Abstract
The pubertal surge in gonadal hormones that occurs during adolescence may impact the long-term effects of early alcohol exposure and sex differences in drinking behavior in adulthood. We investigated this hypothesis by performing sham or gonadectomy surgeries in Long Evans rats around postnatal day (P) 20. From P35–45, males and females were given saline or 3.0 g/kg ethanol using a binge-like model of exposure (8 injections total). As adults (P100), they were trained to self-administer ethanol via a sucrose-fading procedure and then given access to different unsweetened concentrations (5–20% w/v) for 5 days/concentration. We found that during adolescence, ethanol-induced intoxication was similar in males and females that underwent sham surgery. In gonadectomized males and females, however, the level of intoxication was greater following the last injection compared to the first. During adulthood, females drank more sucrose per body weight than males and binge-like exposure to ethanol reduced sucrose consumption in both sexes. These effects were not seen in gonadectomized rats. Ethanol consumption was higher in saline-exposed females compared to males, with gonadectomy reversing this sex difference by increasing consumption in males and decreasing it in females. Exposure to ethanol during adolescence augmented ethanol consumption in both sexes, but this effect was statistically significant only in gonadectomized females. Together, these results support a role for gonadal hormones during puberty in the short- and long-term effects of ethanol on behavior and in the development of sex differences in consummatory behavior during adulthood.
Keywords: adolescence, gonadectomy, ethanol self-administration, sex differences, ovariectomy
Introduction
Alcohol consumption varies widely by age and sex [28, 89]. Approximately 85% of the population in the United States has their first drink by age 21 and the incidence of binge and heavy alcohol use is significantly higher during adolescence compared to adulthood [66]. Sex differences in alcohol intake, with males consuming more and having higher rates of alcohol dependence, are most notable in adulthood [29]. However, examination of drinking patterns in adolescents suggests a role for gonadal steroid hormones in the development of sex differences in drinking behavior. For example, early-maturing girls and boys have been shown to have higher rates of alcohol use [54, 83]. Furthermore, early menarche is associated with a higher incidence of drinking and smoking in adolescent girls [16, 88]. Studies in rodent models also show that adolescence is a period of increased alcohol consumption compared to adulthood [19, 44, 85]. Evidence for sex differences in adolescent consumption is somewhat equivocal: adolescent males have been reported to drink more than females in some rodent studies [37, 86], whereas others suggest that there is no sex difference [19, 82].
Activation of the hypothalamic-pituitary-gonadal axis, which leads to significant increases in circulating estrogen and testosterone, likely facilitates the structural and functional changes in the central nervous system that occur during adolescence [9, 40]. This includes volumetric changes in brain areas such as the frontal cortex, cerebellum, hippocampus, and amygdala [24, 25, 32, 45, 64, 80]. Also evident are changes in the neurotransmitter systems that mediate alcohol-related behaviors, including GABA [49, 90], glutamate [12, 91], dopamine [2, 5], acetylcholine [20, 41], and serotonin [17, 35]. In rodents, sex-specific drinking patterns coincide with the rise of gonadal hormones during adolescence [18], with females typically consuming higher quantities of alcohol than males post-puberty [19, 37, 82]. Notably, gonadectomy during adulthood does not significantly influence this sex difference in alcohol intake [1, 8]. Thus, rather than modulating alcohol intake directly, gonadal steroids during adolescence may organize mechanisms that lead to sexually disparate alcohol consumption.
Gonadal steroids may also modulate the long-term effects of alcohol when exposure starts during adolescence. In humans, drinking during adolescence is associated with heightened vulnerability to developing alcoholism [27]. This effect is more robust in females, as the duration is shorter between drinking onset and the diagnosis of alcohol dependence [57]. In rodents, adolescent exposure to alcohol has been shown to increase consumption in adulthood for both males and females [6, 53, 75], though this effect is dependent on factors such as strain [51], exposure procedure [72], and the method of assessing drinking [68, 79].
The purpose of the present study was to determine if preventing the rise in gonadal hormones during adolescence would influence sex differences in alcohol (ethanol) consumption during adulthood. Additionally, we determined if this manipulation altered the effects of ethanol exposure during adolescence on drinking behavior in adulthood. Male and female Long Evans rats were gonadectomized before puberty and later, during the peri-adolescent period, we exposed them to saline or ethanol using a binge-like pattern of administration. After animals reached adulthood, they were trained to drink unsweetened ethanol and consumption was measured at various concentrations using a limited-access drinking paradigm.
Materials and Methods
Subjects and surgical procedures
Male (n = 49) and female (n = 60) Long-Evans rats, bred in our animal facility from stock rats obtained from Simonson Labs (Gilroy, CA), were housed in groups of 2–3 rats/cage on a 12 hr light/dark cycle (lights on at 0800 h). They were maintained in a temperature and humidity controlled room, with food available ad libitum throughout the duration of the study. Water was also available ad libitum, except for 16 hrs before the first day of the sucrose-fading procedure (see below). All procedures were consistent with the `Principles of Laboratory Animal Care' (NIH Publication no. 85-23) and were approved by the IACUC at the University of Illinois, Urbana-Champaign, USA.
Bilateral gonadectomy (GDX and OVX for males and females, respectively) or sham surgeries were performed on postnatal day (P) 20 ± 1 day. Rats were anesthetized (2.5% isoflurane) and small incisions were made through the skin and intramuscular wall of the ventral torso. The testes or ovaries were removed and the skin was sutured with nylon thread. Sham surgeries were preformed the same way except the gonads were left intact. After the surgery, rats were returned to the dam until weaning on P25.
Binge-like ethanol exposure
From P35–45, rats were exposed intermittently to saline or 3.0 g/kg ethanol (25% v/v) via intraperitoneal (i.p.) injection. The exposure schedule consisted of one injection per day for two consecutive days, followed by one day with no injections. This cycle repeated four times for a total of 8 injections across 11 days. A similar exposure method has been previously shown to produce enduring behavioral and neurophysiological changes that persist into adulthood [53]. A subset of rats in the ethanol-treated groups (n = 24 males and 29 females) were monitored following the first and last ethanol injections using a previously established intoxication rating system [10, 43]. For these 60-min sessions, rats were placed individually into a clear plastic enclosure (43×22×20 cm) lined with hardwood bedding. These enclosures and bedding were comparable to those used for the rat's home cage. Observers that were blind to surgery condition scored behavior on a scale from 0–5, with a score of 0 given for no overt signs of intoxication, 1 for general sedation, 2 for mild ataxia and staggering gate, 3 for severe motor impairment, 4 for little to no motor behavior without loss of the righting reflex (LORR), and 5 for LORR, which was defined as an inability of the animal to right itself twice within 30 sec of being placed in the supine position. Intoxication level was assessed at 5, 10, and 15 min post-injection, with the mean of these scores used in subsequent analyses. The total amount of time each animal displayed LORR was also recorded.
