Abstract
Increased reactive oxygen species (ROS) production is involved in the pathogenesis of hypertension and stroke. The effects of ROS on cerebral vessels from hypertensive rats have not been studied. We hypothesized that tempol, a superoxide dismutase mimetic, would prevent middle cerebral artery (MCA) remodeling in stroke-prone spontaneously hypertensive rats (SHRSP). Six-week-old male SHRSP were treated with tempol (1mM) for six weeks. The MCA was then removed and mounted in a pressure myograph to study tone generation, vessel reactivity and passive vessel structure. Data are shown as mean±SEM, tempol vs control. Plasma thiobarbituric acid reactive substances (TBARS) were decreased by tempol treatment (14.15±1.46 vs 20.55±1.25, p=0.008 nM of malondialdehyde [MDA]/ml). Maximum serotonin induced constriction was increased by tempol treatment, without changes in dilation to adenosine diphosphate or tone generation. At an intralumenal pressure of 80 mmHg, tempol caused a dramatic increase in the MCA lumen diameter (246±5 vs 207±3μm, p<0.001), outer diameter (281±5 vs 241±3μm, p<0.001), lumen cross-sectional area and vessel cross-sectional area. Collagen IV mRNA expression were increased by 2.4-fold after tempol treatment. These results suggest that ROS are involved in the remodeling of the cerebral vasculature of SHRSP and that ROS scavenging can attenuate this process.
Keywords: tempol, vascular remodeling, SHRSP, middle cerebral artery, stroke
Introduction
Ischemic strokes are the major cause of adult disability in the United States (Lloyd-Jones et al. 2009). Ischemic strokes are caused by a partial or complete obstruction of a cerebral vessel (Alberts et al. 2007), which results in reduced perfusion and loss of functional brain tissue. There are many risk factors for stroke, including chronic hypertension, aging, gender, ethnicity and diabetes mellitus (Alberts et al. 2007). Among these, hypertension is the primary modifiable risk factor.
Chronic hypertension causes changes in the structure of resistance vessels, through a process known as vascular remodeling. Hypertension leads to inward vessel remodeling, defined as a reduction in vessel cross-sectional area (CSA), and an increase in wall thickness and wall/lumen ratio (Baumbach et al. 1993; Heagerty et al. 1993). Together, these alterations impair the middle cerebral artery's (MCA) auto-regulatory capacity and may account for an increased risk of ischemic stroke and increased damage in the event of cerebral ischemia (Dorrance et al. 2007; Rigsby et al. 2007). Even though it is accepted that vascular remodeling has a blood pressure dependent component, recent studies suggest that a blood pressure independent component is also present (Rigsby et al. 2007; Sakurabayashi-Kitade et al. 2009). Aldosterone and angiotensin-II (Ang-II) have been implicated in pressure independent remodeling of the resistance vasculature. Interestingly, these agents increase oxidative stress (Kumai et al. 2008; Briones et al. 2009). Furthermore, increases in wall stress, as consequence of reduced lumen diameter, activate intracellular cascades that culminate in the production and accumulation of reactive oxygen species (ROS) (Laurindo et al. 1994; De Keulenaer et al. 1998; Paravicini et al. 2006; Touyz 2006).
ROS, particularly the superoxide anion (O2•−), can be generated in both endothelial and vascular smooth muscle cells (VSMC) by multiple pathways. These include the pro-inflammatory cascades, such as cyclooxygenase and lipoxygenase (Planas et al. 1995; Taniyama et al. 2003; Moro et al. 2005), non-phagocytic NADPH oxidase (Fukui et al. 1997; Lipton 1999; Ungvari et al. 2003; Moro et al. 2005), and disruption of the mitochondrial electron-transport chain (Piantadosi et al. 1996; Moro et al. 2005). Under normal conditions, almost all of O2•− produced is dismutated to H2O2 naturally or by the action of the enzyme superoxide dismutase (SOD) (Touyz 2004). However, in hypertension, overproduction of O2•− saturates the cells defenses. Accumulation of O2•− leads to impaired vasodilation (Ungvari et al. 2003; Touyz 2004) and activation of protein tyrosine kinases and metalloproteinases. These pathways are important in controlling endothelial cell function and VSMC proliferation, differentiation, apoptosis and, ultimately, vessel remodeling (Paravicini et al. 2006). Therefore, we hypothesized that ROS scavenging by the membrane permeable SOD mimetic, 4-hydroxy-2,2,6,6-tetramethyl piperidine-1-oxyl (tempol), would improve MCA structure in stroke-prone spontaneously hypertensive rats (SHRSP).
Materials and Methods
Animals and treatments
Six-week-old male stroke-prone spontaneously hypertensive rats (SHRSP) from the colony housed at Medical College of Georgia and Michigan State University were randomized into two groups: untreated SHRSP and SHRSP treated with tempol (1mM, approximately 25mg/kg/day) in their drinking water for 6 weeks. The rats were maintained on a 12:12hr light:dark cycle, with regular chow and water available ad libitum. The experimental protocols were approved by the Institutional Animal Care & Use Committees in accordance with the American Physiological Society's “Guiding Principles in the Care and Use of Animals”.
Blood pressure measurement
Blood pressure was measured by tail-cuff using a RTBP1001 rat-tail blood pressure system (Kent Scientific, Torrington CT).
