Abstract
Recent evidence showed that 17 β-estradiol (E2) decreased cytokine-induced expression of cell adhesion molecules (CAM). Changes in intracellular Ca2+ concentration ([Ca2+]i) has been shown to be associated with CAM expression in endothelial cells. Here, the effects of E2 (1 μM, 24 h) on the expression of intracellular adhesion molecule-1 (ICAM-1) and [Ca2+]i were investigated in a lipopolysaccharide (LPS) (100 ng/mL, 18 h)-stimulated human endothelial cell line, EA.hy926, using real-time PCR and spectrofluorometry, respectively. PCR analysis revealed a significant increase in ICAM-1 expression in calcium ionophore A23187 (1 nM)- or LPS-stimulated cells. Pretreatment of cells with E2 significantly inhibited LPS-induced ICAM-1 mRNA expression. [Ca2+]i was monitored in Fura-2 AM-loaded cells in the presence and absence of extracellular Ca2+ with thapsigargin (TG, 1 μM), a sarco/endoplasmic reticulum ATPase inhibitor or ATP (100 μM). The extent of TG- or ATP-induced [Ca2+]i increase was significantly higher in LPS-stimulated cells than in control cells. Pre-treatment of LPS-stimulated cells with E2 limited the Ca2+ response to the same level as in control cells. Furthermore, ICI 182,780, an estrogen receptor antagonist, attenuated the inhibitory actions of E2 on ICAM-1 mRNA expression and Ca2+ responses, suggesting that estrogen receptors mediate, at least in part, the effects of estrogen. These data suggest a potential underlying mechanism for the protective effect of E2 against atherosclerosis.
Keywords: calcium, endothelial cells, estrogen, endothelial cell adhesion molecule
1. Introduction
Atherosclerosis is a vascular inflammatory disease (Libby et al., 2002). During the early stages of atherosclerosis, inflammatory cells are recruited to the vascular wall through adherence to the endothelium via cell adhesion molecules (CAMs) expressed on the surface of endothelial cells (Matheny et al., 2000). CAMs include intercellular cell adhesion molecules (ICAM), vascular cell adhesion molecules (VCAM), platelet endothelial cellular adhesion molecules (PECAM), and E-selectins. ICAM-1 is an important adhesion molecule continuously present in low concentrations in the membranes of leukocytes and endothelial cells. It is a ligand for lymphocyte function-associated antigen 1 (LFA-1), a receptor found on leukocytes. When activated, leukocytes bind to endothelial cells via ICAM-1/LFA-1 and subsequently transmigrate through the endothelium into tissues. Tumor necrosis factor-alpha (TNF-α) and lipopolysaccharide (LPS) elicit an endothelial cell inflammatory response mediated by activation of nuclear factor-κB (NF-κB) (Kempe et al., 2005), resulting in endothelial CAM expression in vitro (Norata et al., 2006) and in vivo (Hajra et al., 2000). Increased CAM expression and the development of atherosclerosis are associated with an altered intracellular Ca2+ homeostasis in endothelial cells (Chen et al., 2002).
Calcium homeostasis is a major aspect of maintaining endothelial integrity and function. Aortic endothelial cells from cholesterol-fed rabbits exhibited a five-fold increase in intracellular calcium concentration ([Ca2+]) compared to endothelial cells from normal rabbits (Strickberger et al., 1988). Furthermore, treating bovine aortic endothelial cells with LDL increased [Ca2+]i (Negre-Salvayre et al., 1992). Collectively, these observations are consistent with the possibility that increases in [Ca2+]i mediate upregulation of CAMs in endothelial cells in response to hypercholesterolemia and other pro-atherogenic stimuli, resulting in formation of leukocyte-based atheroma.
Studies in ovariectomized rabbits showed that estrogen supplements attenuated aortic accumulation of cholesterol and reduced the degree of cardiovascular disease including atherosclerosis (Haarbo et al., 1991). 17 β-estradiol (E2) inhibited monocyte adhesion and transendothelial migration in hypercholesterolemic rabbits (Nathan et al., 1999). Hormone replacement therapy in postmenopausal patients with coronary artery disease (Seljeflot et al., 2000), hypercholesterolemic female patients (Sbarouni et al., 2000), and healthy postmenopausal patients (Koh et al., 1997) resulted in decreased CAM expression. The inhibitory effect of E2 on CAM expression has been demonstrated following cytokine-induced CAM overexpression in endothelial cells (Simoncini et al., 2000). The effect of E2 on CAM expression and a possible link between CAM expression and Ca2+ homeostasis (Amrani et al., 2000) contribute to the hypothesis that the anti-atherogenic effect of E2 is mediated, in part, by the modulatory effects on Ca2+ homeostasis in human endothelial cells. Estrogen has been shown to affect Ca2+ homeostasis in human umbilical vein endothelial cells (HUVEC) (Wang et al., 2006). In a very recent report, we showed that treatment of human endothelial cells, EA.hy926, with E2 (1 μM, 24 h) altered Ca2+ homeostasis (Thor et al., 2010). Here, we investigated whether the same treatment of EA.hy926 cells with E2 would alter the expression of adhesion molecule-1 (ICAM-1) and Ca2+ homeostasis in LPS-stimulated EA.hy926 cells. Furthermore, the role of estrogen receptors (ER) in E2-mediated effects was analyzed with the nonselective ER inhibitor, ICI 182,780.
2. Material and methods
2.1 Cell culture
The immortalized EA.hy926 cell line was the kind gift of Dr. Cora-Jean Edgell (University of North Carolina). Cells were cultured at 37°C in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 units/mL), and streptomycin (100 μg/mL). Prior to initiating drug treatments, cells were sub-cultured in phenol red-free, serum-free DMEM for 18 h to induce quiescence. Cells were then assigned to one of the treatment protocols before studying ICAM-1 mRNA expression and intracellular Ca2+ homeostasis. Before treatments, cells were shifted to media containing 10% FBS treated with charcoal stripping to remove estrogen metabolites and other steroid hormones.
| Protocol | Treatment | 24 h | 18 h |
| 1 | control | vehicle | vehicle |
| 2 | LPS-stimulated | vehicle | LPS |
| 3 | E2/LPS-stimulated | E2 | LPS |
| 4 | ICI/E2/LPS-stimulated | ICI + E2 | LPS |
In protocol 1 (control), cells were pretreated with vehicle (0.09% ethanol) for 24 h followed by additional incubation in vehicle for 18 h. In protocol 2 (LPS-stimulated), cells were pretreated with vehicle for 24 h followed by treatment with 100 ng/mL LPS from Escherichia coli (serotype 0111:B4, Sigma, St. Louis, MO) for 18 h. The endotoxin level for the LPS serotype was 3,000,000 endotoxin units per mg. The concentration as well as time course for LPS fell within previously reported data (Simoncini et al., 2000). In protocol 3 (E2/LPS-stimulated), cells were pretreated with 1 μM 17 β-estradiol (E2, Sigma, St. Louis, MO) for 24 h followed by treatment with LPS (100 ng/mL) for 18 h. In protocol 4 (ICI/E2/LPS-stimulated), ICI 182,780 (10 μM) was added one hour prior to the addition of 1 μM E2 for 24 h followed by treatment with LPS (100 ng/mL) for 18 h.
