Abstract
Diabetes and obesity are associated with activation of endoplasmic reticulum (ER) stress; however a direct link between ER stress and increased hepatic gluconeogenesis remains unclear. Here we show that ER stress triggers a significant increase in expression of CCAAT/enhancer-binding protein (C/EBPβ) and phosphorylated CREB together with reduced phospho-AMP-activated protein kinase (pAMPK) in hepatoma cells. ER stress contributed to transcriptional activation of the gluconeogenic phosphoenolpyruvate carboxykinase (PEPCK) promoter in Huh7 and HepG2 cells via cAMP binding motif (CRE site). Chromatin immunoprecipitation assays demonstrate that C/EBPβ is recruited to the PEPCK promoter during ER stress and is reversed by pre-treatment with a JNK inhibitor that relieves ER stress. C/EBPβ but not pCREB were suppressed by the AMPK-activator AICAR or constitutively active AMPK, while dominant negative AMPK increased C/EBPβ expression. These data suggest that ER stress triggers suppression of AMPK while increasing C/EBPβ and pCREB expression which activates PEPCK gene transcription. Understanding how ER stress suppresses AMPK activation and increases C/EBPβ expression could lead to a potentially novel pathway for treatment of diabetes.
Keywords: ER stress, C/EBPβ, pAMPK, gluconeogenesis, XBP1-spliced
1. Introduction
Type 2 diabetes is one of the most prevalent chronic metabolic diseases and a principal source of morbidity and mortality in contemporary society (Hossain et al., 2007). Increased hepatic gluconeogenesis is a hallmark feature of type 2 diabetes, and thus factors (dietary, hormonal, and genetic) that contribute to regulation of the key gluconeogenic enzyme phosphoenolpyruvate carboxykinase (PEPCK) are the subject of intense investigation. It is now widely acknowledged that endoplasmic reticulum (ER) stress is induced in various tissues including liver during obesity and diabetes and may play crucial roles in the insulin resistance pathways (Nakatani et al., 2005; Ozcan et al., 2006). Indeed, reversing ER stress using chemical chaperones, such as 4-phenylbutyrate (PBA), have been shown to lower blood glucose in animals (Ozcan et al., 2006) and improves insulin resistance in type 2 diabetes (Ozawa et al., 2005). However, the molecular mechanisms involved in the regulation of gluconeogenesis during ER stress and their links with diabetes are less clear.
The ER is a membranous network that synthesizes various secretory and membrane proteins. Under nutrient overload conditions, an increase in misfolded protein levels can occur whereby the ER fails to fold and export newly synthesized proteins which induces ER stress signaling in order to reverse the protein overload (Harding et al., 1999; Wang et al., 1998). While recent studies reveal the importance of ER stress in insulin resistance and hepatic steatosis, it remains unknown how ER stress modulates transcriptional events that control hepatic gluconeogenesis. AMP-activated protein kinase (AMPK) is an important energy sensor involved in the regulation of hepatic gluconeogenesis. AMPK suppresses gluconeogenesis in part by suppressing major transcription factors that control PEPCK gene expression, such as HNF4α and FOX01 (Barthel et al., 2002; Horike et al., 2008; Leclerc et al., 2001). Recently, phosphorylation of CREB-regulated transcription coactivator 2 (CRTC2), also referred to as transducer of regulated CREB activity 2 (TORC2) and a co-activator of cAMP-responsive element binding (CREB) protein, was reported to enter the nucleus under conditions of ER stress (Wang et al., 2009); while AMPK was reported to reduce PEPCK gene expression (Horike et al., 2008; Screaton et al., 2004; Shaw et al., 2005).
On the other hand, stress kinase signaling such as p38 MAPK is responsible for stimulation of several transcription factors, for example ATF2, CREB, and C/EBPβ, which up-regulate PEPCK gene transcription (Baan et al., 2006; Chen et al., 1998; Yemelyanov et al., 2007). Park et al. reported a role for C/EBPβ at the cAMP-response element (CRE) in the induction of PEPCK by cAMP (Park et al., 1993). C/EBPβ is rapidly induced by glucagon and binds avidly to the CRE (Arizmendi et al., 1999; Nizielski et al., 1996). Indeed, mice with a global C/EBPβ knockout show a phenotype similar to liver-specific CREB knockout mice (Herzig et al., 2001), including reduced gluconeogenesis, fasting hypoglycemia, and reduced expression of gluconeogenic genes (Arizmendi et al., 1999; Liu et al., 1999). When crossed with genetically obese diabetic Leprdb/db mice, C/EBPβ × Leprdb/db double knockout mice demonstrate reduced fasting blood glucose (Schroeder-Gloeckler et al., 2007), consistent with a role for C/EBPβ in controlling PEPCK gene transcription and gluconeogenesis. Here we demonstrate that ER stress, in addition to increasing C/EBPβ and pCREB expression, suppresses AMPK activation which together increases PEPCK gene transcription. We also demonstrate that C/EBPβ is highly sensitive to AMPK activation, suggesting a possible novel pathway whereby AMPK activation suppresses C/EBPβ expression and inhibits transcriptional activation of the PEPCK promoter in diabetes.
2. Materials and methods
2.1. Reagents
Primary antibodies used in this study were C/EBPβ (sc-150) and actin (sc-7210) from Santa Cruz Biotechnology (Santa Cruz, CA); CREB (9192), AMPK (2532), pAMPK-Thr172 (2531), pCREB-Ser133 (9191), and pACC (3661) from Cell Signaling Technology (Danvers, MA). AICAR was obtained from Toronto Research Chemicals, Inc. (North York, Ontario, Canada), thapsigargin (TPG), tunicamycin (TUN), sodium azide, and FBS from Sigma-Aldrich (St. Louis, MO), and JNK inhibitor from A.G. Scientific (San Diego, CA). Culture media and supplements were obtained from Mediatech, Inc. (Herndon, VA).