MCA reactivity, tone generation and passive structure
MCA structure was assessed by pressure myography (Living Systems Instrumentation, Burlington, VT). The first branch-free segment of the MCA most proximal to the Circle of Willis was mounted in a pressure myograph and kept in oxygenated warm physiological salt solution (PSS) (in mM: 141.9 NaCl, 4.7 KCl, 1.7 MgSO4, 0.5 EDTA, 2.8 CaCl2, 10.0 HEPES, 1.2 KH2PO4, and 5.0 glucose). The vessels were allowed to equilibrate for 30 minutes at an intralumenal pressure of 75 mmHg. To assess MCA reactivity, increasing concentrations of serotonin (5-HT, 10−9 to 10−5M) were added to the bath in a cumulative fashion. The MCA was then washed back to baseline, and the vasodilator adenosine diphosphate (ADP, 10−9 to 10−5M) was added to the bath in a cumulative fashion. The capacity of the MCA to generate tone was tested at intralumenal pressures of 80 mmHg and 140 mmHg. Tone was calculated using the following formula: %tone = [1−(active lumen diameter/passive lumen diameter)*100]. For the analysis of vessel passive structure, the vessels were bathed in calcium-free PSS containing 2mM EGTA and the intralumenal pressure was increased from 3 to 180 mmHg in 20 mmHg increments. Lumen diameter, external diameter and wall thickness were measured at each pressure after a 5-minute equilibration. The wall/lumen ratio, circumferential wall stress and wall strain were calculated using the method of Baumbach and Hadju (Baumbach et al. 1993). The elastic modulus (β-coefficient) was calculated from the stress/strain curves for the individual vessels, these curves were fitted to an exponential model (y=aeβx) where β is the slope of the curve: the higher the β-coefficient the stiffer the vessel.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
The MCA, posterior and anterior communicating ophthalmic and basilar arteries were used for qRT-PCR. These vessels were selected because they are similar in caliber to the MCA. Total mRNA was isolated using TRIzol® Reagent (Invitrogen, Carlsbad, CA) and reverse-transcribed (Superscript® VILO™, Invitrogen, Carlsbad, CA). Quantitative PCR was then performed using Taqman® ABI assays on demand probes in a 7500 Real Time PCR System (Applied Biosystem, Foster City, CA). Fold changes from control were calculated using the 2−ΔΔCT method (Livak et al. 2001), β-2 microglobulin was used for normalization.
Dyhidroethidium (DHE) Staining
Superoxide accumulation in the MCA wall was measured using DHE staining. Briefly, MCAs were isolated and incubated in PSS containing 2×10−6M DHE (Molecular Probes, Eugene, OR) for 45 minutes at 37°C in the dark. The vessels were then washed with PSS and placed on a microscope slide with mounting media (ProLong® Gold antifade reagent, Invitrogen, Eugene, OR) and images were obtained using a confocal microscope (excitation: 510nm, emission: 595nm, Leica TCS SL, Leica Mycrosystems Heidelberg GmbH, Germany). MCAs from untreated SHRSP were used as controls to adjust gain settings. Four regions of interest (ROI) were randomly chosen in each image, and mean fluorescence intensity (MFI) of vascular smooth muscle cells and background were analized using the Leica Confocal Software Lite. Background values were subtracted from MFI for each ROI. The 4 ROIs in each vessel were then averaged to produce a n of 1.
Carotid Artery Collagen, Elastin and Cross-sectional Area
Carotid arteries were excised and placed into 2% papaverin-saline to allow vessel dilation of the vessel. They were then fixed in 4% paraformaldehyde and paraffin-embedded. Serial sections (5μm) were cut and deparaffinized for Hematoxylin and Eosin (H&E) staining to measure vessel cross-sectional area. Total collagen in the vessel wall was measured using Picrossirius red staining and polarized microscopy as described previously (Junqueira et al. 1979; Junquiera et al. 1979). Quantification of total collagen content in the vessel wall was performed using AxioVision Rel. 4.6 software (Axioskop 40, Carl Zeiss Inc., Mexico), and data is expressed as total area of collagen/area of vessel wall. Elastin fibers stain was performed using a commercially available kit (Chromaview® Elastic Stain Kit, Richard-Allan Scientific, Kalamazoo, MI) following manufacturer's directions. Total elastin area was analyzed as described above, and data are expressed as area of elastin fibers/area of vessel wall.
Plasma Thiobarbituric Acid Species (TBARS)
Plasma TBARS was measured using a commercial kit (ZeptoMetrix Corporation, Buffalo, NY), following the manufacturer's instructions.
Statistics
All results are represented as a mean ± standard error of the mean. MCA structure data were analyzed by two-way repeated measures ANOVA with a Bonferroni post-test. Blood pressure, DHE staining, plasma TBARS, β-coefficients and qRT-PCR were compared using Student's t-test. A p-value ≤ 0.05 was considered significant.
Chemicals and Supplies
Unless otherwise stated, all chemicals and supplies were purchased from Sigma Chemical Company (St. Louis, MO).
Results
Blood pressure, DHE staining and plasma TBARS
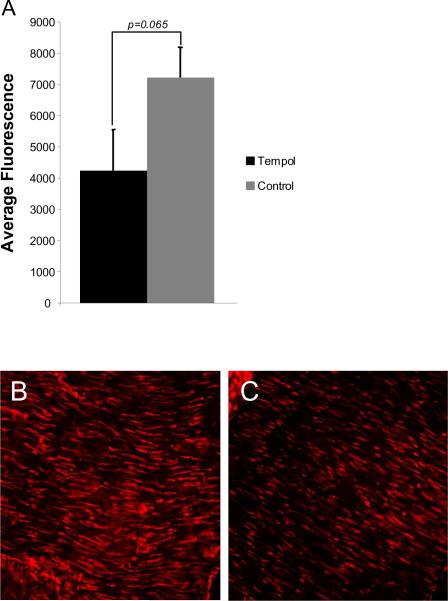
Tempol treatment had no effect on systolic blood pressure in SHRSP (197±4 vs 186±4mmHg, SHRSP+tempol vs SHRSP). Superoxide accumulation in the MCA wall, measured by mean fluorescence intensity after incubation with DHE, showed a trend towards a decrease in tempol-treated SHRSP (Fig 1). Plasma concentration of thiobarbituric acid was significantly decreased by tempol treatment (expressed as nM of malondialdehyde [MDA]/ml, 14.15±1.46 vs 20.55±1.25, tempol vs control, p=0.008).
Fig 1.
Antioxidant treatment with tempol caused a trend towards a reduction in superoxide accumulation in vascular smooth muscle cells from MCA wall observed as a reduction in mean fluorescence intensity after DHE staining in the MCA of tempol treated and untreated SHRSP (Tempol, n=10; Control, n=5) (A). Representative images of DHE staining in the MCA of untreated SHRSP (B) and tempol-treated SHRSP (C) (n=7 for both groups). The images were captured using a confocal microscope with a 40× objective.