In a second set of experiments, to demonstrate the effect of calcium mobilizing agent on the ICAM-1 expression, endothelial cells were assigned into two additional treatment groups as follows:
| Protocol | Treatment | 6 h |
| 1 | control | vehicle |
| 2 | calcium ionophore-stimulated | A23187 |
In protocol 1 (control), cells were treated with vehicle (0.1% DMSO) for 6 h. In protocol 2 (calcium ionophore-stimulated), cells were treated with calcium ionophore A23187 (1 nM, Sigma, St. Louis, MO) for 6 h.
2.2 Real-time PCR
Cells grown on 60 mm Petri dishes were assigned to the treatment protocols as described in 2.1. After removing the medium, cells were washed twice with Hank’s balanced salt solution (HBSS) (Invitrogen, Carlsbad, CA). Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). First strand cDNA was synthesized by reverse transcription of 2 μg of total RNA using Omniscript RT Kit (Qiagen, Valencia, CA) in a volume of 20 μl at 37°C for 60 minutes. The ICAM-1 expression was analyzed with real-time RT-PCR (MyIQ, ICycler, BioRad) using ICAM-1 specific primers and iQ™ SYBR Green Supermix (BioRad, Hercules, CA). Internal variations were normalized using human glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and results were analyzed using the Delta Delta Ct model (Pfaffl, 2001; Yuan et al., 2006). The following primers were used for detection of gene expression: 5′–GGCCTCCAAGGAGTAAGACC- 3′ (forward) and 5′–AGGGGTCTACATGGCAACTG-3′ (reverse) for human GAPDH; 5′–CAGGGAATATGCCCAAGCTA-3′ (forward) and 5′–GAACCATGATTGCACCACTG- 3′ (reverse) for human ICAM-1. Specificity of primers was verified by electrophoresis of the PCR products on a 2% agarose gel and staining with ethidium bromide.
2.3 Intracellular calcium measurements
Cells grown on poly-L-lysine coated glass coverslips were loaded with 1.5 μM Fura-2 acetoxymethyl ester (Fura-2/AM, Molecular Probes, Carlsbad, CA) dissolved in HBSS from a stock solution of 1.5 mM in DMSO containing 20% pluronic acid F-127, at room temperature, for 30 minutes. The average fluorescence of a whole population of Fura-2 AM-loaded cells was measured using continuous rapid alternating excitation (340 and 380 nm) and emission (510 nm) in a fluorescence spectrophotometer equipped with a xenon lamp (PTI, Lawrenceville, NJ). The ratio of fluorescence signals emitted in response to the two excitation wavelengths was recorded at 3 Hz using Felix Fluorescence Analysis Software (PTI, Lawrenceville, NJ). The fluorescence ratio provides an index of [Ca2+]i as a function of the dissociation constant between Fura-2/AM and calcium.
2.4 Area under the curve
The area under the fluorescence ratio curve (AUC) was determined by applying the trapezoidal method Σ[(ratio2+ratio1)/2*(time2−time1)] as previously reported by us (Padar et al., 2004). Depending on the comparison being made, the AUC integration bounds contained either 300, 400, or 500 seconds of recorded signal. However, the length of integration was the same for the test and control experimental data sequences being compared. Calcium traces from cell populations were normalized prior to calculating the AUC by subtracting the average baseline value for the first 75 seconds.
2.5 Calcium Extrusion
Calcium extrusion was calculated from Ca2+ traces obtained in Ca2+-free HBSS in the presence of agonist. It is important to note that the cells were kept in buffer containing Ca2+ right until the point at which the calcium traces were performed. The cells were then shifted to zero Ca2+ buffer for only 75 seconds to get a baseline before adding agonist. Under these conditions the height of the peak reflects the amount of Ca2+ released from the endoplasmic reticulum and the downward slope of a best-fit line from the Ca2+ peak reflects the rate of Ca2+ extrusion from the cytoplasm. One hundred data points above and below the approximate midpoint of the Ca2+ decline between Ca2+ peak and Ca2+ basal level were used to generate a best-fit line.
2.6 Statistical analysis
Values were expressed as mean ± standard deviation. Two-group comparisons were performed by the unpaired Student t-test. Multiple comparisons were made using one-way ANOVA, and individual differences were analyzed using the Bonferroni post hoc test after demonstration of significant intergroup differences by ANOVA. P<0.05 was considered significant. Statistical analyses were performed using the statistic analysis software SPSS (version 14).
3. Results
3.1 Effect of 17 β-estradiol on LPS-induced ICAM-1 expression
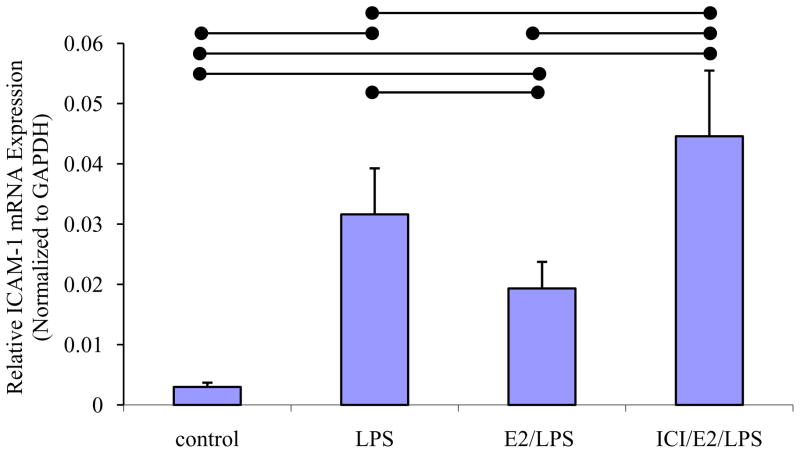
Real-time PCR analysis revealed that ICAM-1 mRNA expression was significantly higher in LPS-stimulated cells than in control cells (P < 0.05, n=8; Fig. 1). Treatment of cells with E2 for 24 h prior to LPS stimulation (E2/LPS-stimulated cells) significantly inhibited ICAM-1 mRNA expression compared to LPS-stimulated control cells (P < 0.05, n=8; Fig. 1). ICI 182,780-pretreatment attenuated the inhibitory effect of E2 on LPS-induced ICAM-1 expression (Fig. 1).
Fig. 1. Real-time PCR analysis of ICAM-1 mRNA expression in ERA.hy926.
The means ± standard deviation of the ICAM-1 mRNA expression in control, LPS-stimulated, E2/LPS-stimulated, and ICI/E2/LPS stimulated cells are shown. ICAM-1 mRNA level was normalized to GAPDH. Significant differences among individual treatment groups are indicated (•—•, P<0.05, n=8) as analyzed using a one-way ANOVA, followed by a Bonferroni post hoc test.