2.2. Cell culture and Western blot analysis
Rat hepatoma FAO (Sigma), human hepatoma HepG2 (ATCC, Manassas, VA), and Huh7 cells (from C. Rice, Rockefeller University) were cultured in MEM or DMEM media, pH 7.4, 1x nonessential amino acids, and 10% supplemented with 25 mM NaHCO3, 4 mM glutamine, FBS and maintained at 37°C in a 5% CO2 incubator. In viral experiments, cells were serum-starved overnight and infected with adenovirus containing dominant negative AMPK (Ad-DN-AMPK), constitutively active AMPK (Ad-CA-AMPK), or Ad-GFP each at 50 pfu/cell as described previously (Minokoshi et al., 2004), or Ad-XBP1-unspliced or Ad-XBP1-spliced at 50 pfu/cell (Ozcan et al., 2004) for 24 h prior to study. Cytosolic and nuclear extracts were prepared and Western blot analysis performed as described previously (Choudhury and Shukla, 2008; Rahman et al., 2007).
2.3. Luciferase activity assay
Cultures were set up 24 h prior to transfections in 6-well plates at 3 × 105 cells/well. Cells were transfected with the 490-Luc luciferase expression vector containing the functional -490-PEPCK promoter (Routes et al., 2000) or the CRE-Luc luciferase expression vector containing the PEPCK promoter with a mutated CRE element (Liu et al., 1991) (constructs from D. Klemm, University of Colorado) for 48 h using Fugene 6 (Roche Applied Science, Indianapolis, IN) at 2:1 DNA/lipid ratio. TPG treatment (300 nM) was carried out for 2 h before harvesting the cells for luciferase assays using a dual luciferase reporter assay system (Promega, Madison, WI) as described (Qadri et al., 2009).
2.4. ChIP assay
The association of C/EBPβ protein with the PEPCK promoter during ER stress was measured by chromatin immunoprecipitation (ChIP). FAO cells were treated with 300 nM TPG alone or in combination with 90 nM JNK inhibitor and ChIP assays were performed according to manufacturer’s protocol (Upstate Biotechnology, Lake Placid, NY). The shearing condition was optimized until it gave a smear of DNA in the desired range of 200–1000 bp. After determining the optimal conditions, unsheared and sheared chromatin from the cell was electrophoresed through a 2% agarose gel and stained with ethidium bromide. The majority of the DNA was sheared to a length between 200 and 1000 bp. ChIPed DNA was used in real time quantitative PCR to measure PEPCK and GAPDH. The primers used were as follows: PEPCK (from −209 to +66) 5'-GAGGCCTCCCAACATTCAT-3' & 5'-CTCAGAGCGTCTCGCCGGA-3' and GAPDH 5'-ACCACAGTCCATGCCATCAC-3' & 5'-TCCACCACCCTGTTGCTGTA-3'.
2.5. Statistical analysis
Data were combined from three separate experiments. Statistical analyses were performed using a standard one-way ANOVA (Newman-Keuls Multiple Comparison Test) or Student’s t test. Differences with P < 0.05 were considered significant.
3. Results
3.1. Exposure to ER stress inhibits AMPK activity
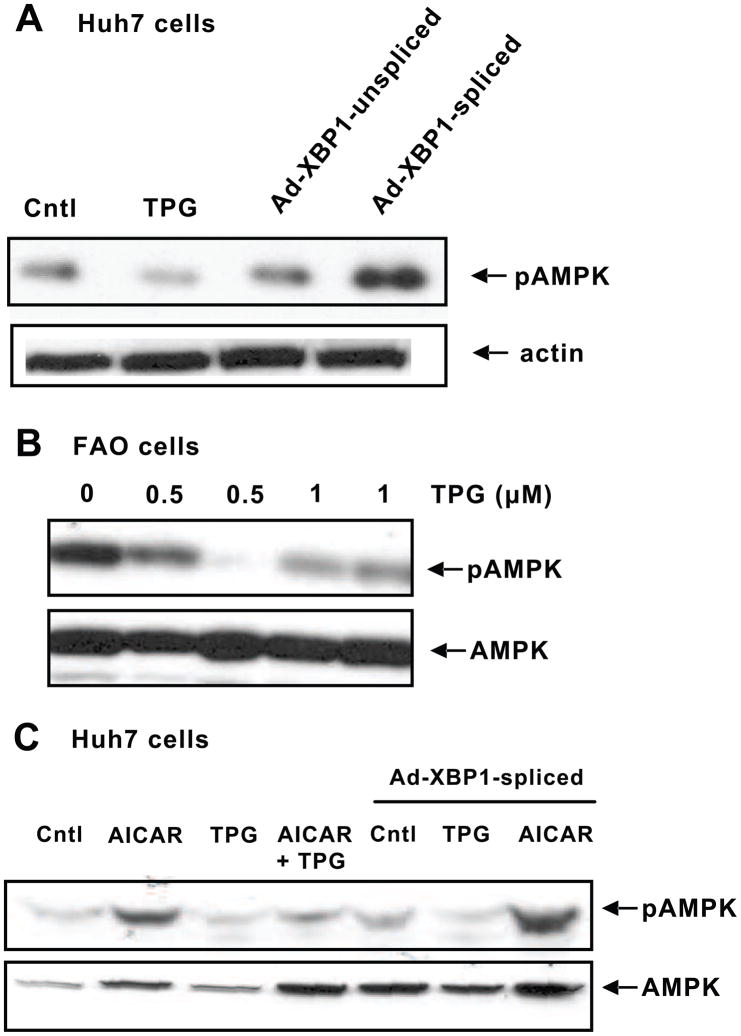
Using the ER stress activator TPG (300 nM), we found that it suppressed phosphorylation of AMPK in Huh7 cells, a human hepatocellular carcinoma cell line (Fig. 1A), and in the rodent-derived FAO cell line (500 nm and 1 μM TPG treatment, Fig. 1B). To examine the down-stream effectors of ER stress, we used a specific ER stress-induced protein X-box-binding protein 1 (XBP1) on AMPK activation. XBP1 is a key transcription factor that when spliced increases the expression of proteins that supply the ER with resident-folding assistants, thereby rebalancing protein load with increased folding capacity in the ER (Back et al., 2005) and relieving ER stress (Ozcan et al., 2004). Cells infected with Ad-XBP1-spliced showed increased pAMPK compared to unspliced XBP1 adenovirus.