MCA reactivity and tone
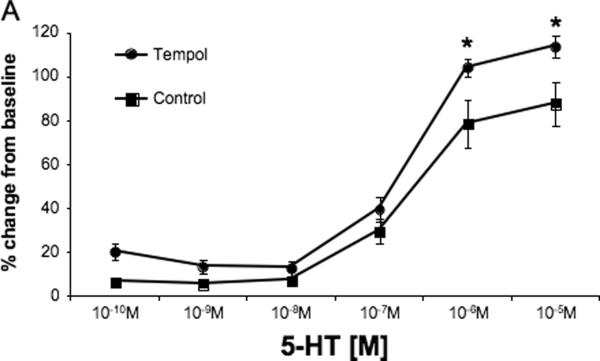
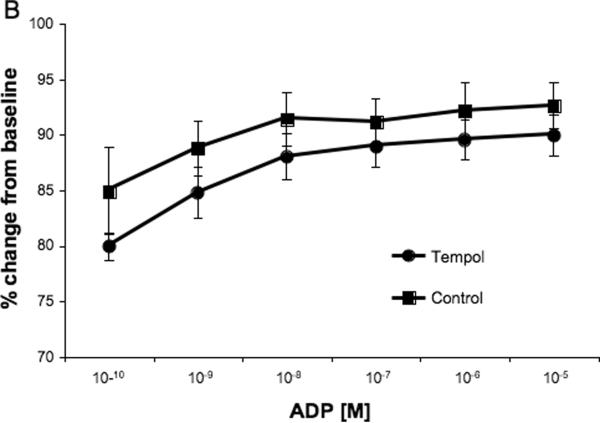
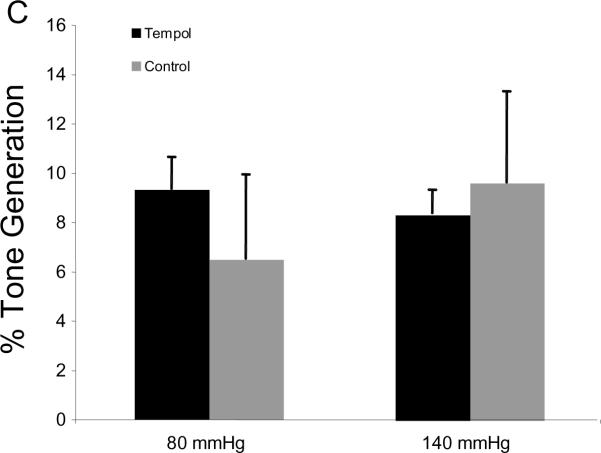
MCA reactivity to 5-HT is presented as the change in lumen diameter from baseline. Surprisingly, tempol treatment increased the MCA constriction to the highest concentration of serotonin (p<0.05, Fig 2A) without changing the logEC50 (−6.68±0.065 vs −6.64±0.121, SHRSP+tempol vs SHRSP). No differences were seen in the concentration-response curve to the endothelium-dependent vasodilator ADP (Fig 2B) and tone generation at 80 mmHg and 140 mmHg (Fig 2C).
Fig 2.
Tempol treatment increased MCA reactivity to higher doses of 5-HT (A), but did not change endothelium-dependent vasodilation (B) or tone generation and 80 mmHg and 140 mmHg (C). *p<0.05, Tempol vs Control, ANOVA. Values are mean±SEM. The MCA was cannulated between two glass cannulas in a pressure arteriograph and kept in oxygenated warm PSS under no-flow conditions throughout the experiment (Tempol, n=10; Control, n=5).
MCA passive structure
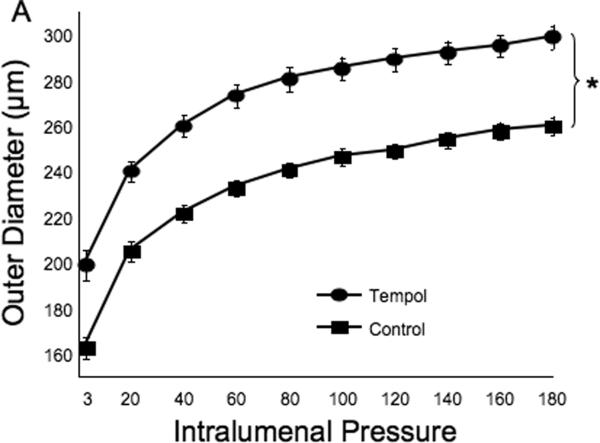
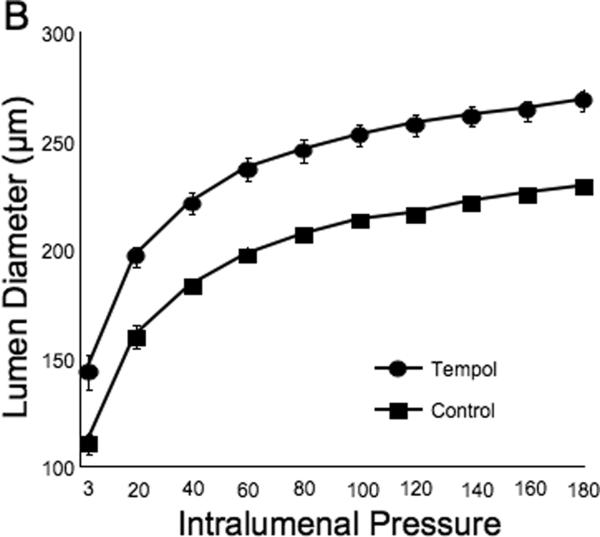
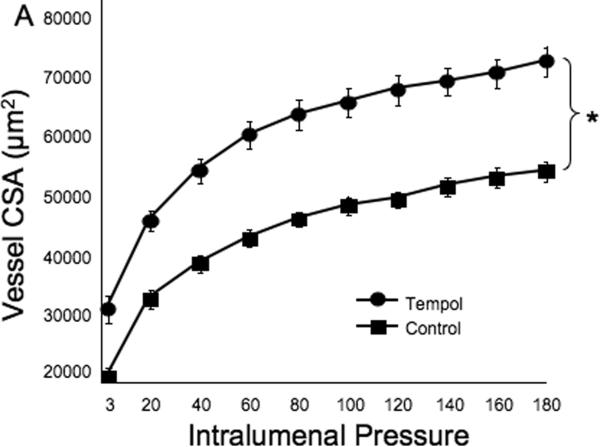
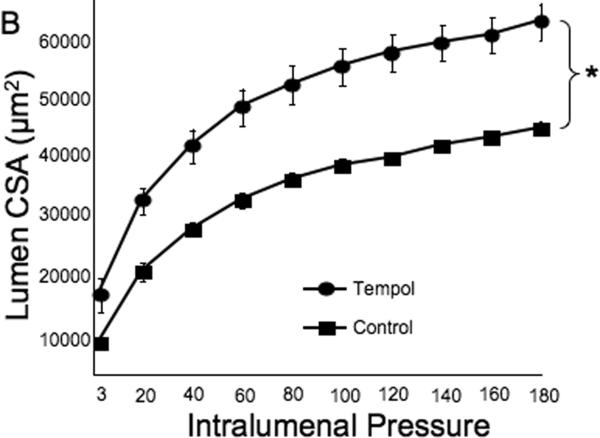
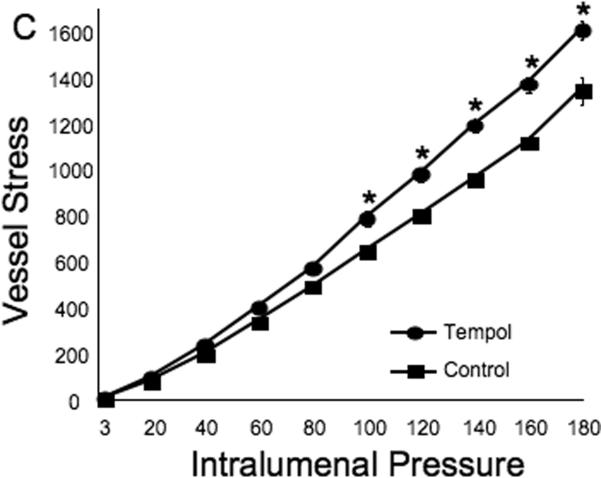
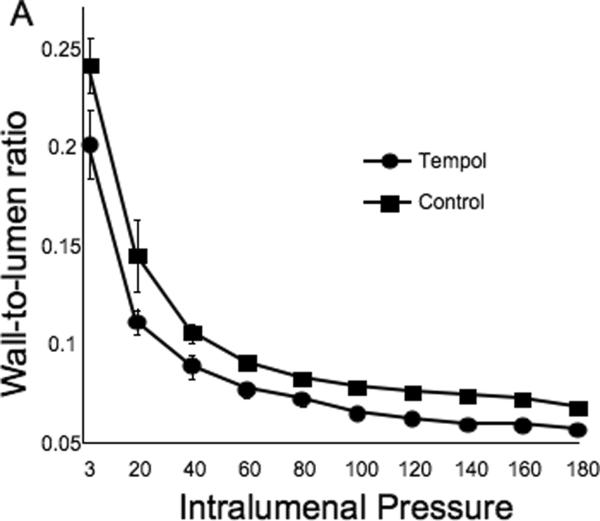
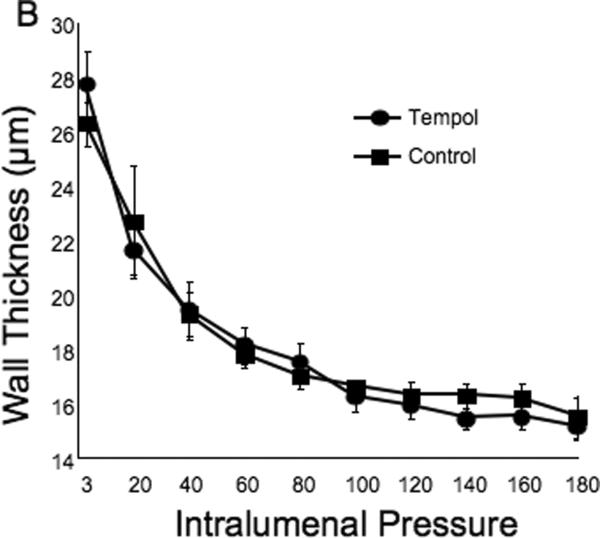
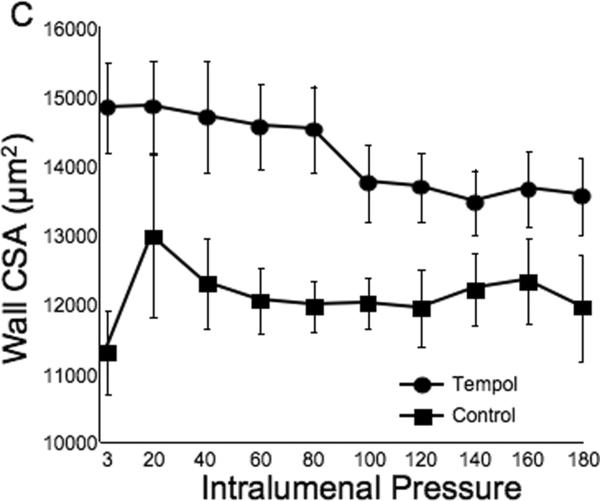
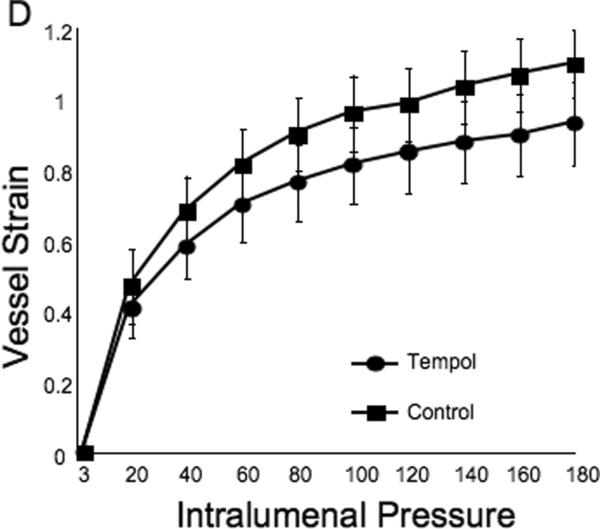
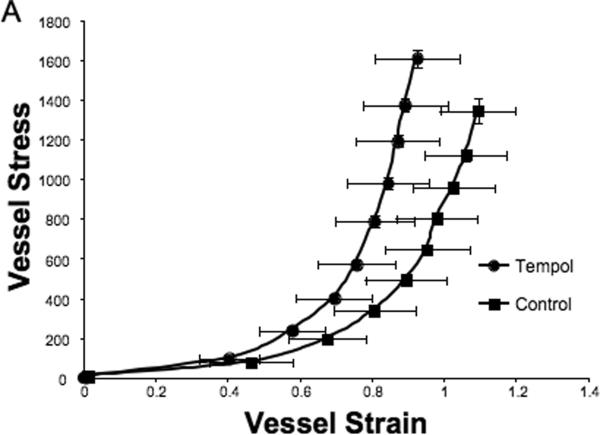
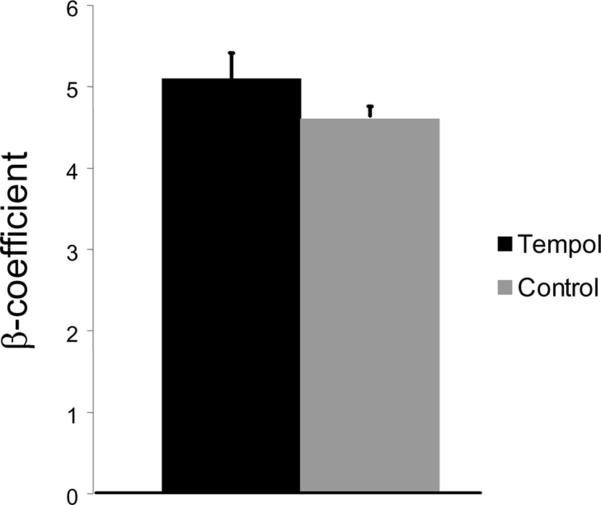
Tempol treatment improved MCA structure in SHRSP, as shown by an increase in outer and lumen diameter (p<0.001, Fig 3A and B, respectively) and vessel and lumen cross-sectional area (CSA) (p<0.001, Fig 4A and B, respectively) over the range of pressures analyzed. Vessel stress was increased in the tempol treated vessels at intralumenal pressures greater than 100 mmHg (p<0.001, Fig 4C). No