3.2 Effects of calcium-mobilizing agent on ICAM-1 expression
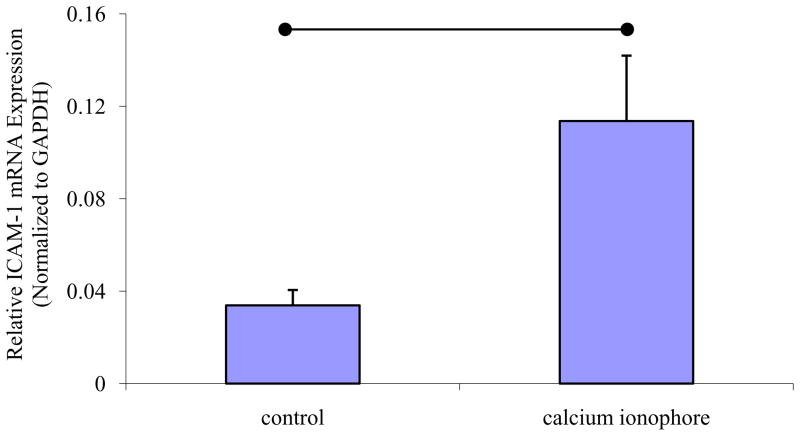
The calcium ionophore A23187 (1 nM) significantly increased the basal level of ICAM-1 expression in EA.hy926, compared to control cells (P < 0.01, n=3) (Fig. 2).
Fig. 2. Effect of calcium ionophore A23187 on ICAM-1 mRNA expression in EA.hy926.
The means ± standard deviation of the ICAM-1 mRNA expression in et al control and calcium ionophore-stimulated cells are shown. ICAM-1 mRNA level was normalized to GAPDH. Significant difference between two groups is indicated (•—•, P<0.05, n=3) as analyzed using the unpaired Student t-test.
3.3 Effect of 17 β-estradiol on LPS-induced altered Ca2+ homeostasis
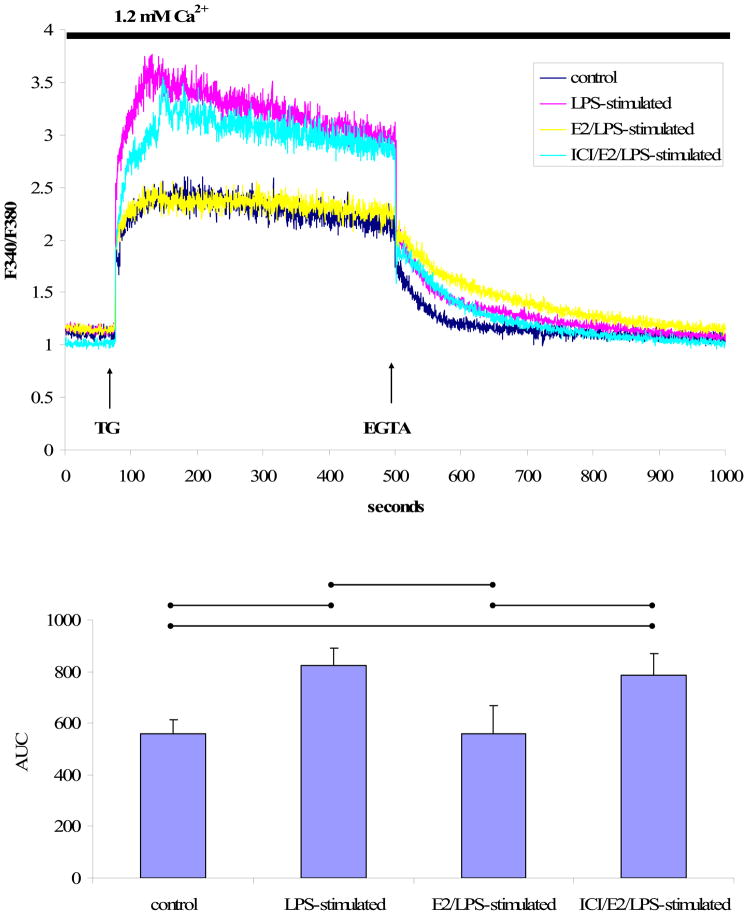
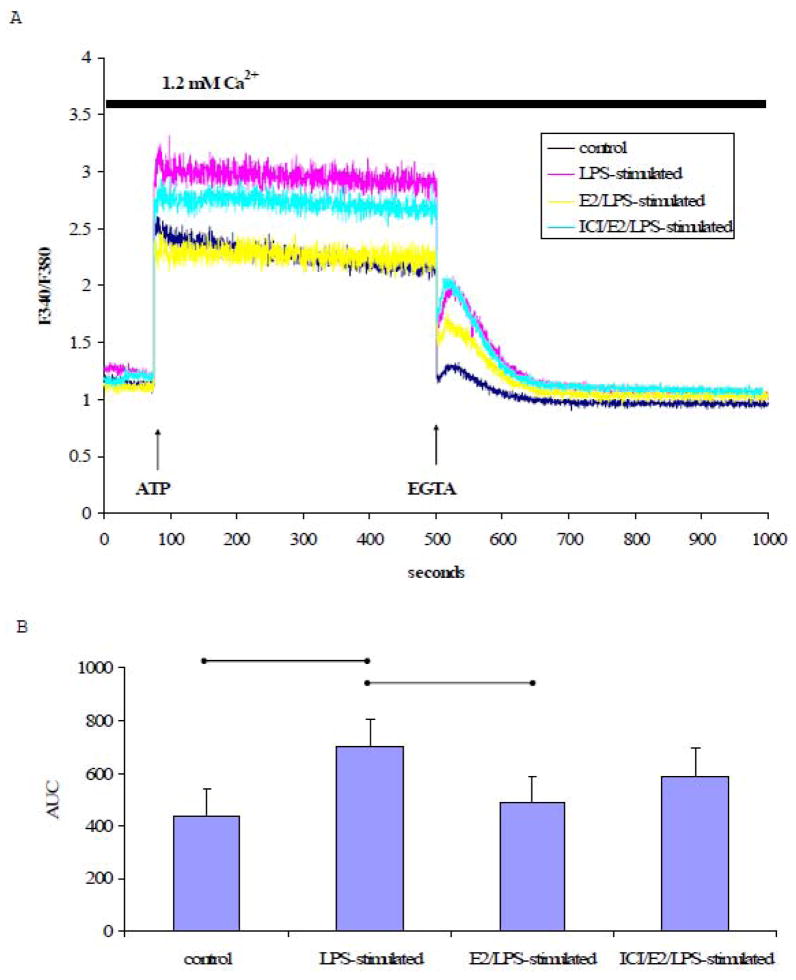
To explore the effects of LPS on Ca2+ homeostasis, [Ca2+]i in response to thapsigargin (TG), a sarcoplasmic/endoplasmic reticulum Ca2+ ATPase (SERCA) inhibitor, was measured in LPS-stimulated or control cells loaded with Fura-2AM. In the presence of extracellular Ca2+ (1.2 mM), TG (1 μM) induced a rapid increase in and subsequent prolonged elevation of [Ca2+]i (Fig. 3A). The plateau phase of TG-induced [Ca2+]i increase was completely abolished by removing extracellular free Ca2+ using EGT A (2 mM), a Ca2+ chelator.