Fig. 1.
ER stress down-regulates pAMPK levels. A, Huh7 cells were treated with 300 nM TPG or infected with Ad-XBP1-unspliced or Ad-XBP1-spliced. Cytosolic proteins were used for Western blot analysis to detect pAMPK and β-actin levels. B, TPG (500 nm and 1μM) suppresses pAMPK levels in FAO cells. pAMPK and total AMPK levels were detected in cytosolic proteins of control and treated cells after 24 h treatment with TPG. C, Huh7 cells were treated with 500 μM AICAR or 300 nM TPG alone or in combination for 2 h. The cells were also infected with Ad-XBP1-spliced for 24 h followed by treatment with either TPG or AICAR for 2 h. Cytosolic proteins were run on SDS-PAGE to detect pAMPK and total AMPK levels. Blots shown are representative of three separate experiments with similar results.
To further examine the interaction between ER stress and AMPK, Huh7 cells were treated with AICAR, a pharmacological activator of AMPK. AICAR promptly increased pAMPK in cells treated for 2 h (Fig. 1C); however, TPG suppressed pAMPK either alone or in the presence of AICAR. In cells infected with Ad-XBP1-spliced, pAMPK tended to increase, however pAMPK was again suppressed in the presence of TPG + Ad-XBP1-spliced. The addition of AICAR to cells expressing Ad-XBP1-spliced significantly increased pAMPK levels above AICAR alone.
3.2. ER stress increases C/EBPβ and CREB expression
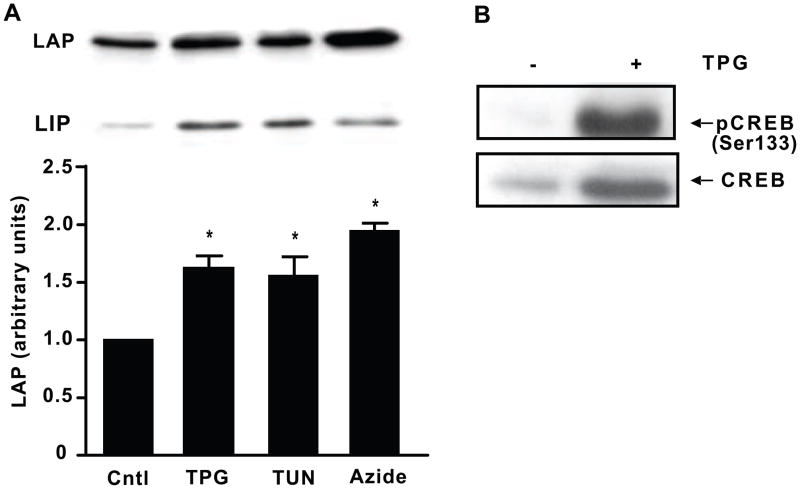
We next examined the effects of ER stress on C/EBPβ, a member of the C/EBP family which regulates transcription of genes that control gluconeogenesis, lipogenesis, and the inflammatory response in liver (Chen et al., 2005; Li et al., 2008; Oyadomari et al., 2008; Rahman et al., 2007). C/EBPβ mRNA translation produces a full length transcriptional activator form, liver activating protein (LAP), and the truncated inhibitory form, liver inhibitory protein (LIP). FAO cells were treated with various inducers of ER stress. Within 2 h, 300 nM TPG, 5 μg/ml TUN, and 1 mM sodium azide all significantly increased the levels of LAP compared to untreated cells (*P < 0.05, Fig. 2A). We also examined the effect of ER stress on CREB and pCREB expression and found that TPG stimulated phosphorylation of CREB as well as CREB protein levels in nuclear extracts (Fig. 2B).
Fig. 2.
ER stress increases C/EBPβ expression (LAP and LIP) and pCREB levels. A, FAO cells were treated with 300 nM TPG, 5 μg/ml TUN, or 1 mM sodium azide for 2 h. Nuclear proteins were prepared and levels of the C/EBPβ isoforms LAP and LIP were detected by Western blot analysis using C/EBPβ antibody. Control set to 1. Data is represented as mean ± SEM of three independent experiments. *P < 0.05 vs. control. B, FAO cells were treated with or without 300 nM TPG for 2 h and nuclear extracts were prepared. CREB and pCREB were detected by Western blot analysis. Blots shown are representative of three separate experiments with similar results.