differences were observed in the wall-to-lumen ratio (Fig 5A), wall thickness (Fig 5B), wall CSA (Fig 5C) and vessel strain (Fig 5D). The stress/strain curve showed a slight leftward shift, but this was not statistically significant (Fig 6A), and the β-coefficient was unchanged by tempol treatment (5.1±1.0 vs 4.6±0.3, SHRSP+tempol vs SHRSP, Fig 6B), indicating that vessel wall stiffness was unchanged. At 80 mmHg the remodeling index of tempol treated SHRSP was 1.17, showing an outward remodeling (increase in the lumen CSA), whilst the growth index was 0.17, indicating that tempol treatment prevented the atrophy of the MCA that is observed in hypertension. This tendency was maintained at the range of pressures (data not shown).
Fig 3.
Antioxidant treatment greatly improved MCA passive structure as observed by an increase in vessel outer and lumen diameter (A and B, respectively). *p<0.05, Tempol vs Control, ANOVA. Values are mean±SEM. The MCA was cannulated between two glass cannulas in a pressure arteriograph and kept in oxygenated warm calcium-free PSS under no-flow conditions throughout the experiment (Tempol, n=10; Control, n=5).
Fig 4.
Tempol treatment improved MCA passive structure as observed by an increase in vessel and lumen cross-sectional area (A and B, respectively). Vessel stress was increased in the MCA of tempol-treated SHRSP at intralumenal pressures over than 100 mmHg (C). *p<0.05, Tempol vs Control, ANOVA. Values are mean±SEM. The MCA was cannulated between two glass cannulas in a pressure arteriograph and kept in oxygenated warm calcium-free PSS under no-flow conditions throughout the experiment (Tempol, n=10; Control, n=5).
Fig 5.