Fig. 3. TG-induced Ca2+ signals in the presence of extracellular Ca2+.
A) A representative tracing of the Ca2+ signal from EA.hy926 cells in response to TG (1 μM) in the presence of 1.2 mM extracellular Ca2+ followed by EGTA (2 mM) treatment. B) The mean ± standard deviation of the Ca2+ AUC traced during the response to TG in the presence of extracellular Ca2+. Significant differences among individual treatment groups are indicated (•—•, P<0.05, n=5) as analyzed using a one-way ANOVA, followed by a Bonferroni post hoc test.
As shown in Fig. 3A, TG treatment increased [Ca2+]i in both LPS-stimulated and [Ca2+]i control cells. However, the extent of TG -induced [Ca2+]i increase was significantly higher in LPS-stimulated cells than in control cells as indicated by the AUC (P<0.05, n=5, Fig. 3B). Moreover, the elevated Ca2+ signals observed in LPS-stimulated cells were attenuated when cells were treated with E2 for 24 h prior to stimulation with LPS (Fig. 3A). ICI 182,780-pretreatment attenuated the inhibitory effect of E2 on the LPS-[Ca2+]i increase in response to TG (F igs. 3A and 3B).
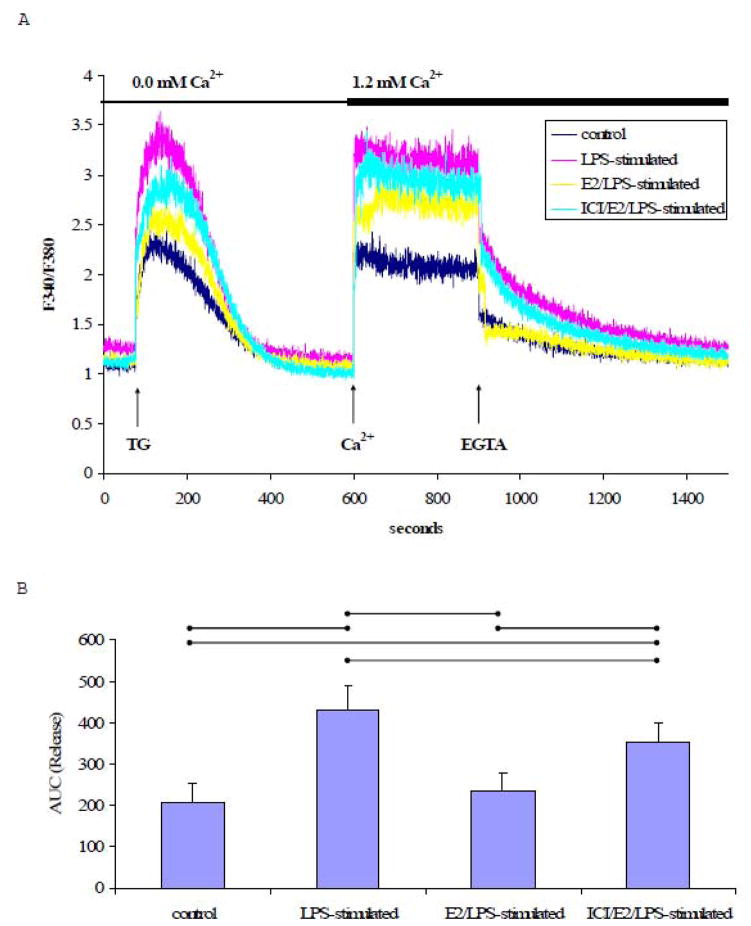
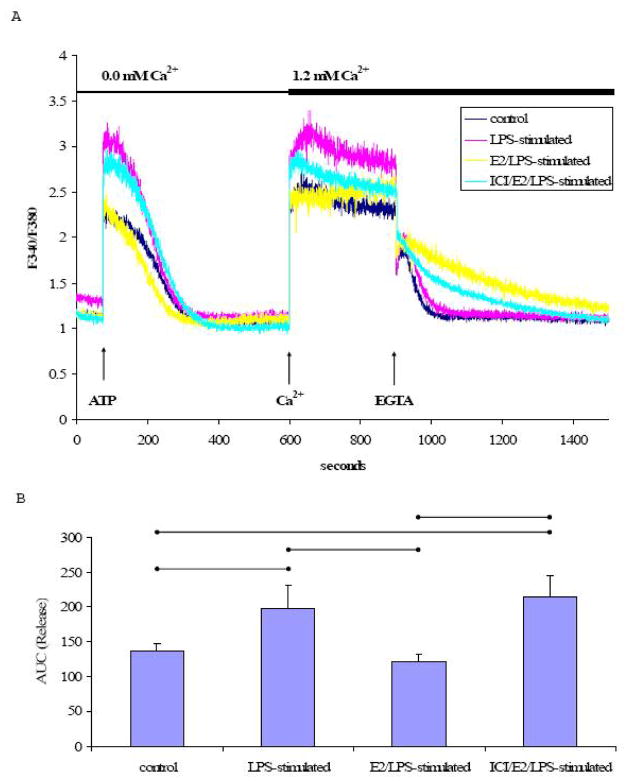
In the absence of extracellular Ca2+, TG treatment resulted in a single Ca2+ transient in both LPS-stimulated and control cells (Fig. 4A). The AUC of the TG-induced Ca2+signal in Ca2+-free buffer was significantly greater (p<0.05, n=5) in LPS-stimulated cells than control cells (Fig. 4B). In addition, the rate of Ca2+ extrusion was significantly faster in LPS-stimulated cells (−11.1×10−3± 1.73×10−3 s−1) compared to control cells (−5.93×10−3± 1.87×10−3 s−1) (p<0.05, n=5). Estrogen pretreatment significantly attenuated the effects of LPS on the rate of Ca2+ extrusion (−7.48×10−3 ± 0.85×10−3 s−1). The rate of Ca2+ extrusion in ICI/E2/LPS-stimulated cells (−9.540×10−3± 1.32×10−3 s−1) was not significantly different from E2/LPS-stimulated cells (−7.48×10−3± 0.85×10−3 s−1).
Fig. 4. TG-induced Ca2+ signal in the absence of extracellular Ca2+.