3.3. AMPK regulates C/EBPβ levels
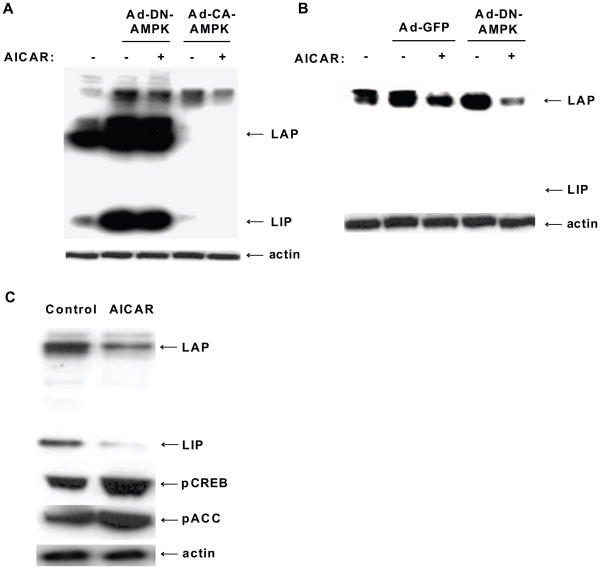
To determine whether AMPK regulates C/EBPβ expression, FAO cells were infected with Ad-DN-AMPK, Ad-CA-AMPK, or Ad-GFP as control adenovirus and treated with or without 2 mM AICAR for 24 h. Over-expression of AMPK-DN led to a large increase in expression of both isoforms of LAP (33 and 38 kD) and the 16 kD LIP isoform (Fig. 3A), while AICAR showed no further increase in C/EBPβ expression. By contrast, over-expression of AMPK-CA, in either the absence or presence of 2 mM AICAR, dramatically suppressed the expression of both the LAP and LIP isoforms of C/EBPβ. β-actin levels remained unchanged in all cases. Using a lower titer of Ad-DN-AMPK in cells (10 pfu/cell, Fig. 3B), we demonstrated that AMPK-DN increased LAP protein expression but that AICAR was capable of suppressing LAP expression. Simultaneously, we also showed that AICAR (500 μM for 24 h) alone increased pCREB and acetyl CoA carboxylase (pACC, one of the targets of AMPK) phosphorylation (Fig. 3C).
Fig. 3.
C/EBPβ responds to AMPK activation or inhibition in FAO cells. A, Cells expressing AMPK-DN or AMPK-CA (50 pfu/cell) were treated with or without 2 mM AICAR for 24 h. Levels of LAP and LIP isoforms of C/EBPβ were compared and loading was standardized to β-actin. B, Cells expressing either Ad-GFP or Ad-DN-AMPK at a lower titer (10 pfu/cell) were treated with or without 2 mM AICAR for 24 h. Cells were lysed and the levels of the LAP and LIP isoforms were detected by Western blot analysis using β-actin as a loading control. C, Cells treated with 500 μM AICAR for 24 h showed reduced C/EBPβ protein and increased pCREB and pACC levels. Blots are representative of three separate experiments with similar results.
3.4. Involvement of AMPK activity and ER stress in C/EBPβ regulation
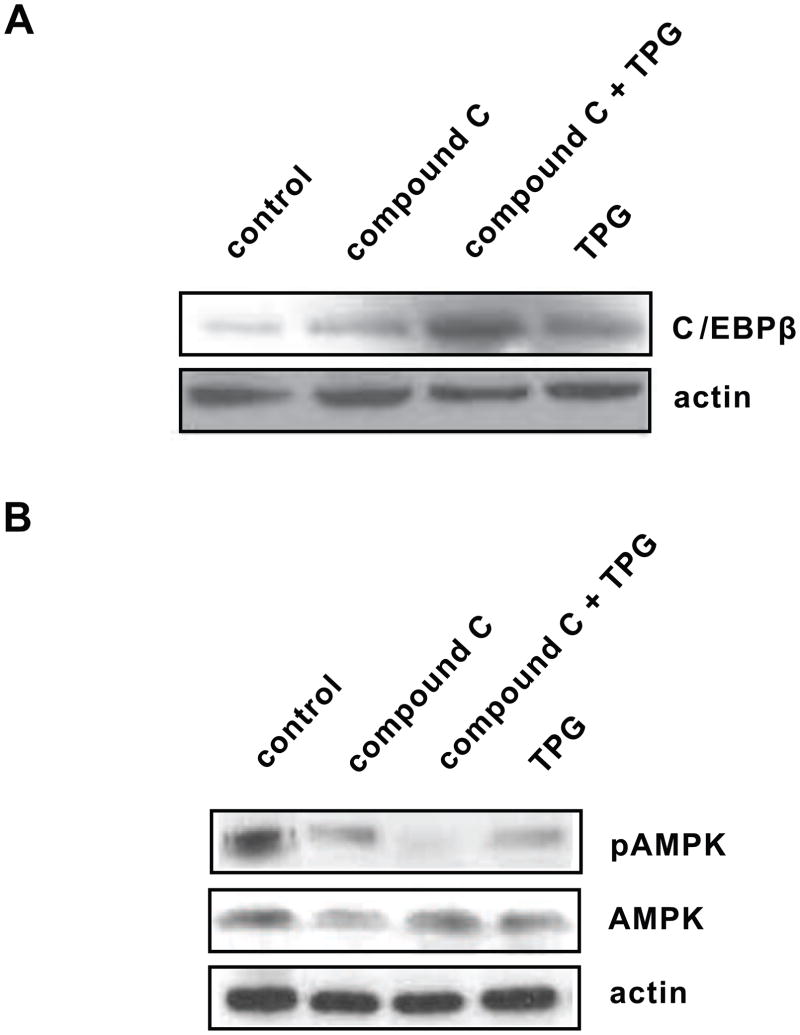
To further determine whether down-regulation of the AMPK pathway was the major mechanism for ER stress to induce C/EBPβ protein levels, we examined the effects of the pAMPK inhibitor compound C with and without the ER stress inducer TPG in Huh7 cells. Cells treated with 10 μM compound C alone tended to show increased C/EBPβ (Fig. 4A), consistent with the effect seen using DN-AMPK in Fig. 3. When compound C was combined with TPG, C/EBPβ increased significantly (P < 0.05) compared to either control cells or cells treated with TPG alone, suggesting that C/EBPβ is sensitive to both AMPK inhibition and ER stress. As expected, compound C reduced pAMPK activation under all conditions tested (Fig. 4B).
Fig. 4.
