PubMed] [Google Scholar]
- 12.Pilankatta R, Lewis D, Adams CM, Inesi G. High yield heterologous expression of wild-type and mutant Cu+-ATPase (ATP7B, Wilson disease protein) for functional characterization of catalytic activity and serine residues undergoing copper-dependent phosphorylation. J Biol Chem. 2009;284:21307–21316. doi: 10.1074/jbc.M109.023341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz P, Garcia-Celma JJ, Fendler K. SSM-based electrophysiology. Methods. 2008;46:97–103. doi: 10.1016/j.ymeth.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Weber K, Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969;244:4406–4412. [PubMed] [Google Scholar]
- 16.Pintschovius J, Fendler K. Charge translocation by the Na+/K+-ATPase investigated on solid supported membranes: Rapid solution exchange with a new technique. Biophys J. 1999;76:814–826. doi: 10.1016/S0006-3495(99)77245-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Pilankatta R, Lewis D, Inesi G, Tadini-Buoninsegni F, Bartolommei G, Moncelli MR. High-yield heterologous expression of wild type and mutant Ca2+ ATPase: Characterization of Ca2+ binding sites by charge transfer. J Mol Biol. 2009;391:858–871. doi: 10.1016/j.jmb.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tadini-Buoninsegni F, Bartolommei G, Moncelli MR, Guidelli R, Inesi G. Pre-steady state electrogenic events of Ca2+/H+ exchange and transport by the Ca2+-ATPase. J Biol Chem. 2006;281:37720–37727. doi: 10.1074/jbc.M606040200. [DOI] [PubMed] [Google Scholar]
- 19.Bartolommei G, Buoninsegni FT, Moncelli MR. Calcium transport by sarcoplasmic reticulum Ca-ATPase can be investigated on a solid-supported membrane. Bioelectrochemistry. 2004;63:157–160. doi: 10.1016/j.bioelechem.2003.07.010. [DOI] [PubMed] [Google Scholar]