A) A representative tracing of the Ca2+ signal from EA.hy926 cells in response to TG (1 μM) in Ca2+-free medium, followed by the addition of extracellular Ca2+ (1.2 mM) and EGTA (2 mM). B) The mean ± standard deviation of the Ca2+ AUC traced during the response to TG in Ca2+-free medium. C) The mean ± standard deviation of the Ca2+ AUC traced during the response to TG upon addition of extracellular Ca2+. Significant differences among individual treatment groups are indicated by (•—•, P<0.05, n=5) as analyzed using a one-way ANOVA, followed by a Bonferroni post hoc test.
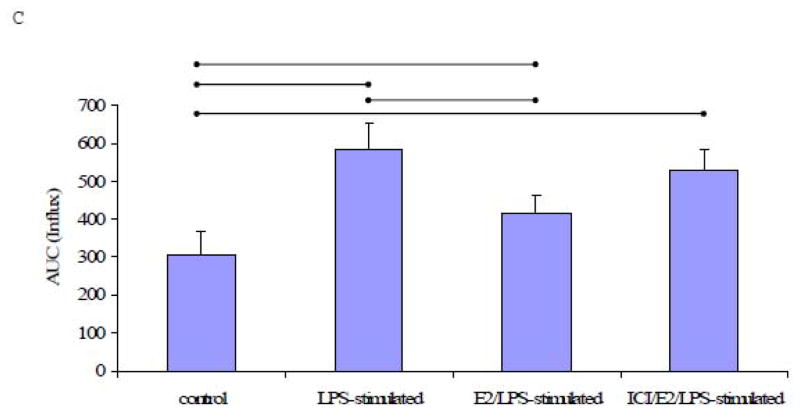
Subsequently increasing the extracellular Ca2+ concentration to 1.2 mM led to a robust increase in [Ca2+]i which was completely reversed by EGTA (Fig. 4A). The elevation of [Ca2+]i in response to added extracellular Ca2+ (1.2 mM) was significantly higher in LPS-stimulated cells relative to control cells, suggesting an enhanced Ca2+ influx in LPS-stimulated cells (P<0.05, n=5, Figs. 4A & 4C). The increase in [Ca2+]i in LPS-stimulated cells was attenuated by E2 treatment prior to stimulation with LPS (E2/LPS-stimulated cells). Although, ICI 182,780 pretreatment tended to limit the E2-mediated effect on the TG-induced Ca2+ influx, ICI 182,780 did not significantly block the E2’s inhibitory effect (Fig. 4C).
To further explore the efects of LPS on the endoplasmic reticulum Ca2+ pools, LPS-stimulated cells and control cells were treated with ATP (100 μM). ATP induced increase in [Ca2+]i in both LPS-stimulated and control cells, likely due to calcium release from intracellular stores. Both LPS-stimulated and control cells exhibited a sustained increase in [Ca2+]i upon ATP treatment in the presence of extracellular Ca2+, consistent with calcium influx. This increase was reversed by EGTA (Fig. 5A). The ATP-induced increase in [Ca2+]i was significantly higher in LPS-stimulated cells compared to control cells (Fig. 5B). Moreover, a comparison of the AUC shows that E2-pretreatment significantly attenuated the efects of LPS on the ATP-mediated increase in [Ca2+]i (E2/LPS-stimulated cells), and that ICI 182,780-pretreatment had little or no effect against the E2-attenuated [Ca2+]i increase (Fig. 5B).
Fig. 5. ATP-induced Ca2+ signals in the presence of extracellular Ca2+.
A) A representative tracing of the Ca2+ signal from EA.hy926 cells in response to ATP (100 μM) in the presence of 1.2 mM extracellular Ca2+, followed by EGTA (2 mM) treatment. B) The mean ± standard deviation of the Ca2+ AUC traced during the response to ATP in the presence of extracellular Ca2+. Significant dierences among individual treatment groups are indicated (•—•, P<0.05, n=5) as analyzed using a one-way ANOVA, followed by a Bonferroni post hoc test.
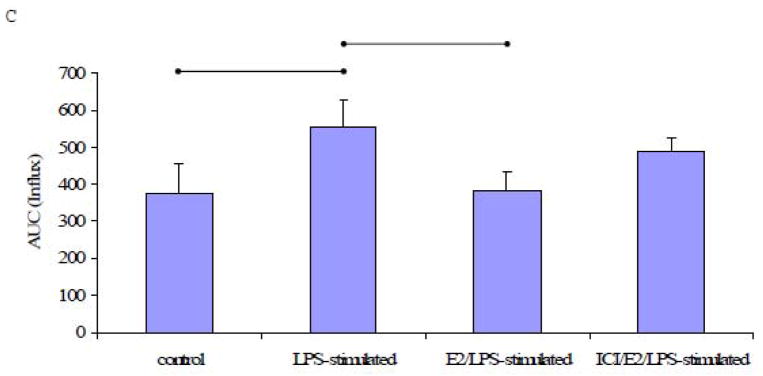
When cells were treated with ATP in the absence of extracellular Ca2+, the amount of Ca2+ released from the endoplasmic reticulum was significantly higher in LPS-stimulated cells than in control cells (P<0.05, n=5, Figs. 6A and 6B). In cells pre-treated with E2, the ATP-induced increase in [Ca2+]i was significantly attenuated in LPS-stimulated cells. Exposure to ICI 182,780 prior to E2 treatment significantly attenuated the inhibitory effect of E2 (Fig. 6B).
Fig. 6. ATP-induced Ca2+ signal in the absence of extracellular Ca2+.
A) A representative tracing of the Ca2+ signal from EA.hy926 cells in response to ATP (100 μM) in Ca2+-free medium, followed by the addition of extracellular Ca2+ (1.2 mM) and EGTA (2 mM). B) The mean ± standard deviation of the Ca2+ AUC traced during the response to ATP in Ca2+-free medium. C) The mean ± standard deviation of the Ca2+ AUC traced during the response to ATP upon the addition of extracellular Ca2+. Significant differences among individual treatment groups are indicated by (•—•, P<0.05, n=5) as analyzed using a one-way ANOVA, followed by a Bonferroni post hoc test.
A subsequent addition of Ca2+ (1.2 mM) caused a rapid and robust increase in [Ca2+]i in both LPS-stimulated and control cells, but [Ca2+]i reached a higher level in LPS-treated cells. The [Ca2+]i returned to baseline upon subsequent addition of EGTA (Fig. 6A). Although E2 pretreatment significantly inhibited the [Ca2+]i response to ATP in the presence of extracellular Ca2+ (P<0.05, n=5), ICI 182,780-pretreatment failed to block this effect of E2 in ICI/E2/LPS-stimulated cells (Fig. 6C).
The rate of Ca2+ extrusion in cells treated with ATP was not significantly different between LPS-stimulated (−13.7×10−3± 2.37×10−3 s−1) and control cells (−9.48×10−3± 2.71×10 s−1). The rate of calcium extrusion in response to ATP followed a pattern similar to that in TG-induced cells when LPS-stimulated cells were pretreated with E2 alone or ICI 182,780 plus E2. Calcium extrusion was significantly lower in LPS-stimulated cells treated with E2 (−8.18×10−3± 2.39×10−3 s−1) than in the absence of E2 (−13.7×10−3 + 2.37×10−3 s−1) (P<0.05, n=5). The rate of Ca2+ extrusion in ICI/E2/LPS-stimulated cells (−8.98×10−3 + 1.25×10−3 s−1) was not significantly different from E2/LPS-stimulated cells (−8.18×10−3 ±2.39×10−3 s−1).