Involvement of AMPK activity and ER stress in C/EBPβ regulation. A, Huh7 cells were treated with 10 μM compound C (pAMPK inhibitor) in the presence or absence of ER stress inducer TPG (300 nM). TPG and compound C showed increased C/EBPβ level. Compound C in combination with TPG significantly (P < 0.05) increased C/EBPβ compared to control. The levels of β-actin remained unchanged. B, Huh7 cells were treated with the same reagents as above and pAMPK levels was measured. pAMPK was reduced (P < 0.05) when treated with TPG or compound C alone. The levels of β-actin remained unchanged.
3.5. ER stress activates the PEPCK promoter via CRE site
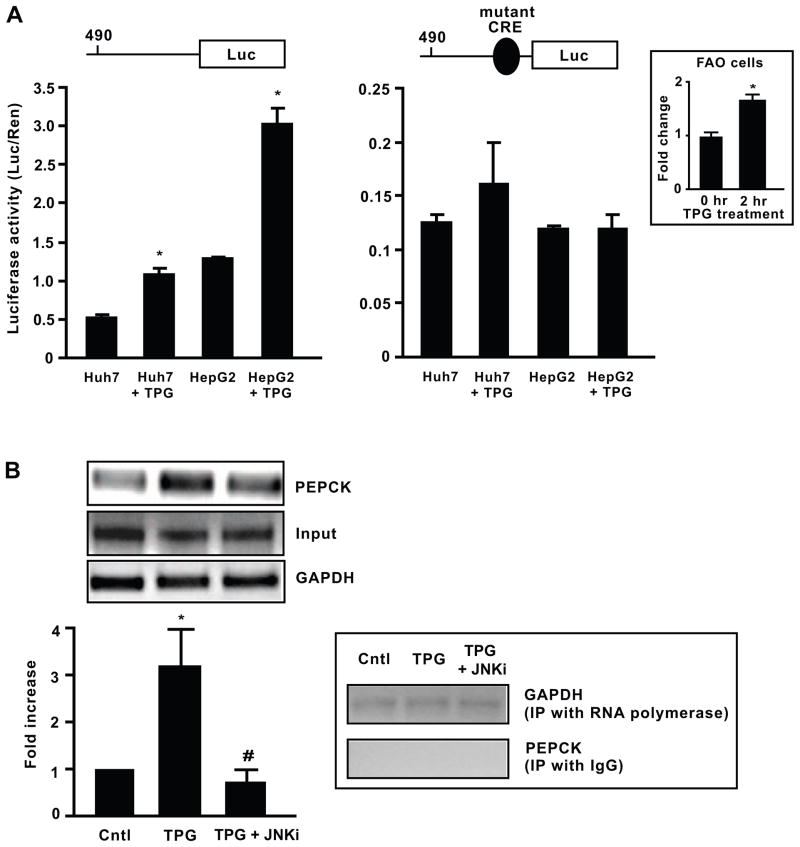
Since C/EBPβ is stimulated by agents that induce ER stress, it was important to determine whether the expression of gluconeogenic genes downstream of C/EBPβ, such as PEPCK, was also increased under ER stress. TPG increased PEPCK reporter gene transcription in both Huh7 and HepG2 cell lines containing the functional 490 bp PEPCK promoter with the CRE site intact (490-Luc) by ~1.5- and 3-fold respectively (P < 0.05, Fig. 5A). However, when the 490 bp PEPCK promoter containing a mutant CRE (PEPCK CRE-mutant-Luc) construct was used, there was low basal luciferase activity and no significant promoter activation by TPG in either cell line. We also demonstrated that the endogenous PEPCK mRNA was inducible by 1.8-fold with the TPG treatment in FAO cells (Fig. 5A inset).
Fig. 5.
ER stress activates the PEPCK promoter via CRE site and recruits C/EBPβ. A, ER stress activated transcription from the 490 bp PEPCK promoter but not the PEPCK promoter lacking the CRE site. Huh7 and HepG2 cells were transfected with a luciferase expression vector containing the functional −490-PEPCK promoter or an expression vector containing the −490-PEPCK promoter with a mutant CRE construct and their relative levels of luciferase activity were measured and normalized to a Renilla control. Cells were treated either with vehicle or TPG for 2 h prior to measurement of luciferase activity. Data represent mean ± SEM of three independent experiments. *P < 0.05 vs. control. ER stress inducer TPG also increased PEPCK mRNA in FAO cells within 2 h (*P < 0.05). B, C/EBPβ binds to PEPCK promoter during ER stress. ChIP assays were performed using C/EBPβ-specific antibody to test C/EBPβ binding to PEPCK promoter in FAO cells. Cells were treated with 300 nM TPG alone or in combination with 90 nM JNK inhibitor. ChIPed DNA was subjected to RT-PCR for PEPCK (−209 to +66) and GAPDH and run on an agarose gel, with input DNA run for normalization. *P < 0.05 vs. control, #P < 0.05 vs. TPG-treated cells. As a technical positive control, DNA was immunoprecipitated with RNA polymerase and subjected to PCR analysis for GAPDH and it remained unchanged under all treatments. As a negative control, DNA was immunoprecipitated with normal IgG and subjected to PCR analysis for targeted PEPCK promoter region and the resulting DNA did not show any binding with PEPCK promoter.
3.6. C/EBPβ is recruited to the PEPCK promoter during ER stress
The ability of ER stress to increase C/EBPβ and stimulate PEPCK promoter activity prompted us to examine C/EBPβ recruitment to endogenous PEPCK chromatin templates under ER stress. Using ChIP and real time quantitative PCR analysis, C/EBPβ occupancy over the PEPCK promoter increased significantly in FAO cells after 2 h TPG (300 nM) treatment (P < 0.05, Fig. 5B). However, when the JNK inhibitor SP600125 (90 mM) was added to relieve the TPG-induced ER stress (Verma and Datta, 2010), very negligible C/EBPβ was bound to the PEPCK promoter (P < 0.05). GAPDH remained unchanged for all treatments. As a technical positive control, DNA was immunoprecipitated with RNA polymerase and subjected to PCR analysis for GAPDH and it remained unchanged under all treatments. As a negative control, DNA was immunoprecipitated with normal IgG and subjected to PCR analysis for targeted PEPCK promoter region as above and the resulting DNA did not show any binding with PEPCK promoter.