Ethanol self-administration in adulthood
When the males (n = 49) and a subset of the females (n = 45) reached P100, they were trained to drink ethanol using a modified sucrose-fading procedure [23, 67]. Daily drinking sessions took place in a separate drinking chamber that was comparable to the rats' home cages and the enclosures used during binge-like ethanol exposure. All solutions were presented for 30 min in a single 100 ml bottle fitted with a double ball-bearing sipper tube (Ancare; Bellmore, NY) that minimized leakage. Initially, animals were water deprived for 16 hrs before they were given a session with access to 20% sucrose. They were then returned to ad libitum water access in their homecage and given a single session with 20% sucrose. During the next training phase, rats were given two sessions each with a sucrose-ethanol solution that had the concentration of sucrose reduced from 20, 10, 5, and 2.5% over successive sessions while the concentration of ethanol was kept constant at 5% (w/v). Following three sessions of 5% ethanol alone, rats were given two sessions with access to 5% sucrose + 10% (w/v) ethanol, two sessions with access to 2.5% sucrose + 10% ethanol, and three sessions with 10% ethanol alone. Lastly, rats were given two sessions with access to 5% sucrose + 20% (w/v) ethanol, two sessions with access to 2.5% sucrose + 20% ethanol, and three sessions with 20% ethanol alone.
In the next phase of training, a stable baseline of ethanol consumption was determined by providing rats ten daily 30 min sessions with access to 10% ethanol. This was followed by a concentration-response analysis wherein rats were presented with various concentrations of ethanol (5–20%) during their daily 30 min drinking sessions. Ethanol concentrations were presented in random order for 5 consecutive days each. Lastly, rats underwent ten days without drinking sessions (i.e., forced abstinence) and were then given five, once daily 30-min sessions where a single bottle of 10% ethanol was available.
Data analysis
Ratings of intoxication were obtained at 5, 10, and 15 min post-injection on the first and last day of binge-like ethanol exposure (P35 and 45, respectively). The mean of these ratings was taken as each rat's intoxication score. These scores, along with the amount of time each rat displayed LORR, were analyzed using mixed factorial ANOVA with sex (male or female) and surgery (sham or gonadectomy) as the between-subjects factor and injection number (1 or 8) as the repeated measure. A mixed factorial ANOVA (surgery × adolescent exposure × age, with P35, P45, and P100 as the levels of the repeated measure of age) was used to analyze body weights. Sucrose intake in adult males and females was analyzed with between-subjects ANOVA (sex × surgery × adolescent exposure), whereas ethanol intake was analyzed using a mixed factorial ANOVA (sex × surgery × adolescent exposure × ethanol concentration, with 10, 5, 8, 15, 20, and 10% as the levels of the repeated measure of ethanol concentration). Significant interactions were further analyzed with one, two, or three-way ANOVA and Holm-Sidak post-hoc tests.
Results
Binge-like exposure to ethanol during adolescence
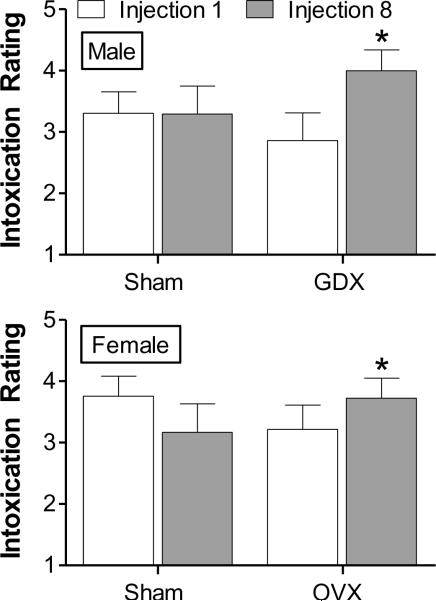
Exposure to saline or 3.0 g/kg ethanol began on P35 and continued through P45. As shown in Fig. 1, males and females showed similar levels of intoxication following ethanol treatment at injection 1 and 8. Overall ANOVA revealed a significant interaction between surgery and injection number [F(1,98) = 4.01, p < 0.05]. This was because gonadectomized rats achieved higher levels of intoxication following the last compared to the first injection [F(1,51) = 4.52, p < 0.05]. Approximately 60% of rats (30 out of 53) became sufficiently intoxicated to reach LORR following the first or last injection (12.3 ± 2.50 and 13.7 ± 2.74 min, respectively, for all rats regardless of sex or treatment group). However, the duration of LORR in these animals was not statistically different across injection, sex, or surgery, nor were there any significant interactions among these factors.
Figure 1.
Intoxication ratings following the first and last injection (1 and 8, respectively) during binge-like ethanol exposure during adolescence. Males (top; n = 24) and females (bottom; n = 29) were rated every 5 min for 15 min following 3.0 g/kg ethanol (i.p.). A maximum score of 5 was given in each bin and the group averages of the mean scores across the 15 min period are shown here (see Methods for a description of the scoring procedure). * p < 0.05, compared to injection 1 within the same group.
Body weights during adolescence and adulthood
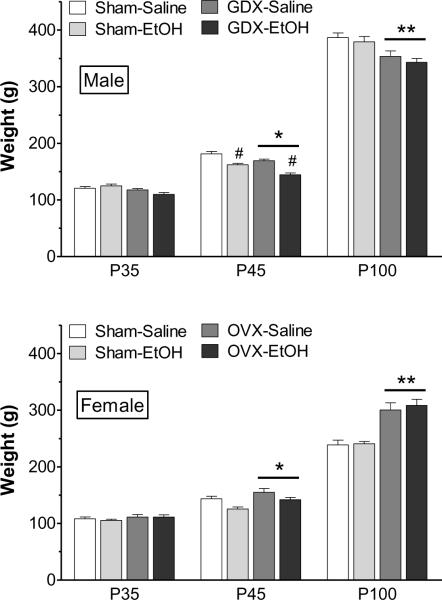
Prepubertal gonadectomy and ethanol exposure influenced growth rates during the peri-adolescent exposure period, particularly in males. ANOVA revealed a statistically significant interaction between adolescent exposure and age [F(2,135) = 3.40, p < 0.05]. As shown in Fig. 2, males that were exposed to ethanol during adolescence had significantly reduced weights on P45 compared to saline controls. This was particularly pronounced in GDX males. Nonetheless, in adulthood (P100) there were no significant weight differences between ethanol-exposed and saline-exposed rats. Females' weights were not significantly affected by adolescent ethanol exposure, although there was a trend for female ethanol-exposed rats to weigh less than controls [F(1,122) = 1.10, p = 0.08]. As expected, prepubertal gonadectomy significantly influenced weights in both sexes. In males, there was a significant interaction between surgery and age [F(2,135) = 5.84, p < 0.01], with GDX males weighing significantly less than sham controls after reaching P45. There was also a significant interaction between surgery and age in females [F(2,122) = 23.9, p < 0.001], with OVX females weighing significantly more than intact controls after reaching P45. The effects of gonadectomy on weight were most pronounced after males and females reached P100.