Tempol treatment did not prevent the increase in the MCA wall thickness observed in the SHRSP, as seen by no changes in wall-to-lumen ratio (A), wall thickness (B) and wall cross-sectional area (C). MCA strain was not altered by tempol treatment (D). *p<0.05, Tempol vs Control, ANOVA. Values are mean±SEM. The MCA was cannulated between two glass cannulas in a pressure arteriograph and kept in oxygenated warm calcium-free PSS under no-flow conditions throughout the experiment (Tempol, n=10; Control, n=5).
Fig 6.
Tempol treatment caused a slightly leftward shift of the MCA stress-strain curve, although not statiscally significant (A). Vessel stiffness was not changed by tempol treatment, as seen by no differences in the β-coefficient values (B). Values are mean±SEM. The MCA was cannulated between two glass cannulas in a pressure arteriograph and kept in oxygenated warm calcium-free PSS under no-flow conditions throughout the experiment (Tempol, n=10; Control, n=5).
mRNA expression analysis by qRT-PCR
The results of the qRT-PCR are summarized in Table 1. We examined a pannel of extracelular matrix components, inflammatory, proliferation and apoptotic markers. Tempol treatment increased the expression of collagen IV (p<0.05). The mRNA expression of the other markers was unchanged by tempol treatment.
Table 1.
mRNA expression in CBV of Tempol-treated SHRSP
| mRNA | Fold Change from non-Treated SHRSP | p Value |
|---|---|---|
| ICAM-1 | 1.083±0.41 | 0.382 |
| CD68 | 1.297±0.24 | 0.369 |
| CCL-2 | 3.324±1.16 | 0.054 |
| TNF-α | 2.175±0.51 | 0.060 |
| Osteopontin | 1.670±0.45 | 0.205 |
| MKi-67 | 1.250±0.23 | 0.416 |
| Bcl-2 | 1.867±0.74 | 0.284 |
| BAD | 1.738±0.34 | 0.080 |
| Collagen I | 1.072±0.74 | 0.376 |
| Collagen III | 1.448±0.23 | 0.152 |
| Collagen IV | 2.353±0.53 | 0.034* |
| MMP-2 | 1.051±0.06 | 0.374 |
| MMP-9 | 1.196±0.20 | 0.890 |
| MMP-13 | 3.152±0.85 | 0.136 |
| TIMP-2 | 2.318±0.61 | 0.055 |
All values are mean ± SEM and are fold change from non-treated SHRSP. β-2 microglobulin was used for normalization. The data was analyzed by Student's T test.
Statistically significant.