4. Discussion
This is the first report showing that ER stress regulates AMPK and C/EBPβ expression in human hepatoma and rodent-derived FAO cells. Our findings suggest that the ER stress-inducer TPG is a potent suppressor of AMPK activity in the presence of either AICAR or XBP1-spliced. Given XBP1-spliced is known to relieve ER stress; it was surprising that it was unable to prevent pAMPK reduction in response to TPG (Fig. 1, A and C). However, over-expression of XBP1-spliced has been shown recently to increase hepatic triglycerides and a number of lipid synthesis genes (Lee et al., 2008) completely separate from its function as a mediator of the ER stress response. Therefore, it could be that down-regulation of pAMPK in the presence of XBP1-spliced is secondary to increased lipid synthesis. Exposure to fatty acids has also been shown previously to suppress pAMPK levels in liver cells (Rahman et al., 2009). While the mechanisms underlying the early suppression of pAMPK by ER stress or fatty acids requires further investigation, reduced pAMPK may provide another important explanation for the link between ER stress and insulin resistance.
The present study is the first to show that C/EBPβ expression levels are highly suppressed by AMPK activity in hepatoma cells. This is in contrast to adipocytes, where AICAR augmented the expression of C/EBPβ in 3T3-L1 preadipocytes (Arai et al., 2007). Compared with pre-adipocytes in which C/EBPβ is expressed transiently, in liver C/EBPβ is essential for induction of inflammation, gluconeogenesis, and lipogenesis (Liu et al., 1999; Rahman et al., 2007; Schroeder-Gloeckler et al., 2007). The C/EBP family of transcription factors is regulated by a variety of hormones, cytokines, and nutrients (Ramji and Foka, 2002). The strong association between C/EBPβ and genes that control inflammation is consistent with a role for C/EBPβ in insulin resistance of obesity. Given that AMPK promotes anti-inflammatory phenotype (Sag et al., 2008) as well as suppression of gluconeogenesis, it suggests the inhibition of C/EBPβ by AMPK may be an important property for prevention of steatohepatitis, as well as diabetes at the transcriptional level.
Our data also clearly shows that ER stress activates PEPCK expression through the CRE site and this effect is likely due to factors binding to the CRE (Fig. 4A). C/EBPβ is indeed involved in activating PEPCK gene expression and appears to bind to the PEPCK gene promoter under ER stress conditions. In addition, we show that ER stress induced phosphorylation of CREB at serine 133 and total CREB content in the nucleus. C/EBPβ and CREB are both b-zip transcription factors that bind to the CRE of the PEPCK promoter and are increased in Leprdb/db and high fat-fed mice (Rahman et al., 2007; Schroeder-Gloeckler et al., 2007). Exogenous fatty acids, especially palmitate, cause ER stress and are associated with increased expression of C/EBPβ and also CHOP (Guo et al., 2007). Certain pathological stress conditions disrupt ER homeostasis and lead to accumulation of unfolded or misfolded proteins in the ER lumen (Harding et al., 2002; Mori, 2000), such as abnormal lipid accumulation and lipid biosynthesis (Borradaile et al., 2006; Wang et al., 2006). Although our study found that both ER stress (Fig. 1) and AICAR (Fig. 3) activated pCREB, Horike et al. showed that AICAR alone had no effect on pCREB levels (Horike et al., 2008). However, AICAR strongly inhibits CRE-containing promoter activity in HepG2 cells, despite no change in CREB levels (Horike et al., 2008; Screaton et al., 2004). This is consistent with an effect of AMPK on TORC2, a CREB co-activator (Screaton et al., 2004). While AICAR increases TORC2 phosphorylation and suppresses CREB-mediated gluconeogenesis (Koo et al., 2005), recently an article appeared showing that chronic ER stress for 12–15 h stimulated an arm of the unfolded protein response pathway ATF6α in hepatocytes which can sequester the CREB co-activator TORC2, thereby reducing gluconeogenic gene expression (Wang et al., 2009). However, in TORC2 knockout mouse glucose homeostasis in the whole body was not affected in the animals (Le Lay et al., 2009). Thus, the role of TORC2 in control of hepatic gluconeogenesis in vivo may need to be re-examined.