Figure 2.
Body weight of males (top) and females (bottom) before (P35) and after (P45) binge-like ethanol exposure, and also in adulthood (P100). The groups (n = 11–13 each) are sham operated, saline-exposed (Sham-Saline), sham operated, ethanol-exposed (Sham-EtOH), gonadectomy (males) or ovariectomy (females), saline-exposed (GDX-Saline or OVX-Saline), and GDX or OVX, ethanol-exposed (GDX-EtOH or OVX-EtOH). * p < 0.05, ** p < 0.001, compared to sham operated rats of same age, collapsed across exposure type; #p < 0.001, compared to saline-exposed rats of same age.
Ethanol self-administration in adulthood
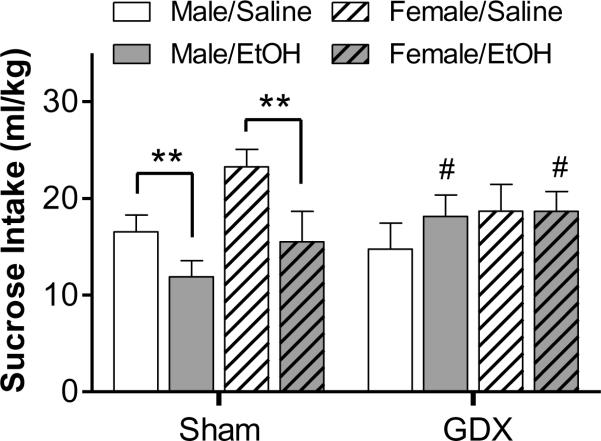
In order to assess if these adolescent manipulations influenced general consummatory behavior, we first measured intake of a highly palatable 20% w/v sucrose solution, which was offered unadulterated during the early stages of the sucrose-fading procedure. A three-way ANOVA of these data revealed a significant main effect of sex [F(1,86) = 5.23, p < 0.05] and a significant interaction between surgery and adolescent exposure [F(1,86) = 5.94, p < 0.05]. As shown in Fig. 3, saline-exposed females that underwent sham surgery consumed more sucrose per body weight than males in this control group. Exposure to ethanol during adolescence reduced sucrose consumption in males and females. The statistically significant sex difference in sucrose consumption, and the effect of ethanol exposure, was not seen in gonadectomized rats. Nonetheless, male and female gonadectomized rats exposed to ethanol did consume significantly more sucrose than sham, ethanol-exposed controls (Fig. 3).
Figure 3.
Sucrose consumption during the initial phase of the sucrose-fading procedure. Shown is the intake of 20% sucrose during a 30-min access period when rats had ad libitum access to food and water in their homecage. ** p < 0.01 compared to same sex ethanol-exposed shams; #p < 0.05, compared to same sex, ethanol-exposed sham rats
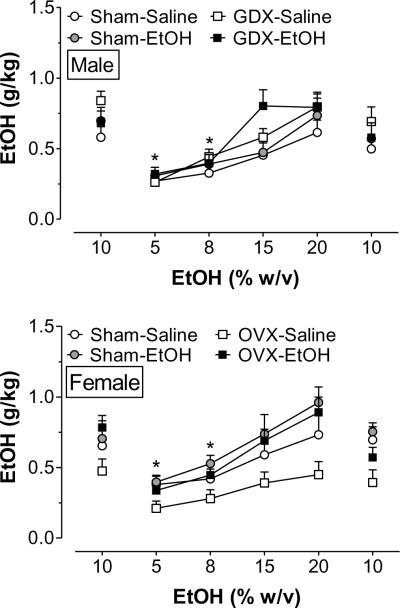
Following the sucrose-fading procedure, rats were given daily access to various concentrations of ethanol ranging from 5–20% w/v. Overall ANOVA revealed a significant main effect of ethanol concentration [F(5,516) = 29.0, p < 0.001] and a significant sex × surgery × adolescent exposure interaction [F(1, 516) = 4.93, p < 0.05]. The data for males and females were subsequently analyzed using separate one-way ANOVAs. As shown in Fig. 4, rats tended to achieve the highest level of ethanol intake when presented the higher concentrations of ethanol, with males consuming the most when they were given concentrations over 10% [F(5,288) = 18.0, p < 0.001] and females likewise escalating intake as ethanol concentration increased [F(5,264) = 10.4, p < 0.001].
Figure 4.
Concentration response curves for ethanol consumption in adult male (top) and female (bottom) rats. Initially, rats were given access to 10 % ethanol during 10 daily 30 min drinking sessions. When intake was stable at 10%, different ethanol concentrations (5–20 % w/v) were presented in random order for 5 consecutive days each. Finally, rats were given 5 more days of access to 10% ethanol following 10 days of alcohol deprivation. Data are presented as mean ± SEM, averaged across the final 3 days that each concentration was presented. Group labels are defined as in Fig. 3. * p < 0.05, compared to intake during the first presentation of 10% ethanol.
To further assess sex differences in ethanol intake, the data were collapsed across ethanol concentration. This revealed significant interactions of surgery × sex [F(1,556) = 18.8, p < 0.001] and adolescent exposure × sex [F(1,556) = 6.85, p < 0.01]. As shown in Fig. 5, males that underwent sham surgery consumed significantly less ethanol than sham females. Sex differences were also evident in response to prepubertal gonadectomy and adolescent ethanol exposure. In adulthood, GDX males consumed significantly more ethanol than shams. OVX females, in contrast, consumed less ethanol than their same-sex sham surgery controls. This effect of gonadectomy in females was reversed by adolescent alcohol exposure. Thus, ethanol exposure during adolescence augmented ethanol consumption in females, but had no significant effect on intake in males. This was true for both surgery types, but statistically significant from saline-exposed rats only in those that underwent OVX surgery.
Figure 5.