Carotid Artery Collagen, Elastin and Cross-sectional Area
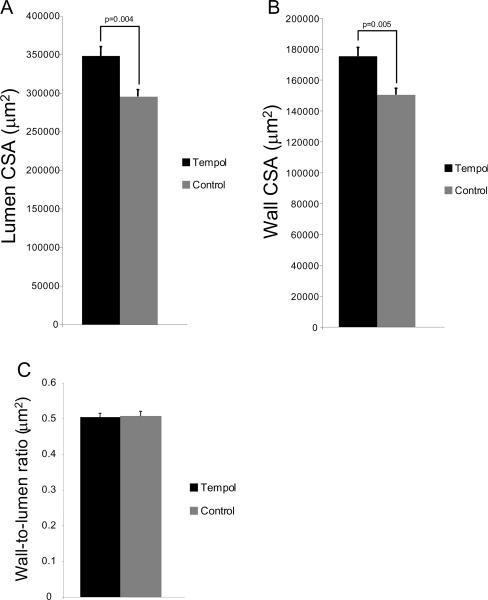
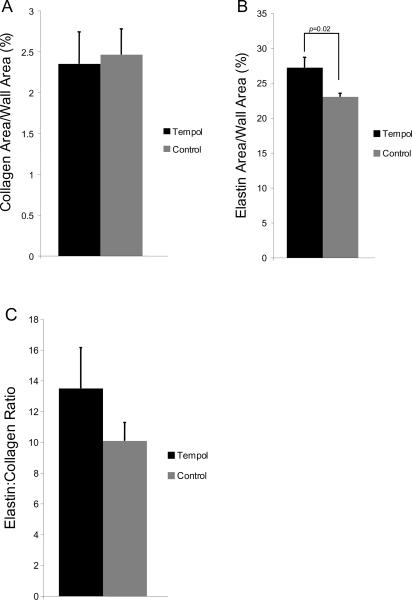
Tempol treatment significantly increased the outer and lumen cross-sectional area of carotid artery (Figs 7A and B), without changing the wall-to-lumen ratio (Fig 7C). Wall collagen content was not changed by tempol treatment (Fig 8A), but the elastin content in the vessel wall was increased (Fig 8B). Elastin:collagen ratio was not changed by tempol treatment (Fig 8C).
Fig 7.
Tempol treatment increased the lumen (A) and wall cross-sectional area (B) of the carotid artery in SHRSP (n=7 for both groups). Surprisingly, the wall-to-lumen ratio was not altered by tempol treatment (C). Morphometry was performed with images captured at 4× objective and analyzed using AxioVision Rel 4.6 (Carl Zeiss Inc).
Fig 8.
Tempol treatment did not change the area of total collagen in the vessel wall, as measured by percentage of wall area occupied by collagen (A) after Picrosirius red staining and analysis by polarized microscopy. Percent area occupied by elastin in the vessel wall was increased by tempol treatment in SHRSP (B). Elastin fibers were identified using Van Gieson's method for elastic fibers staining. Elastin:collagen ratio, an indicator of vascular compliance, was not altered by tempol treatment (C) (n=7 for both groups).
Discussion
The present study shows that tempol treatment improves MCA structure in 12 week-old male SHRSP. We observed an increase in the outer and lumen diameter, in vessel and lumen CSA and in vessel stress. These changes occurred independently of blood pressure. These data suggest that ROS, particularly the O2•− anion, is involved in hypertensive cerebral vessel remodeling. To our knowledge, this is the first report to show the benefits of ROS scavenging in the cerebral vasculature.
Despite observing a remarkable improvement in MCA structure, tempol did not reduce the blood pressure in the SHRSP. In agreement with this, tempol treatment did not prevent the Ang-II dependent increase in blood pressure in transgenic (mRen2) rats (Wei et al. 2007; DeMarco et al. 2008). However, tempol was shown to have anti-hypertensive effects in two-kidney, one-clip hypertensive rats (2K-1C) (Castro et al. 2009). 2K-1C is entirely dependent on activation of the rennin-angiotensin-aldosterone system, whereas hypertension in the SHRSP is polygenic and multifactorial, making it possible that mechanisms independent of ROS maintain the elevated blood pressure.
Tempol treatment caused a small and statistically insignificant reduction in O2•− accumulation in MCA wall, as measured by DHE. This technique has caveats that might explain the results obtained. MCAs were incubated ex vivo with DHE and, consequently, unable to perform de novo absorption of tempol. Furthermore, tempol action as an antioxidant rests on its activity as a scavenger of O2•−, and not by inhibition of enzymes that generate ROS. Therefore, it is possible that, during incubation, the intracellular pool of tempol might have been saturated, leading to the results we observed.
ROS have been implicated in the impaired reactivity of vascular beds to vasoconstrictor and vasodilator agents in hypertensive subjects. Increased intracellular O2•− impairs nitric oxide (NO) dependent dilation, by reacting with NO to produce peroxinitrite. Studies have suggested that tempol ameliorates endothelial dysfunction in the periphery in hypertensive rats. In Dahl-sensitive rats, tempol treatment improved both sustained and transient endothelium-dependent vasodilation of renal arterioles (Ozawa et al. 2004). We did not observe an improvement in endothelium-dependent dilation after tempol treatment. The lack of improvement in vasodilation could be because ADP was not perfused into the lumen to directly activate endothelium-dependent dilation. However, it is also possible that ADP-dependent vasodilation is simply not affected by ROS.
MCA reactivity to serotonin was increased after tempol treatment. Other studies suggest that ROS scavenging reduces vessel responsiveness to contractile agents. Renal afferent arterioles incubated with tempol showed reduced constriction to Ang-II and endothelin-1 (Wang et al. 2004). Basilar arteries from Mn-SOD knock-out mice showed enhanced constriction to arginine vasopressin, and this effect was abolished by addition of tempol to the bath (Faraci et al. 2006). The discrepancy between our findings and those of Wang and Faraci could be explained by the nature of tempol treatment. Tempol was not present in the pressure myograph bath during our studies. It is possible that acute effects of tempol would be diminished by the time the 5-HT curve was performed. Consequently, the MCA might have been more sensitive to the effects of superoxide produced by constricting agents.
Many studies show that O2•− is involved in vessel remodeling in peripheral vessels. NADPH-oxidase mediated O2•− generation is involved in coronary vascular remodeling in SHR (Bonacasa et al. 2008). Treatment with vitamin C and E (Chen et al. 2001) and the mineralocorticoid receptor antagonist spironolactone (Virdis et al. 2002) improved mesenteric resistance artery (MRA) structure by reducing O2•− in SHRSP and Sprague-Dawley rats, respectively. Tempol reduced the wall-to-lumen ratio in MRA of high-salt fed SHRSP (Park et al. 2002). However, alterations in peripheral vessels do not always translate into alterations in cerebral arteries, since they are structurally different (Lee 1995). In this regard, little is known about the effects of ROS and O2•− scavenging in the cerebral vasculature. Our data clearly shows O2•− involvement in MCA remodeling. Interestingly, we did not observe a reduction in the wall-to-lumen ratio of the MCA as has been reported in MRA. This could be a consequence of the type of remodeling observed in different vascular beds. Hypertension causes hypertrophic remodeling in the MRA and eutrophic remodeling in the MCA.