4. Discussion
The major findings of the above experimental observations were that 1) LPS stimulation of endothelial cells increases ICAM-1 mRNA expression and agonist-induced Ca2+ response, and 2) the effects of LPS on ICAM-1 and Ca2+ response were abrogated by E2-pretreatment. These data suggest a potential underlying mechanism for the protective effect of E2 against atherosclerosis.
Deterioration in endothelial health and integrity is an early and important contributor to the pathogenesis of atherosclerosis. Atherosclerosis has many features of a chronic inflammatory disease. Recent evidence suggests that common bacteria and viruses can contribute to the development of atherosclerosis, probably by triggering inflammation (Melnick et al., 1993; Chiu et al., 1997). Chronic infection can be mimicked by infusion of bacterial endotoxins such as LPS. This bacterial endotoxin may cause a local inflammation after incorporation of the lipoproteins into the vascular wall. LPS administration has been shown to aggravate atherosclerosis in apoE-deficient mice (Ostos et al., 2002). Adhesion molecules are important factors during inflammation because they enable inflammatory cells to migrate to inflamed compartments. In concert with a decrease in bioavailable nitric oxide (NO), numerous factors including oxidized LDL and various pro-inflammatory cytokines such as LPS contribute to endothelial activation (Libby, 2003) and promote the induction of adhesion molecules. Iiyama et al., (1999) used LPS injection to induce the expression of ICAM-1 and VCAM-1 in the regions of aortic endothelial cells predisposed to lesion in LDL receptor knockout mice. In agreement with previous studies demonstrating upregulation of CAM expression by LPS in human endothelial cells (Norata et al., 2006), we observed that LPS induces ICAM-1 mRNA expression in endothelial cells.
Calcium had been implicated as an important second messenger and regulator of endothelial cell homeostasis (Plank et al., 2006) and VCAM and E-selectin expression (Allen et al., 1998). In line with previous reports that demonstrate the involvement of Ca2+ in CAM expression (Allen et al., 1998; Juan et al., 1999), we also found that calcium ionophore A23187 significantly induces ICAM-1 mRNA expression in EA.hy926 cells. Next, Ca2+ homeostasis of LPS-stimulated EA.hy926 cells was studied using ATP or TG to elicit mobilization of intracellular and extracellular calcium pools. ATP releases Ca2+ by activating inositol triphosphate receptors (IP3R) (Dubyak & el-Moatassim, 1993; Ralevic & Burnstock, 1998), while TG inhibits SERCA (Thastrup et al., 1990) and allows Ca2+ to leak out of the endoplasmic reticulum. In the presence of extracellular Ca2+, the common cellular response to ATP or TG was biphasic with an initial rapid increase in [Ca2+]i followed by a plateau phase of elevated [Ca2+]i. Since the magnitude of the initial peaks (Figs. 3A & 5A) were similar to the responses seen in the absence of extracellular Ca2+ (Figs. 4A & 6A), it could be concluded that the first phase of the response to TG or ATP was mainly due to Ca2+ release from the endoplamic reticulum. In addition, the plateau phase appeared only in the presence of extracellular Ca2+ suggesting that this phase was due to Ca2+ influx. The basal levels of [Ca2+]i were similar between control and LPS-stimulated cells (data not shown), but TG or ATP induced much larger increases in cytosolic Ca2+ levels, with both enhanced release and influx components, in LPS-stimulated cells as compared to control cells.
Endoplasmic reticulum Ca2+ store depletion under physiological conditions typically triggers a large increase in [Ca2+]i due t et al o Ca2+ influx via plasma membrane calcium channels, which serves to refill the intracellular Ca2+ stores (Putney, 2001). Our data suggested that the enhanced Ca2+ response in LPS-stimulated cells may be related to the enhanced Ca2+ influx and is most likely not due to less Ca2+ extrusion activity, since we did not observe slower Ca2+ decline rates. In fact, Ca2+ extrusion in cells treated with TG was faster in LPS-stimulated cells than that of control cells. Calcium extrusion from endothelial cells is an active process which appears to depend primarily on the plasmamembrane Ca2+-ATPase (PMCA) (Liang et al., 2004) and to a lesser extent on the Na+/Ca2+ exchanger (NCX) (Li & van Breemen, 1995) and SERCA. Whether the faster rate of Ca2+ extrusion observed in LPS-stimulated cells was related to increased expression or activity of PMCA, NCX, or SERCA remains to be investigated. Calcium extrusion is essential for restoring and maintaining a low resting [Ca2+]i following Ca2+ entry. Therefore, the increase in Ca2+ extrusion in TG-treated cells associated with LPS-stimulated cells may be important for cell viability since increases in [Ca2+]i have been associated with apoptosis (Mattson & Chan, 2003). Further studies will be needed to gain a better understanding of the functional consequence of an increased Ca2+ extrusion rate by LPS stimulation in endothelial cells. It is important to note that, despite the faster extrusion of Ca2+, the agonist-induced increase in [Ca2+]i in LPS-stimulated cells was enhanced.
Estrogen has been implicated as an atherosclerotic protective agent (Haarbo et al., 1991). Our data show that E2 (1 μM) decreased LPS-induced ICAM-1 mRNA expression in EA.hy926. Although, we did not measure ICAM protein expression, this in vitro action of E2 has been studied by other investigators with conflicting results. For instance, in studies using HUVEC, E2 decreased the VCAM-1 mRNA or protein expression induced by either LPS (Nathan et al., 1999; Simoncini et al., 2000) or by interleukin-1 (Nakai et al., 1994; Caulin-Glaser et al., 1996). However, Cid et al. (1994) observed that E2 increased TNF-α-induced expression of adhesion molecules including ICAM-1. These contrasting observations may be due to differences in the cytokines used to induce CAM expression or differences in the concentration of E2 used. The effect of E2 on CAM expression and a possible link between CAM expression and Ca2+ homeostasis contribute to the hypothesis that the anti-atherogenic effect of E2 is mediated, in part, by modulation of Ca2+ homeostasis in human endothelial cells. Recently, we reported that E2 (1 μM, 24 h) modulates Ca2+ homeostasis in EA.hy926 (Thor et al., 2010). Here, we sought to determine whether the same treatment of EA.hy926 cells with E2 has a detectable effect on agonist-induced increases in [Ca2+]i in LPS-stimulated cells. Pretreatment of LPS-stimulated EA.hy926 cells with E2 clearly altered the Ca2+ response profile. These data support the hypothesis that E2 may inhibit CAM expression in LPS-stimulated endothelial cells secondary to lower [Ca2+]i. Although we did not elucidate the underlying mechanisms (e.g. change in activity or expression of Ca2+ channels and pumps) responsible for the effects of LPS or E2 on Ca2+ homeostasis in this study, our data suggest a potential underlying mechanism for the protective effect of E2 against atherosclerosis.