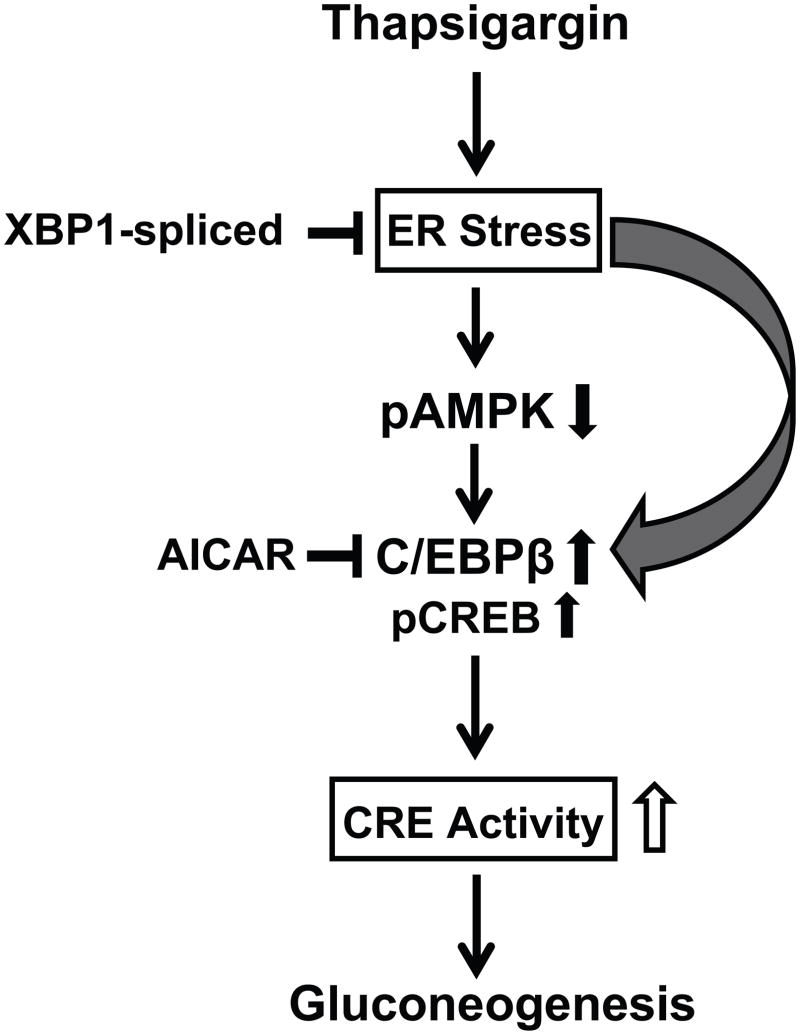
The present results implicate a model whereby ER stress can down-regulate AMPK and this may trigger an increase in C/EBPβ expression. This conclusion is tempered by the fact that simply blocking AMPK activity in the absence of ER stress induces C/EBPβ expression. These results imply that C/EBPβ is sensitive to both AMPK and ER stress, as depicted in Fig. 6. C/EBPβ binds avidly to the PEPCK promoter and increases transcription of the PEPCK gene which in turn can stimulate gluconeogenesis in the liver. C/EBPβ is a key regulator of a number of lipogenic, gluconeogenic, and inflammatory genes in the liver and is increased in a variety of liver cell lines upon exposure to fatty acids (Schroeder-Gloeckler et al., 2007). The present findings emphasize a critical role for C/EBPβ in governing inflammation, lipogenesis (Rahman et al., 2007; Schroeder-Gloeckler et al., 2007), and gluconeogenesis (Shao et al., 2005; Wang et al., 2000) under conditions of ER stress and suggest that inhibition of C/EBPβ by AMPK could ameliorate the liver steatosis and excess hepatic glucose production that are the hallmarks of diabetes and obesity.
Fig. 6.
Model for the up-regulation of gluconeogenesis via modification of AMPK and C/EBPβ by ER stress in liver cells. ER stress leads to a rapid decrease in pAMPK activity and consequently an increase in C/EBPβ expression and increased pCREB levels. Increased C/EBPβ binds to the PEPCK promoter via the CRE and thereby increases transcription of genes coding for increased gluconeogenesis.
Acknowledgments
This work was supported by NIH grant: RO1-DK059767. We thank Dr. Charles Rice, Laboratory of Virology and Infectious Disease, Center for the Study of Hepatitis C, The Rockefeller University for providing the Huh7 cell line. We thank Dr. Morris Birnbaum, University of Pennsylvania, for the AMPK-DN and AMPK-CA adenoviruses and Dr. Umet Ozcan, Boston Children’s Hospital, for Ad-XBP1-unspliced, Ad-XBP1-spliced, and Ad-GFP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arai N, Masuzaki H, Tanaka T, Ishii T, Yasue S, Kobayashi N, Tomita T, Noguchi M, Kusakabe T, Fujikura J, Ebihara K, Hirata M, Hosoda K, Hayashi T, Sawai H, Minokoshi Y, Nakao K. Ceramide and adenosine 5'-monophosphate-activated protein kinase are two novel regulators of 11beta-hydroxysteroid dehydrogenase type 1 expression and activity in cultured preadipocytes. Endocrinology. 2007;148:5268–77. doi: 10.1210/en.2007-0349. [DOI] [PubMed] [Google Scholar]
- 2.Arizmendi C, Liu S, Croniger C, Poli V, Friedman JE. The transcription factor CCAAT/enhancer-binding protein beta regulates gluconeogenesis and phosphoenolpyruvate carboxykinase (GTP) gene transcription during diabetes. J Biol Chem. 1999;274:13033–13040. doi: 10.1074/jbc.274.19.13033. [DOI] [PubMed] [Google Scholar]
- 3.Baan B, van Dam H, van der Zon GC, Maassen JA, Ouwens DM. The role of c-Jun N-terminal kinase, p38, and extracellular signal-regulated kinase in insulin-induced Thr69 and Thr71 phosphorylation of activating transcription factor 2. Mol Endocrinol. 2006;20:1786–95. doi: 10.1210/me.2005-0289. [DOI] [PubMed] [Google Scholar]
- 4.Back SH, Schroder M, Lee K, Zhang K, Kaufman RJ. ER stress signaling by regulated splicing: IRE1/HAC1/XBP1. Methods. 2005;35:395–416. doi: 10.1016/j.ymeth.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Barthel A, Schmoll D, Kruger KD, Roth RA, Joost HG. Regulation of the forkhead transcription factor FKHR (FOXO1a) by glucose starvation and AICAR, an activator of AMP-activated protein kinase. Endocrinology. 2002;143:3183–6. doi: 10.1210/endo.143.8.8792. [DOI] [PubMed] [Google Scholar]
- 6.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Chen A, Mumick S, Zhang C, Lamb J, Dai H, Weingarth D, Mudgett J, Chen H, MacNeil DJ, Reitman ML, Qian S. Diet induction of monocyte chemoattractant protein-1 and its impact on obesity. Obes Res. 2005;13:1311–20. doi: 10.1038/oby.2005.159. [DOI] [PubMed] [Google Scholar]
- 8.Chen KD, Chen LY, Huang HL, Lieu CH, Chang YN, Chang MD, Lai YK. Involvement of p38 mitogen-activated protein kinase signaling pathway in the rapid induction of the 78-kDa glucose-regulated protein in 9L rat brain tumor cells. J Biol Chem. 1998;273:749–55. doi: 10.1074/jbc.273.2.749. [DOI] [PubMed] [Google Scholar]
- 9.Choudhury M, Shukla SD. Surrogate alcohols and their metabolites modify histone H3 acetylation: involvement of histone acetyl transferase and histone deacetylase. Alcohol Clin Exp Res. 2008;32:829–39. doi: 10.1111/j.1530-0277.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Wong S, Xie W, Lei T, Luo Z. Palmitate modulates intracellular signaling, induces endoplasmic reticulum stress, and causes apoptosis in mouse 3T3-L1 and rat primary preadipocytes. Am J Physiol Endocrinol Metab. 2007;293:E576–586. doi: 10.1152/ajpendo.00523.2006. [DOI] [PubMed] [Google Scholar]