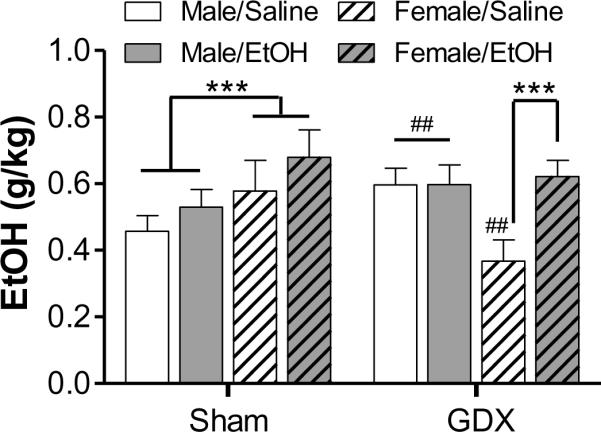
Drinking behavior collapsed across ethanol concentration (5–20% w/v). The average intake during the last 3 days each concentration was used for this analysis. *** p < 0.001; ##p < 0.01, compared to same-sex, sham-operated controls (collapsed across treatment)
Discussion
The results of these experiments suggest that preventing the rise in gonadal steroids during puberty alters responses to repeated ethanol exposure during adolescence and has significant effects on consummatory behavior in adulthood. In adolescence, gonadectomized males and females achieved heightened levels of intoxication following the last exposure to ethanol. In adulthood, females consumed more sucrose than males and ethanol exposure during adolescence decreased sucrose consumption in both sexes. Gonadectomy partially reversed these effects of ethanol exposure, with saline- and ethanol-exposed rats of both sexes consuming similar amounts of sucrose. Ethanol consumption during adulthood was also higher in females compared to males and these sex differences were significantly modulated by prepubertal gonadectomy and, to a lesser extent, by adolescent ethanol exposure. GDX males consumed significantly higher quantities of ethanol than same-sex sham surgery controls, while OVX females consumed less than their controls.
Previous reports indicate that rodents repeatedly exposed to high doses of ethanol during adolescence develop tolerance to the hypothermic, sedative, and hypnotic effects of ethanol [46, 78]. Similarly, we found no changes in ethanol intoxication in adolescent male and female rats given binge-like ethanol exposure and sham surgeries. Gonadectomy, however, appeared to enhance ethanol impairment in both sexes following repeated ethanol exposure. Ethanol-induced locomotor impairment is mediated, at least in part, via activation of GABAA receptors located within the cerebellum [14, 77]. Human imaging studies indicate that the cerebellum is one of the brain regions that is still developing during adolescence [52, 80]. Rodent studies are consistent with these findings and suggest that gonadal hormones modulate cerebellar development [42, 76] and GABAA function [7]. Furthermore, gonadal hormones are thought to be protective against ethanol-induced neurotoxicity and withdrawal via GABAergic activation [31, 34, 60, 61]. Thus, altered cerebellar development and GABAergic function in gonadectomized rats may contribute to the increased sensitivity to ethanol intoxication we observed here.
Although we found effects of gonadectomy on ethanol-induced intoxication, we did not see differences in LORR duration. In fact, just over half of the rats we tested displayed LORR to the 3.0 g/kg dose of ethanol. Although it is not clear why gonadectomy influenced ethanol-induced locomotor impairment but not LORR, previous reports indicate that adolescents generally display decreased sensitivity to the sedative-hypnotic effects of ethanol [69] and show only modest LORR in response to ethanol doses below 4.0 g/kg [46]. Notably, similar doses (2.0 – 3.0 g/kg) given during a binge pattern of exposure have been shown previously to produce significant blood ethanol concentrations (BECs; ~200 mg%) and lead to long-term behavioral changes and neuroadaptations within the mesocorticolimbic system [53, 56] in adolescent rats.
Significant, acute effects of exposure to ethanol on body weight in adolescent rats have been reported previously following an intermittent exposure regimen [70]. In the present study, ethanol treatment-induced decreases in body weight were statistically significant in males, but not in females. We also found no evidence of gross, morphological changes when rats reached adulthood, as the weights of ethanol-exposed rats from both sexes where not different from their saline-exposed controls. Previous studies have suggested that binge-like exposure to ethanol during adolescence can produce long-term effects on bone mass and skeletal development [38, 87]. We did not measure these parameters in the current study, but it is unlikely that they played a significant role in adult drinking behavior because we found a greater influence of ethanol exposure during adolescence in the rats that did not exhibit significant ethanol-induced decreases in body weight at P45 (i.e., females). The reduced body weights of males and females that were gonadectomized before puberty, which were expected based on previous studies [73], are indicative of successful removal of sex hormones via the pre-pubertal gonadectomy.
In adulthood, we found that females consumed higher doses of ethanol than males. This finding is consistent with the majority of reports indicating that adult female rats consume greater quantities of ethanol than males per body weight [19, 37, 39]. Interestingly, this is an effect that tends to emerge following puberty [19, 37, 82]. Previous studies have demonstrated inconsistent effects of gonadectomy on sex-specific patterns of ethanol intake during adulthood. For example, some studies have shown that adult gonadectomy has little to no effect on male and female rat drinking behavior [1, 8], whereas others [4, 22] show that ovariectomy reduces ethanol consumption in females. In these studies, the effect of ovariectomy was reduced by subsequent replacement therapy with estradiol, but not progesterone. In males, gonadectomy in adulthood had no consistent effects on ethanol consumption, but subsequent administration of either testosterone or estrogen increased ethanol drinking under certain conditions [8, 30, 36]. Differences in strain, ethanol drinking protocol, or age of assessment, all of which varied across these studies, may have contributed to the lack of consistency in these findings, but one factor they all had in common was that gonadectomies were performed after puberty. Here, we addressed whether the sex-specific patterns of ethanol intake in adulthood were influenced by the rising hormone levels during puberty by preventing this hormonal surge through prepubertal gonadectomy. We found that the effects of gonadectomy on drinking behavior persist well into the adult time period, thereby supporting the hypothesis that sex differences in ethanol intake during adulthood are established by gonadal hormones during puberty. Future studies that employ hormone replacement procedures in rats given prepubertal gonadectomy will be important for determining if sex differences in ethanol intake that are blocked by prepubertal gonadectomy can be reversed. Furthermore, they will help identify if puberty is a critical period during which sex differences in drinking behavior emerge. In addition to the issue of timing, further experiments that replace the gonadal steroids both chronically or cyclically are needed to elucidate the role that specific hormones have in the effect.