To address the involvement of ERs in mediating inhibitory effects of E2 on ICAM-1 mRNA expression and Ca2+ during LPS-stimulation, cells were treated with an excess of the non-selective ER antagonist, ICI 182,780. Pretreatment of cells with ICI 182, et al 780 attenuated the effect of E2 on LPS-induced ICAM-1 expression. Furthermore, the abrogation of some of the inhibitory effects of E2 on increased [Ca2+]i by ICI 182,780 indicates that the ERs likely mediate one or more Ca2+ mobilization process (e.g., Ca2+ release) responsible for observed increases in [Ca2+]i in response to TG and ATP in the absence of extracellular Ca2+ (Figs. 4B and 6B). However, the failure of ICI 182,780 to impact inhibition by E2 of the [Ca2+]i increase upon replacement of extracellular Ca2+ suggests that E2 inhibited Ca2+ influx, at least in part, via a non-ER-mediated mechanism. At present, we cannot explain why ICI 182,780 did not block the effects of E2 on Ca2+ influx component in our studies. We cannot rule out the possibility that the effects of E2 on Ca2+ influx were mediated by an ER subtype that is insensitive to ICI 182,780 (Biewenga et al., 2005) or that E2 inhibited Ca2+ influx via a non-specific mechanism (Castillo et al., 2006). Clearly, further studies are required to provide a more complete understanding of the role that ERs play in mediating changes in Ca2+ homeostasis.
We are aware that the concentration of E2 used in this study may not be considered physiologically relevant since free estrogens may not reach micromolar levels in the plasma. It has been shown that E2 attenuates the expression of VCAM-1 in vivo at physiological levels, whereas supraphysiological concentrations of E2 are required in vitro (Nathan et al., 1999) to show the same effect. Mukherjee et al. (2003) assessed whether a metabolite of E2, which could only be generated in vivo, might be a more potent inhibitor of VCAM-1 expression and thereby explain this discrepancy. In support of this hypothesis, they demonstrated that 17-epiestriol, an estrogen metabolite, was about 400 fold more potent than E2 in suppressing TNFα-induced VCAM-1 expression in HUVEC. In the same study, they showed that E2 did not affect VCAM-1 mRNA expression at lower concentrations (100 and 300 pM), whereas at higher concentrations (1–300 nM) it decreased VCAM-1 mRNA. These results are in accordance with observations of other investigators (Nakai et al., 1994; Caulin-Glaser et al., 1996; Simoncini et al., 2000) who reported that the concentrations of E2 required for suppression of VCAM-1 expression in endothelial cell cultures were in a supraphysiological range (1 nM-10 μM). It is also important to note that the experimental model (an immortalized cultured endothelial cell line derived from human umbilical endothelial cells) used in the current study could very likely be much less sensitive to the effects of E2 than cells in primary culture or even isolated microvessels in vitro.
In conclusion, we provide data demonstrating that LPS-stimulated human endothelial cells are characterized by increased ICAM-1 expression and agonist-induced [Ca2+]i compared to control cells. These effects of LPS are attenuated by pretreatment of the cells with E2. Our study indicates that E2 regulated [Ca2+]i homeostasis in LPS-stimulated human endothelial cells and suggests a potential mechanism for the anti-atherogenic activity of E2. However, a major conceptual issue that remains is whether these relatively short-term (24 h) effects of E2 in vitro can be used as an explanation for the long-term beneficial effects of E2 in atherosclerosis. It is possible that the effects of E2 reported in the current study are merely transient effects and that the protective effects of E2 reported in vivo are mainly due to alterations in the plasma lipid profile.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute [R15 HL-71520 to R.R.]; and the National Institute of Dental and Craniofacial Research [R15DE016587 to L.A. and R.R.].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen S, Khan S, Al-Mohanna F, Batten P, Yacoub M. Native low density lipoprotein-induced calcium transients trigger VCAM-1 and E-selectin expression in cultured human vascular endothelial cells. J Clin Invest. 1998;101:1064–1075. doi: 10.1172/JCI445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrani Y, Lazaar AL, Hoffman R, Amin K, Ousmer S, Panettieri RA., Jr Activation of p55 tumor necrosis factor-alpha receptor-1 coupled to tumor necrosis factor receptor-associated factor 2 stimulates intercellular adhesion molecule-1 expression by modulating a thapsigargin-sensitive pathway in human tracheal smooth muscle cells. Mol Pharmacol. 2000;58:237–245. doi: 10.1124/mol.58.1.237. [DOI] [PubMed] [Google Scholar]
- Biewenga E, Cabell L, Audesirk T. Estradiol and raloxifene protect cultured SN4741 neurons against oxidative stress. Neurosci Lett. 2005;373:179–183. doi: 10.1016/j.neulet.2004.09.067. [DOI] [PubMed] [Google Scholar]
- Castillo C, Ceballos G, Rodriguez D, Villanueva C, Medina R, Lopez J, Mendez E, Castillo EF. Effects of estradiol on phenylephrine contractility associated with intracellular calcium release in rat aorta. Am J Physiol Cell Physiol. 2006;291:C1388–1394. doi: 10.1152/ajpcell.00556.2005. [DOI] [PubMed] [Google Scholar]
- Caulin-Glaser T, Watson CA, Pardi R, Bender JR. Effects of 17beta-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest. 1996;98:36–42. doi: 10.1172/JCI118774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KH, Chang BH, Younan P, Shlykov SG, Sanborn BM, Chan L. Increased intracellular calcium transients by calmodulin antagonists differentially modulate tumor necrosis factor-alpha-induced E-selectin and ICAM-1 expression. Atherosclerosis. 2002;165:5–13. doi: 10.1016/s0021-9150(01)00768-7. [DOI] [PubMed] [Google Scholar]
- Chiu B, Viira E, Tucker W, Fong IW. Chlamydia pneumoniae, cytomegalovirus, and herpes simplex virus in atherosclerosis of the carotid artery. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS. Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest. 1994;93:17–25. doi: 10.1172/JCI116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak GR, el-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- Haarbo J, Leth-Espensen P, Stender S, Christiansen C. Estrogen monotherapy and combined estrogen-progestogen replacement therapy attenuate aortic accumulation of cholesterol in ovariectomized cholesterol-fed rabbits. J Clin Invest. 1991;87:1274–1279. doi: 10.1172/JCI115129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra L, Evans AI, Chen M, Hyduk SJ, Collins T, Cybulsky MI. The NF-kappa B signal transduction pathway in aortic endothelial cells is primed for activation in regions predisposed to atherosclerotic lesion formation. Proc Natl Acad Sci U S A. 2000;97:9052–9057. doi: 10.1073/pnas.97.16.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iiyama K, Hajra L, Iiyama M, Li H, DiChiara M, Medoff BD, Cybulsky MI. Patterns of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 expression in rabbit and mouse atherosclerotic lesions and at sites predisposed to lesion formation. Circ Res. 1999;85:199–207. doi: 10.1161/01.res.85.2.199. [DOI] [PubMed] [Google Scholar]