An increase in lumen diameter without a reduction in blood pressure could result in increased wall stress. This was the case in this study and this could increase the risk of a hemorrhagic stroke. However, wall thickness was not reduced by tempol treatment, and collagen IV mRNA was increased, suggesting an increase in collagen deposition in the basement membrane. Together, these factors might increase the vessel structural resistance to increased stress and protect the wall against rupture. It should be noted that we did not observe any evidence of hemorrhagic stroke in the tempol treated rats.
O2•− scavenging did not reduce MCA stiffness. Increased stiffness is a consequence of increased fibrillar collagen deposition and a decrease in elastic fibers. Cultured vascular smooth muscle cells produce collagen I in response to Ang-II via a ROS dependent mechanism. MRA stiffness was also increased in rats with Ang-II induced hypertension. This was also attributed to increased ROS generation in the vessel wall (Briones et al. 2009). The discrepancy between our results and those reported by Briones et al. might again be explained by the vascular bed studied. Unlike MRA, the MCA does not have an external elastic lamina (Lee 1995), and this might account for an increased stiffness and reduced compliance. It is also possible that fibrillar collagen (type I and III) deposition does not play a major role in MCA remodeling. The absence of a change in collagen I and III mRNA expression add weight to this argument.
Tempol treatment significantly increased the carotid artery outer, lumen and wall cross-sectional area. This was associated with an increase in the area of elastic fibers in the vessel wall. In aortas elastin corresponds to approximately 50% of the dry weight of the vessel (D'Armiento 2003). Therefore, it is possible that the increase in wall cross-sectional area observed in tempol-treated is a consequence of increased elastin content. Interestingly, carotid artery outer and lumen cross-sectional area were increased by tempol treatment, suggesting that in SHRSP carotid arteries undergo ROS-dependent inward remodeling, similar to MCA. Interestingly ex vivo ROS generation in carotid arteries has been linked to shear stress (Lemarie et al. 2006), which may be increased in SHRSP. In this environment, the increase in elastin could be a mechanical adaptation of the tempol-treated carotid arteries to increased blood flow due to an increase in lumen diameter in order to maintain a healthy level of vascular compliance. This possibility, however, needs further elucidation.
The protective activity of tempol in the cerebral vasculature could be through many pathways. Ang-II acts through NADPH-oxidase and generate ROS within the endothelial and VSMC (Touyz et al. 2003). Aldosterone increases ROS generation in human endothelial vein umbilical cells (Nagata et al. 2006). Endothelin-1 was shown to increase ROS production in rat aortic rings through NADPH oxidase (Loomis et al. 2005). Proinflammatory cytokines, like TNF-α, act through NADPH oxidase to generate ROS within endothelial cells (Sprague et al. 2009). During chronic hypertension, it has been suggested that these mediators are increased and, consequently, the intracellular pathways they activate are also upregulated. Moreover, it is known that these mediators are involved in remodeling of small arteries. Therefore, it is possible that the beneficial effects of tempol in the cerebral vasculature are not due to a reduction of one mechanism in particular, but rather by inhibiting all these pathways at once.
Currently, the only therapy available for stroke treatment is tissue plasminogen activator, is only administered to a small percentage of stroke patients. Hence, it is essential to develop therapies for primary stroke prevention rather than treatment. The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) showed that administration of rosuvastatin to apparently healthy patients reduced the incidence of stroke by approximately 50% (Everett et al.; Ridker et al. 2008). From the JUPITER trial, it became clear that primary prevention might be a key for stroke risk management. Many studies show that improving cerebrovascular structure results in a reduced infarct after cerebral ischemia. In this regard, antioxidants might be a tool for primary prevention of ischemic stroke.
Acknowledgements
The present study was supported by grants from the National Institutes of Health (HL077385:AMD) and the American Heart Association (0840122N:AMD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts MJ, Ovbiagele B. Current strategies for ischemic stroke prevention: role of multimodal combination therapies. J Neurol. 2007;254(10):1414–26. doi: 10.1007/s00415-007-0569-9. [DOI] [PubMed] [Google Scholar]
- Baumbach GL, Ghoneim S. Vascular remodeling in hypertension. Scanning Microsc. 1993;7(1):137–42. discussion 143. [PubMed] [Google Scholar]
- Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21(6 Pt 1):816–26. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- Bonacasa B, Sanchez ML, Rodriguez F, Lopez B, Quesada T, Fenoy FJ, Hernandez I. 2-Methoxyestradiol attenuates hypertension and coronary vascular remodeling in spontaneously hypertensive rats. Maturitas. 2008;61(4):310–6. doi: 10.1016/j.maturitas.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Briones AM, Rodriguez-Criado N, Hernanz R, Garcia-Redondo AB, Rodrigues-Diez RR, Alonso MJ, Egido J, Ruiz-Ortega M, Salaices M. Atorvastatin prevents angiotensin II-induced vascular remodeling and oxidative stress. Hypertension. 2009;54(1):142–9. doi: 10.1161/HYPERTENSIONAHA.109.133710. [DOI] [PubMed] [Google Scholar]
- Castro MM, Rizzi E, Rodrigues GJ, Ceron CS, Bendhack LM, Gerlach RF, Tanus-Santos JE. Antioxidant Treatment Reduces Matrix Metalloproteinase-2-Induced Vascular Changes in Renovascular Hypertension. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38(3 Pt 2):606–11. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- D'Armiento J. Decreased elastin in vessel walls puts the pressure on. J Clin Invest. 2003;112(9):1308–10. doi: 10.1172/JCI20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82(10):1094–101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- DeMarco VG, Habibi J, Whaley-Connell AT, Schneider RI, Heller RL, Bosanquet JP, Hayden MR, Delcour K, Cooper SA, Andresen BT, Sowers JR, Dellsperger KC. Oxidative stress contributes to pulmonary hypertension in the transgenic (mRen2)27 rat. Am J Physiol Heart Circ Physiol. 2008;294(6):H2659–68. doi: 10.1152/ajpheart.00953.2007. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Pollock DM, Romanko OP, Stepp DW. A high-potassium diet reduces infarct size and improves vascular structure in hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R415–22. doi: 10.1152/ajpregu.00438.2005. [DOI] [PubMed] [Google Scholar]
- Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) Circulation. 121(1):143–50. doi: 10.1161/CIRCULATIONAHA.109.874834. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Modrick ML, Lynch CM, Didion LA, Fegan PE, Didion SP. Selective cerebral vascular dysfunction in Mn-SOD-deficient mice. J Appl Physiol. 2006;100(6):2089–93. doi: 10.1152/japplphysiol.00939.2005. [DOI] [PubMed] [Google Scholar]
- Fukui T, Ishizaka N, Rajagopalan S, Laursen JB, Capers Q. t., Taylor WR, Harrison DG, de Leon H, Wilcox JN, Griendling KK. p22phox mRNA expression and NADPH oxidase activity are increased in aortas from hypertensive rats. Circ Res. 1997;80(1):45–51. doi: 10.1161/01.res.80.1.45. [DOI] [PubMed] [Google Scholar]
- Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertension. 1993;21(4):391–7. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J. 1979;11(4):447–55. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Junquiera LC, Junqueira LC, Brentani RR. A simple and sensitive method for the quantitative estimation of collagen. Anal Biochem. 1979;94(1):96–9. doi: 10.1016/0003-2697(79)90795-4. [DOI] [PubMed] [Google Scholar]
- Kumai Y, Ooboshi H, Ago T, Ishikawa E, Takada J, Kamouchi M, Kitazono T, Ibayashi S, Iida M. Protective effects of angiotensin II type 1 receptor blocker on cerebral circulation independent of blood pressure. Exp Neurol. 2008;210(2):441–8. doi: 10.1016/j.expneurol.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Laurindo FR, Pedro Mde A, Barbeiro HV, Pileggi F, Carvalho MH, Augusto O, da Luz PL. Vascular free radical release. Ex vivo and in vivo evidence for a flow-dependent endothelial mechanism. Circ Res. 1994;74(4):700–9. doi: 10.1161/01.res.74.4.700. [DOI] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66(1):149–73. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- Lemarie CA, Tharaux PL, Esposito B, Tedgui A, Lehoux S. Transforming growth factor-alpha mediates nuclear factor kappaB activation in strained arteries. Circ Res. 2006;99(4):434–41. doi: 10.1161/01.RES.0000237388.89261.47. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart Disease and Stroke Statistics--2010 Update. A Report From the American Heart Association. Circulation. 2009 doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- Loomis ED, Sullivan JC, Osmond DA, Pollock DM, Pollock JS. Endothelin mediates superoxide production and vasoconstriction through activation of NADPH oxidase and uncoupled nitric-oxide synthase in the rat aorta. J Pharmacol Exp Ther. 2005;315(3):1058–64. doi: 10.1124/jpet.105.091728. [DOI] [PubMed] [Google Scholar]
- Moro MA, Almeida A, Bolanos JP, Lizasoain I. Mitochondrial respiratory chain and free radical generation in stroke. Free Radic Biol Med. 2005;39(10):1291–304. doi: 10.1016/j.freeradbiomed.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48(1):165–71. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Hayashi K, Kanda T, Homma K, Takamatsu I, Tatematsu S, Yoshioka K, Kumagai H, Wakino S, Saruta T. Impaired nitric oxide- and endothelium-derived hyperpolarizing factor-dependent dilation of renal afferent arteriole in Dahl salt-sensitive rats. Nephrology (Carlton) 2004;9(5):272–7. doi: 10.1111/j.1440-1797.2004.00292.x. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71(2):247–58. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Park JB, Touyz RM, Chen X, Schiffrin EL. Chronic treatment with a superoxide dismutase mimetic prevents vascular remodeling and progression of hypertension in salt-loaded stroke-prone spontaneously hypertensive rats. Am J Hypertens. 2002;15(1 Pt 1):78–84. doi: 10.1016/s0895-7061(01)02233-6. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27(2):327–31. doi: 10.1161/01.str.27.2.327. discussion 332. [DOI] [PubMed] [Google Scholar]
- Planas AM, Soriano MA, Rodriguez-Farre E, Ferrer I. Induction of cyclooxygenase-2 mRNA and protein following transient focal ischemia in the rat brain. Neurosci Lett. 1995;200(3):187–90. doi: 10.1016/0304-3940(95)12108-g. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- Rigsby CS, Pollock DM, Dorrance AM. Spironolactone improves structure and increases tone in the cerebral vasculature of male spontaneously hypertensive stroke-prone rats. Microvasc Res. 2007;73(3):198–205. doi: 10.1016/j.mvr.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurabayashi-Kitade S, Aoka Y, Nagashima H, Kasanuki H, Hagiwara N, Kawana M. Aldosterone blockade by Spironolactone improves the hypertensive vascular hypertrophy and remodeling in angiotensin II overproducing transgenic mice. Atherosclerosis. 2009;206(1):54–60. doi: 10.1016/j.atherosclerosis.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78(6):539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–81. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Reactive oxygen species and angiotensin II signaling in vascular cells -- implications in cardiovascular disease. Braz J Med Biol Res. 2004;37(8):1263–73. doi: 10.1590/s0100-879x2004000800018. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Mitochondrial redox control of matrix metalloproteinase signaling in resistance arteries. Arterioscler Thromb Vasc Biol. 2006;26(4):685–8. doi: 10.1161/01.ATV.0000216428.90962.60. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Tabet F, Schiffrin EL. Redox-dependent signalling by angiotensin II and vascular remodelling in hypertension. Clin Exp Pharmacol Physiol. 2003;30(11):860–6. doi: 10.1046/j.1440-1681.2003.03930.x. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108(10):1253–8. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Virdis A, Neves MF, Amiri F, Viel E, Touyz RM, Schiffrin EL. Spironolactone improves angiotensin-induced vascular changes and oxidative stress. Hypertension. 2002;40(4):504–10. doi: 10.1161/01.hyp.0000034738.79310.06. [DOI] [PubMed] [Google Scholar]
- Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane prostanoid receptors, and endothelium. Circ Res. 2004;94(11):1436–42. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- Wei Y, Whaley-Connell AT, Chen K, Habibi J, Uptergrove GM, Clark SE, Stump CS, Ferrario CM, Sowers JR. NADPH oxidase contributes to vascular inflammation, insulin resistance, and remodeling in the transgenic (mRen2) rat. Hypertension. 2007;50(2):384–91. doi: 10.1161/HYPERTENSIONAHA.107.089284. [DOI] [PubMed] [Google Scholar]