The mechanisms of the effects of pre-pubertal gonadectomy on ethanol drinking behavior in adulthood are not clear. One factor that potentially contributed to the effects we observed was an influence of early life experience. Early life stress, for example, is well known to alter behavioral outcomes in adulthood [33]. Rats that underwent pre-pubertal gonadectomy were subjected to brief (~ 90 min) maternal separation and, upon their return to the dams, may have been subjected to increased maternal attention (i.e. licking and grooming). However, we controlled for this by using comparison groups that underwent identical procedures except their gonads were left intact. Furthermore, the effects of maternal behaviors on pups are considerably more robust in the early postnatal period compared to just prior to weaning [47, 50]. Another potential contributing factor to our observed effects is sex differences in hepatic alcohol dehydrogenase (ADH) activity and ethanol metabolism. These have been reported in humans and rodent models, with females typically showing more rapid ethanol clearance than males [48]. However, the effects of gonadectomy in adulthood on these differences in ethanol metabolism have been inconsistent. In rodents, gonadectomy in males has been shown to produce no change or an increase in hepatic activity and ethanol clearance, whereas ovariectomy had no effect in females [26, 59]. Furthermore, ethanol pharmacokinetics in the blood and the brain have been shown to be unaltered across the estrous cycle in female Sprague-Dawley rats [62]. BEC was not measured in the present study, so it is unclear if the effect of gonadectomy on male intake reported here is in part due to changes in ethanol metabolism. However, it is unlikely these effects are solely the result of altered ethanol metabolism because we did not observe any sex, surgery, or treatment group differences in LORR duration. It is also noteworthy that the ethanol doses consumed in the present study are consistent with previous reports of intake in outbred rats tested in limited-access home cage and operant paradigms, where doses and BECs typically range between 0.2–0.8 g/kg and 10–50 mg%, respectively [13, 53, 82, 86].
Because we used a sucrose-fading procedure to engender self-administration of significant amounts of ethanol, we were also able to determine potential group differences in consumption of a natural reward. We found that females drank greater quantities of sucrose when intake is adjusted for body weight. Previously, female rats have been shown to be more responsive to sweet tastants [11, 92], which may contribute to the sex difference in sucrose intake shown here. Interestingly, the sex difference in sucrose consumption was abolished by gonadectomy. We also found that adolescent ethanol exposure led to reduced sucrose intake in sham surgery rats of both sexes, but had no effect on gonadectomized animals. This finding suggests gonadectomy may have blocked ethanol-induced `anhedonia'. In adult rats, chronic ethanol exposure has previously been shown to reduce motivation to procure sucrose under both fixed ratio (FR1) and progressive ratio (PR) schedules of reinforcement [71]. Our findings are consistent with other studies showing that gonadectomy influences reward-related behavior in response to drugs like cocaine [65] and nicotine [81].
We found that exposure to ethanol during adolescence also influenced ethanol intake in adulthood, although this effect was statistically significant only in OVX females. Similar findings in mice have recently been reported showing females to be more sensitive than males to binge-like exposure to ethanol during adolescence [75]. It is not clear why sham females in the current study were not affected in the same robust way as OVX females, although this finding is consistent with other studies suggesting that estrogen is protective against the long-term effects of ethanol exposure during adolescence [60]. Furthermore, adolescent ethanol exposure does not always influence self-administration in adult rats [72] and the effects may appear only under certain conditions (e.g. stress [68]). Meanwhile, others have shown that adolescent ethanol exposure modulates multiple ethanol-induced behaviors in adulthood, including novelty response [74], responsiveness to ethanol odor [21], LORR [46], withdrawal [10], and self-administration [53, 63].
Although the mechanisms underlying the effects of early alcohol exposure on drinking behavior in adulthood are not fully understood, one contributing factor may be ethanol-induced disruption of the neurophysiological systems that mediate ethanol consumption. In support of this hypothesis, binge-like exposure to ethanol during adolescence modulates the expression of dopaminergic and glutamatergic receptors in the prefrontal cortex and nucleus accumbens [53]. In addition, recent microdialysis studies indicated that dopaminergic neurotransmission is altered in rats that are exposed to ethanol during adolescence [3, 55, 56]. Changes in hypothalamic pituitary adrenal (HPA) axis regulation were also found in male and female rats after binge-like ethanol exposure during adolescence [58]. Further studies are warranted in order to assess the role these physiological changes play in the long-term behavioral consequences of adolescent exposure. Alternatively, it is possible that changes in ethanol metabolism may have contributed to the effects we found here. Relatively few studies have assessed changes in ethanol metabolism following repeated exposure during adolescence, with one study demonstrating metabolic tolerance in adolescents twelve days following repeated ethanol exposure [70]. Others have reported no changes in BECs following exposure [51, 84].
In summary, our findings highlight the important role that gonadal hormones during puberty play in the acute intoxicating effects of ethanol during adolescence and in sex differences in the consumption of natural rewards (sucrose) and ethanol in adulthood. Future studies will be necessary to determine if it was the lack of hormones during the pubertal period itself that was critical or whether gonadectomy in adulthood would produce the same effect. It is noteworthy, however, that previous studies utilizing gonadectomy in adulthood argue against the latter possibility in regards to gonadectomy effects on ethanol drinking. At the present time, the specific underlying mechanisms of the effects we observed are unclear, but it is possible that gonadal hormones influence ethanol drinking behavior by modulating individual sensitivity to the rewarding, or alternatively the aversive properties, of ethanol [15, 84, 86]. Future studies are warranted to elucidate if adolescents, relative to adults, are especially sensitive to the long-lasting neurophysiological and behavioral effects of ethanol and how gonadal steroids modulate this heightened sensitivity.