- Juan M, Mullol J, Roca-Ferrer J, Fuentes M, Perez M, Vilardell C, Yague J, Picado C. Regulation of ICAM-3 and other adhesion molecule expressions on eosinophils in vitro. Effects of dexamethasone Allergy. 1999;54:1293–1298. doi: 10.1034/j.1398-9995.1999.00251.x. [DOI] [PubMed] [Google Scholar]
- Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33:5308–5319. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KK, Bui MN, Mincemoyer R, Cannon RO., 3rd Effects of hormone therapy on inflammatory cell adhesion molecules in postmenopausal healthy women. Am J Cardiol. 1997;80:1505–1507. doi: 10.1016/s0002-9149(97)00732-7. [DOI] [PubMed] [Google Scholar]
- Li L, van Breemen C. Na(+)-Ca2+ exchange in intact endothelium of rabbit cardiac valve. Circ Res. 1995;76:396–404. doi: 10.1161/01.res.76.3.396. [DOI] [PubMed] [Google Scholar]
- Liang W, Buluc M, van Breemen C, Wang X. Vectorial Ca2+ release via ryanodine receptors contributes to Ca2+ extrusion from freshly isolated rabbit aortic endothelial cells. Cell Calcium. 2004;36:431–443. doi: 10.1016/j.ceca.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Libby P. Vascular biology of atherosclerosis: overview and state of the art. Am J Cardiol. 2003;91:3A–6A. doi: 10.1016/s0002-9149(02)03143-0. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- Matheny HE, Deem TL, Cook-Mills JM. Lymphocyte migration through monolayers of endothelial cell lines involves VCAM-1 signaling via endothelial cell NADPH oxidase. J Immunol. 2000;164:6550–6559. doi: 10.4049/jimmunol.164.12.6550. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–1043. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- Melnick JL, Adam E, Debakey ME. Cytomegalovirus and atherosclerosis. Eur Heart J. 1993;14(Suppl K):30–38. [PubMed] [Google Scholar]
- Mukherjee TK, Nathan L, Dinh H, Reddy ST, Chaudhuri G. 17-epiestriol, an estrogen metabolite, is more potent than estradiol in inhibiting vascular cell adhesion molecule 1 (VCAM-1) mRNA expression. J Biol Chem. 2003;278:11746–11752. doi: 10.1074/jbc.M207800200. [DOI] [PubMed] [Google Scholar]
- Nakai K, Itoh C, Hotta K, Itoh T, Yoshizumi M, Hiramori K. Estradiol-17 beta regulates the induction of VCAM-1 mRNA expression by interleukin-1 beta in human umbilical vein endothelial cells. Life Sci. 1994;54:PL221–227. doi: 10.1016/0024-3205(94)00630-x. [DOI] [PubMed] [Google Scholar]
- Nathan L, Pervin S, Singh R, Rosenfeld M, Chaudhuri G. Estradiol inhibits leukocyte adhesion and transendothelial migration in rabbits in vivo: possible mechanisms for gender differences in atherosclerosis. Circ Res. 1999;85:377–385. doi: 10.1161/01.res.85.4.377. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Fitoussi G, Reaud V, Pieraggi MT, Thiers JC, Salvayre R. A delayed and sustained rise of cytosolic calcium is elicited by oxidized LDL in cultured bovine aortic endothelial cells. FEBS Lett. 1992;299:60–65. doi: 10.1016/0014-5793(92)80101-l. [DOI] [PubMed] [Google Scholar]
- Norata GD, Tibolla G, Seccomandi PM, Poletti A, Catapano AL. Dihydrotestosterone decreases tumor necrosis factor-alpha and lipopolysaccharide-induced inflammatory response in human endothelial cells. J Clin Endocrinol Metab. 2006;91:546–554. doi: 10.1210/jc.2005-1664. [DOI] [PubMed] [Google Scholar]
- Ostos MA, Recalde D, Zakin MM, Scott-Algara D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 2002;519:23–29. doi: 10.1016/s0014-5793(02)02692-3. [DOI] [PubMed] [Google Scholar]
- Padar S, van Breemen C, Thomas DW, Uchizono JA, Livesey JC, Rahimian R. Differential regulation of calcium homeostasis in adenocarcinoma cell line A549 and its Taxol-resistant subclone. Br J Pharmacol. 2004;142:305–316. doi: 10.1038/sj.bjp.0705755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank MJ, Wall DJ, David T. Atherosclerosis and calcium signalling in endothelial cells. Prog Biophys Mol Biol. 2006;91:287–313. doi: 10.1016/j.pbiomolbio.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Putney JW., Jr Pharmacology of capacitative calcium entry. Mol Interv. 2001;1:84–94. [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Sbarouni E, Kroupis C, Kyriakides ZS, Koniavitou K, Kremastinos DT. Cell adhesion molecules in relation to simvastatin and hormone replacement therapy in coronary artery disease. Eur Heart J. 2000;21:975–980. doi: 10.1053/euhj.1999.1988. [DOI] [PubMed] [Google Scholar]
- Seljeflot I, Arnesen H, Hofstad AE, Os I. Reduced expression of endothelial cell markers after long-term transdermal hormone replacement therapy in women with coronary artery disease. Thromb Haemost. 2000;83:944–948. [PubMed] [Google Scholar]
- Simoncini T, Maffei S, Basta G, Barsacchi G, Genazzani AR, Liao JK, De Caterina R. Estrogens and glucocorticoids inhibit endothelial vascular cell adhesion molecule-1 expression by different transcriptional mechanisms. Circ Res. 2000;87:19–25. doi: 10.1161/01.res.87.1.19. [DOI] [PubMed] [Google Scholar]
- Strickberger SA, Russek LN, Phair RD. Evidence for increased aortic plasma membrane calcium transport caused by experimental atherosclerosis in rabbits. Circ Res. 1988;62:75–80. doi: 10.1161/01.res.62.1.75. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor D, Uchizono JA, Lin-Cereghino GP, Rahimian R. The effect of 17 beta-estradiol on intracellular calcium homeostasis in human endothelial cells. Eur J Pharmacol. 2010;630:92–99. doi: 10.1016/j.ejphar.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Z, Wang L, Zhou Y, Zhao Y, Liu L, Yao C, Qiao Z. Estrogen down-regulates nicotine-induced adhesion molecule expression via nongenomic signal pathway in endothelial cells. Int Immunopharmacol. 2006;6:892–902. doi: 10.1016/j.intimp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Yuan JS, Reed A, Chen F, Stewart CN., Jr Statistical analysis of real-time PCR data. BMC Bioinformatics. 2006;7:85. doi: 10.1186/1471-2105-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]