Acknowledgements
This study was supported by a grant from the National Institute on Alcohol Abuse and Alcoholism (R21 AA017354). We thank Danielle Ranney, Tomasz Podobinski, Andrew Barfield, Claire Berthold, Paul Hopkins, and Shanhai Heywood for technical assistance. We also thank Dr. Janice Juraska for advice and helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Almeida OF, Shoaib M, Deicke J, Fischer D, Darwish MH, Patchev VK. Gender differences in ethanol preference and ingestion in rats. The role of the gonadal steroid environment. J Clin Invest. 1998;101:2677–2685. doi: 10.1172/JCI1198. doi: 10.1172/JCI1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. doi: 2-B. [DOI] [PubMed] [Google Scholar]
- [3].Badanich KA, Maldonado AM, Kirstein CL. Chronic ethanol exposure during adolescence increases basal dopamine in the nucleus accumbens septi during adulthood. Alcohol Clin Exp Res. 2007;31:895–900. doi: 10.1111/j.1530-0277.2007.00370.x. doi: 10.1111/j.1530-0277.2007.00370.x. [DOI] [PubMed] [Google Scholar]
- [4].Becker HC, Anton RF, De Trana C, Randall CL. Sensitivity to ethanol in female mice: effects of ovariectomy and strain. Life Sci. 1985;37:1293–1300. doi: 10.1016/0024-3205(85)90244-9. [DOI] [PubMed] [Google Scholar]
- [5].Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- [6].Blizard DA, Vandenbergh DJ, Jefferson AL, Chatlos CD, Vogler GP, McClearn GE. Effects of periadolescent ethanol exposure on alcohol preference in two BALB substrains. Alcohol. 2004;34:177–185. doi: 10.1016/j.alcohol.2004.08.007. [DOI] [PubMed] [Google Scholar]
- [7].Bujas M, Pericic D, Jazvinscak M. Influence of gender and gonadectomy on bicuculline-induced convulsions and on GABAA receptors. Brain Res Bull. 1997;43:411–416. doi: 10.1016/s0361-9230(97)00027-0. [DOI] [PubMed] [Google Scholar]
- [8].Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- [9].Cameron JL. Interrelationships between hormones, behavior, and affect during adolescence: understanding hormonal, physical, and brain changes occurring in association with pubertal activation of the reproductive axis. Introduction to part III. Ann N Y Acad Sci. 2004;1021:110–123. doi: 10.1196/annals.1308.012. doi: 10.1196/annals.1308.012. [DOI] [PubMed] [Google Scholar]
- [10].Chung CS, Wang J, Wehman M, Rhoads DE. Severity of alcohol withdrawal symptoms depends on developmental stage of Long-Evans rats. Pharmacol Biochem Behav. 2008;89:137–144. doi: 10.1016/j.pbb.2007.12.002. doi: 10.1016/j.pbb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clarke SN, Ossenkopp KP. Taste reactivity responses in rats: influence of sex and the estrous cycle. Am J Physiol. 1998;274:R718–24. doi: 10.1152/ajpregu.1998.274.3.R718. [DOI] [PubMed] [Google Scholar]
- [12].Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453:116–130. doi: 10.1002/cne.10376. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- [13].Czachowski CL, Samson HH, Denning CE. Blood ethanol concentrations in rats drinking sucrose/ethanol solutions. Alcohol Clin Exp Res. 1999;23:1331–1335. [PubMed] [Google Scholar]
- [14].Dar MS. Antagonism by intracerebellar Ro15-4513 of acute ethanol-induced motor incoordination in mice. Pharmacol Biochem Behav. 1995;52:217–223. doi: 10.1016/0091-3057(95)00107-8. [DOI] [PubMed] [Google Scholar]
- [15].Diaz-Granados JL, Graham DL. The effects of continuous and intermittent ethanol exposure in adolesence on the aversive properties of ethanol during adulthood. Alcohol Clin Exp Res. 2007;31:2020–2027. doi: 10.1111/j.1530-0277.2007.00534.x. doi: 10.1111/j.1530-0277.2007.00534.x. [DOI] [PubMed] [Google Scholar]
- [16].Dick DM, Rose RJ, Viken RJ, Kaprio J. Pubertal timing and substance use: associations between and within families across late adolescence. Dev Psychol. 2000;36:180–189. [PubMed] [Google Scholar]
- [17].Dinopoulos A, Dori I, Parnavelas JG. The serotonin innervation of the basal forebrain shows a transient phase during development. Brain Res Dev Brain Res. 1997;99:38–52. doi: 10.1016/s0165-3806(96)00198-8. [DOI] [PubMed] [Google Scholar]
- [18].Dohler KD, Wuttke W. Changes with age in levels of serum gonadotropins, prolactin and gonadal steroids in prepubertal male and female rats. Endocrinology. 1975;97:898–907. doi: 10.1210/endo-97-4-898. [DOI] [PubMed] [Google Scholar]
- [19].Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- [20].Doura MB, Gold AB, Keller AB, Perry DC. Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Res. 2008;1215:40–52. doi: 10.1016/j.brainres.2008.03.056. doi: 10.1016/j.brainres.2008.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Eade AM, Youngentob SL. Adolescent ethanol experience alters immediate and long-term behavioral responses to ethanol odor in observer and demonstrator rats. Behav Brain Funct. 2009;5:23. doi: 10.1186/1744-9081-5-23. doi: 10.1186/1744-9081-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ford MM, Eldridge JC, Samson HH. Ethanol consumption in the female Long-Evans rat: a modulatory role of estradiol. Alcohol. 2002;26:103–113. doi: 10.1016/s0741-8329(01)00203-8. [DOI] [PubMed] [Google Scholar]
- [23].Gauvin DV, Moore KR, Holloway FA. Do rat strain differences in ethanol consumption reflect differences in ethanol sensitivity or the preparedness to learn? Alcohol. 1993;10:37–43. doi: 10.1016/0741-8329(93)90051-o. [DOI] [PubMed] [Google Scholar]
- [24].Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. doi: 2-7. [DOI] [PubMed] [Google Scholar]
- [25].Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2:861–863. doi: 10.1038/13158. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- [26].Gililland KR, Finn DA. The impact of gonadectomy and adrenalectomy on acute withdrawal severity in male and female C57BL/6J and DBA/2J mice following a single high dose of ethanol. Alcohol Clin Exp Res. 2007;31:1846–1857. doi: 10.1111/j.1530-0277.2007.00509.x. doi: 10.1111/j.1530-0277.2007.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- [28].Greenfield TK, Rogers JD. Who drinks most of the alcohol in the US? The policy implications. J Stud Alcohol. 1999;60:78–89. doi: 10.15288/jsa.1999.60.78. [DOI] [PubMed] [Google Scholar]
- [29].Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- [30].Juarez J, Vazquez-Cortes C, Barrios-De Tomasi E. Different stages in the temporal course of estrogen treatment produce opposite effects on voluntary alcohol consumption in male rats. Alcohol. 2005;36:55–61. doi: 10.1016/j.alcohol.2005.06.003. doi: 10.1016/j.alcohol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- [31].Jung ME, Yang SH, Brun-Zinkernagel AM, Simpkins JW. Estradiol protects against cerebellar damage and motor deficit in ethanol-withdrawn rats. Alcohol. 2002;26:83–93. doi: 10.1016/s0741-8329(01)00199-9. [DOI] [PubMed] [Google Scholar]
- [32].Juraska JM, Markham JA. The cellular basis for volume changes in the rat cortex during puberty: white and gray matter. Ann N Y Acad Sci. 2004;1021:431–435. doi: 10.1196/annals.1308.058. doi: 10.1196/annals.1308.058. [DOI] [PubMed] [Google Scholar]
- [33].Kaffman A, Meaney MJ. Neurodevelopmental sequelae of postnatal maternal care in rodents: clinical and research implications of molecular insights. J Child Psychol Psychiatry. 2007;48:224–244. doi: 10.1111/j.1469-7610.2007.01730.x. doi: 10.1111/j.1469-7610.2007.01730.x. [DOI] [PubMed] [Google Scholar]
- [34].Kaufman KR, Tanchuck MA, Strong MN, Finn DA. Replacement with GABAergic steroid precursors restores the acute ethanol withdrawal profile in adrenalectomy/gonadectomy mice. Neuroscience. 2010;166:5–14. doi: 10.1016/j.neuroscience.2009.11.075. doi: 10.1016/j.neuroscience.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Knoll J, Miklya I, Knoll B, Dallo J. Sexual hormones terminate in the rat: the significantly enhanced catecholaminergic/serotoninergic tone in the brain characteristic to the post-weaning period. Life Sci. 2000;67:765–773. doi: 10.1016/s0024-3205(00)00671-8. [DOI] [PubMed] [Google Scholar]
- [36].Lakoza GN, Barkov NK. The role of testosterone in the development of experimental alcoholism. Bull Narc. 1980;32:41–48. [PubMed] [Google Scholar]
- [37].Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20:1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- [38].Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ. Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol. 2008;42:649–656. doi: 10.1016/j.alcohol.2008.08.005. doi: 10.1016/j.alcohol.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Le AD, Israel Y, Juzytsch W, Quan B, Harding S. Genetic selection for high and low alcohol consumption in a limited-access paradigm. Alcohol Clin Exp Res. 2001;25:1613–1620. doi: 10.1111/j.1530-0277.2001.tb02168.x. [DOI] [PubMed] [Google Scholar]
- [40].Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72:46–55. doi: 10.1016/j.bandc.2009.10.008. doi: 10.1016/j.bandc.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Levin ED, Lawrence SS, Petro A, Horton K, Rezvani AH, Seidler FJ, Slotkin TA. Adolescent vs. adult-onset nicotine self-administration in male rats: duration of effect and differential nicotinic receptor correlates. Neurotoxicol Teratol. 2007;29:458–465. doi: 10.1016/j.ntt.2007.02.002. doi: 10.1016/j.ntt.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Litteria M. Long-term effects of neonatal ovariectomy on cerebellar development in the rat: a histological and morphometric study. Brain Res Dev Brain Res. 1994;81:113–120. doi: 10.1016/0165-3806(94)90073-6. [DOI] [PubMed] [Google Scholar]
- [43].Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- [44].Maldonado AM, Finkbeiner LM, Alipour KK, Kirstein CL. Voluntary ethanol consumption differs in adolescent and adult male rats using a modified sucrose-fading paradigm. Alcohol Clin Exp Res. 2008;32:1574–1582. doi: 10.1111/j.1530-0277.2008.00733.x. doi: 10.1111/j.1530-0277.2008.00733.x. [DOI] [PubMed] [Google Scholar]
- [45].Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- [46].Matthews DB, Tinsley KL, Diaz-Granados JL, Tokunaga S, Silvers JM. Chronic intermittent exposure to ethanol during adolescence produces tolerance to the hypnotic effects of ethanol in male rats: a dose-dependent analysis. Alcohol. 2008;42:617–621. doi: 10.1016/j.alcohol.2008.09.001. doi: 10.1016/j.alcohol.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [47].Meaney MJ, Aitken DH. The effects of early postnatal handling on hippocampal glucocorticoid receptor concentrations: temporal parameters. Brain Res. 1985;354:301–304. doi: 10.1016/0165-3806(85)90183-x. [DOI] [PubMed] [Google Scholar]
- [48].Mezey E. Influence of sex hormones on alcohol metabolism. Alcohol Clin Exp Res. 2000;24:421. [PubMed] [Google Scholar]
- [49].Mitsushima D, Hei DL, Terasawa E. gamma-Aminobutyric acid is an inhibitory neurotransmitter restricting the release of luteinizing hormone-releasing hormone before the onset of puberty. Proc Natl Acad Sci U S A. 1994;91:395–399. doi: 10.1073/pnas.91.1.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Moore CL, Chadwick-Dias AM. Behavioral responses of infant rats to maternal licking: variations with age and sex. Dev Psychobiol. 1986;19:427–438. doi: 10.1002/dev.420190504. doi: 10.1002/dev.420190504. [DOI] [PubMed] [Google Scholar]
- [51].Moore EM, Mariani JN, Linsenbardt DN, Melon LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) Mice Consume Greater Amounts of Limited-Access Ethanol Compared to Adults and Display Continued Elevated Ethanol Intake into Adulthood. Alcohol Clin Exp Res. 2010 doi: 10.1111/j.1530-0277.2009.01143.x. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT. Developmental changes of cortical and cerebellar motor control: a clinical positron emission tomography study with children and adults. J Child Neurol. 1998;13:550–556. doi: 10.1177/088307389801301105. [DOI] [PubMed] [Google Scholar]
- [53].Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- [54].Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114:e300–6. doi: 10.1542/peds.2003-0626-F. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Philpot R, Kirstein C. Developmental differences in the accumbal dopaminergic response to repeated ethanol exposure. Ann N Y Acad Sci. 2004;1021:422–426. doi: 10.1196/annals.1308.056. doi: 10.1196/annals.1308.056. [DOI] [PubMed] [Google Scholar]
- [56].Philpot RM, Wecker L, Kirstein CL. Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi Int. J Dev Neurosci. 2009;27:805–815. doi: 10.1016/j.ijdevneu.2009.08.009. doi: 10.1016/j.ijdevneu.2009.08.009. [DOI] [PubMed] [Google Scholar]
- [57].Piazza NJ, Vrbka JL, Yeager RD. Telescoping of alcoholism in women alcoholics Int. J Addict. 1989;24:19–28. doi: 10.3109/10826088909047272. [DOI] [PubMed] [Google Scholar]
- [58].Przybycien-Szymanska MM, Rao YS, Pak TR. Binge-pattern alcohol exposure during puberty induces sexually dimorphic changes in genes regulating the HPA axis. Am J Physiol Endocrinol Metab. 2009 doi: 10.1152/ajpendo.00615.2009. doi: 10.1152/ajpendo.00615.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Quintanilla ME, Tampier L, Sapag A, Gerdtzen Z, Israel Y. Sex differences, alcohol dehydrogenase, acetaldehyde burst, and aversion to ethanol in the rat: a systems perspective. Am J Physiol Endocrinol Metab. 2007;293:E531–7. doi: 10.1152/ajpendo.00187.2007. doi: 10.1152/ajpendo.00187.2007. [DOI] [PubMed] [Google Scholar]
- [60].Rewal M, Jung ME, Simpkins JW. Role of the GABA-A system in estrogen-induced protection against brain lipid peroxidation in ethanol-withdrawn rats. Alcohol Clin Exp Res. 2004;28:1907–1915. doi: 10.1097/01.alc.0000148100.78628.e7. [DOI] [PubMed] [Google Scholar]
- [61].Rewal M, Jung ME, Wen Y, Brun-Zinkernagel AM, Simpkins JW. Role of the GABAA system in behavioral, motoric, and cerebellar protection by estrogen during ethanol withdrawal. Alcohol. 2003;31:49–61. doi: 10.1016/j.alcohol.2003.07.005. [DOI] [PubMed] [Google Scholar]
- [62].Robinson DL, Brunner LJ, Gonzales RA. Effect of gender and estrous cycle on the pharmacokinetics of ethanol in the rat brain. Alcohol Clin Exp Res. 2002;26:165–172. [PubMed] [Google Scholar]
- [63].Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- [64].Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Russo SJ, Festa ED, Fabian SJ, Gazi FM, Kraish M, Jenab S, Quinones-Jenab V. Gonadal hormones differentially modulate cocaine-induced conditioned place preference in male and female rats. Neuroscience. 2003;120:523–533. doi: 10.1016/s0306-4522(03)00317-8. [DOI] [PubMed] [Google Scholar]
- [66].SAMHSA . Results from the 2008 National Survey on Drug Use and Health: National Findings (Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434) Rockville, MD: 2009. [Google Scholar]
- [67].Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- [68].Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- [69].Silveri MM, Spear LP. Acute, rapid, and chronic tolerance during ontogeny: observations when equating ethanol perturbation across age. Alcohol Clin Exp Res. 2001;25:1301–1308. [PubMed] [Google Scholar]
- [70].Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- [71].Slawecki CJ. Two-choice reaction time performance in Sprague-Dawley rats exposed to alcohol during adolescence or adulthood. Behav Pharmacol. 2006;17:605–614. doi: 10.1097/01.fbp.0000236272.10418.62. doi: 10.1097/01.fbp.0000236272.10418.62. [DOI] [PubMed] [Google Scholar]
- [72].Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- [73].Slob AK, Van der Werff Ten Bosch JJ. Sex differences in body growth in the rat. Physiol Behav. 1975;14:353–361. doi: 10.1016/0031-9384(75)90044-x. [DOI] [PubMed] [Google Scholar]
- [74].Stansfield KH, Kirstein CL. Chronic cocaine or ethanol exposure during adolescence alters novelty-related behaviors in adulthood. Pharmacol Biochem Behav. 2007;86:637–642. doi: 10.1016/j.pbb.2007.02.008. doi: 10.1016/j.pbb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- [75].Strong MN, Yoneyama N, Fretwell AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Horm Behav. 2009 doi: 10.1016/j.yhbeh.2009.10.008. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Stuart EB, Thompson JM, Rhees RW, Lephart ED. Steroid hormone influence on brain calbindin-D(28K) in male prepubertal and ovariectomized rats. Brain Res Dev Brain Res. 2001;129:125–133. doi: 10.1016/s0165-3806(01)00191-2. [DOI] [PubMed] [Google Scholar]
- [77].Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Alcohols stimulate gamma-aminobutyric acid receptor-mediated chloride uptake in brain vesicles: correlation with intoxication potency. Brain Res. 1988;444:340–345. doi: 10.1016/0006-8993(88)90943-2. [DOI] [PubMed] [Google Scholar]
- [78].Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ. Developmental differences in the acquisition of tolerance to ethanol. Alcohol. 1998;15:311–314. doi: 10.1016/s0741-8329(97)00135-3. [DOI] [PubMed] [Google Scholar]
- [79].Tambour S, Brown LL, Crabbe JC. Gender and age at drinking onset affect voluntary alcohol consumption but neither the alcohol deprivation effect nor the response to stress in mice. Alcohol Clin Exp Res. 2008;32:2100–2106. doi: 10.1111/j.1530-0277.2008.00798.x. doi: 10.1111/j.1530-0277.2008.00798.x. [DOI] [PubMed] [Google Scholar]
- [80].Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Torres OV, Natividad LA, Tejeda HA, Van Weelden SA, O'Dell LE. Female rats display dose-dependent differences to the rewarding and aversive effects of nicotine in an age-, hormone-, and sex-dependent manner. Psychopharmacology (Berl) 2009;206:303–312. doi: 10.1007/s00213-009-1607-3. doi: 10.1007/s00213-009-1607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- [83].Tschann JM, Adler NE, Irwin CE, Jr, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychol. 1994;13:326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- [84].Varlinskaya EI, Spear LP. Sensitization to social anxiolytic effects of ethanol in adolescent and adult Sprague-Dawley rats after repeated ethanol exposure. Alcohol. 2010;44:99–110. doi: 10.1016/j.alcohol.2009.09.036. doi: 10.1016/j.alcohol.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions Alcohol. Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Vetter-O'Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood Alcohol. Alcohol. 2009;44:547–554. doi: 10.1093/alcalc/agp048. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Wezeman FH, Juknelis D, Frost N, Callaci JJ. Spine bone mineral density and vertebral body height are altered by alcohol consumption in growing male and female rats. Alcohol. 2003;31:87–92. doi: 10.1016/j.alcohol.2003.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wilson DM, Killen JD, Hayward C, Robinson TN, Hammer LD, Kraemer HC, Varady A, Taylor CB. Timing and rate of sexual maturation and the onset of cigarette and alcohol use among teenage girls. Arch Pediatr Adolesc Med. 1994;148:789–795. doi: 10.1001/archpedi.1994.02170080019004. [DOI] [PubMed] [Google Scholar]
- [89].Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29:81–95. doi: 10.1016/j.ntt.2006.10.013. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- [90].Yu ZY, Wang W, Fritschy JM, Witte OW, Redecker C. Changes in neocortical and hippocampal GABAA receptor subunit distribution during brain maturation and aging. Brain Res. 2006;1099:73–81. doi: 10.1016/j.brainres.2006.04.118. doi: 10.1016/j.brainres.2006.04.118. [DOI] [PubMed] [Google Scholar]
- [91].Zhang L, Warren RA. Postnatal development of excitatory postsynaptic currents in nucleus accumbens medium spiny neurons. Neuroscience. 2008;154:1440–1449. doi: 10.1016/j.neuroscience.2008.05.002. doi: 10.1016/j.neuroscience.2008.05.002. [DOI] [PubMed] [Google Scholar]
- [92].Zucker I, ZUCKER Hormonal determinants of sex differences in saccharin preference, food intake and body weight. Physiology behavior. 1969;4:595–602. [Google Scholar]