- 11.Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–99. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 12.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–4. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 13.Herzig S, Long F, Jhala US, Hedrick S, Quinn R, Bauer A, Rudolph D, Schutz G, Yoon C, Puigserver P, Spiegelman B, Montminy M. CREB regulates hepatic gluconeogenesis through the coactivator PGC-1. Nature. 2001;413:179–83. doi: 10.1038/35093131. [DOI] [PubMed] [Google Scholar]
- 14.Horike N, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Kamata H, Nishiyama K, Uchijima Y, Kurihara Y, Kurihara H, Asano T. AMP-activated protein kinase activation increases phosphorylation of glycogen synthase kinase 3beta and thereby reduces cAMP-responsive element transcriptional activity and phosphoenolpyruvate carboxykinase C gene expression in the liver. J Biol Chem. 2008;283:33902–10. doi: 10.1074/jbc.M802537200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 16.Koo SH, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, Takemori H, Montminy M. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–11. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- 17.Le Lay J, Tuteja G, White P, Dhir R, Ahima R, Kaestner KH. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 2009;10:55–62. doi: 10.1016/j.cmet.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leclerc I, Lenzner C, Gourdon L, Vaulont S, Kahn A, Viollet B. Hepatocyte nuclear factor-4alpha involved in type 1 maturity-onset diabetes of the young is a novel target of AMP-activated protein kinase. Diabetes. 2001;50:1515–21. doi: 10.2337/diabetes.50.7.1515. [DOI] [PubMed] [Google Scholar]
- 19.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Bevilacqua E, Chiribau CB, Majumder M, Wang C, Croniger CM, Snider MD, Johnson PF, Hatzoglou M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J Biol Chem. 2008;283:22443–56. doi: 10.1074/jbc.M801046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu JS, Park EA, Gurney AL, Roesler WJ, Hanson RW. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991;266:19095–102. [PubMed] [Google Scholar]
- 22.Liu S, Croniger C, Arizmendi C, Harada-Shiba M, Ren J, Poli V, Hanson RW, Friedman JE. Hypoglycemia and impaired hepatic glucose production in mice with a deletion of the C/EBPbeta gene. J Clin Invest. 1999;103:207–13. doi: 10.1172/JCI4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferre P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature. 2004;428:569–74. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 24.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–4. doi: 10.1016/s0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 25.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 26.Nizielski SE, Arizmendi C, Shteyngarts AR, Farrell CJ, Friedman JE. Involvement of transcription factor C/EBP-beta in stimulation of PEPCK gene expression during exercise. Am J Physiol. 1996;270:R1005–12. doi: 10.1152/ajpregu.1996.270.5.R1005. [DOI] [PubMed] [Google Scholar]
- 27.Oyadomari S, Harding HP, Zhang Y, Oyadomari M, Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–32. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ozawa K, Miyazaki M, Matsuhisa M, Takano K, Nakatani Y, Hatazaki M, Tamatani T, Yamagata K, Miyagawa J, Kitao Y, Hori O, Yamasaki Y, Ogawa S. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54:657–63. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 29.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 30.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park EA, Gurney AL, Nizielski SE, Hakimi P, Cao Z, Moorman A, Hanson RW. Relative roles of CCAAT/enhancer-binding protein beta and cAMP regulatory element-binding protein in controlling transcription of the gene for phosphoenolpyruvate carboxykinase (GTP) J Biol Chem. 1993;268:613–9. [PubMed] [Google Scholar]
- 32.Qadri I, Hu LJ, Iwahashi M, Al-Zuabi S, Quattrochi LC, Simon FR. Interaction of hepatocyte nuclear factors in transcriptional regulation of tissue specific hormonal expression of human multidrug resistance-associated protein 2 (abcc2) Toxicol Appl Pharmacol. 2009;234:281–92. doi: 10.1016/j.taap.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Rahman SM, Qadri I, Janssen RC, Friedman JE. Fenofibrate and PBA prevent fatty acid-induced loss of adiponectin receptor and pAMPK in human hepatoma cells and in hepatitis C virus-induced steatosis. J Lipid Res. 2009;50:2193–202. doi: 10.1194/jlr.M800633-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, Friedman JE. CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology. 2007;45:1108–17. doi: 10.1002/hep.21614. [DOI] [PubMed] [Google Scholar]
- 35.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–75. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Routes JM, Colton LA, Ryan S, Klemm DJ. CREB (cAMP response element binding protein) and C/EBPalpha (CCAAT/enhancer binding protein) are required for the superstimulation of phosphoenolpyruvate carboxykinase gene transcription by adenoviral E1a and cAMP. Biochem J. 2000;352(Pt 2):335–42. [PMC free article] [PubMed] [Google Scholar]
- 37.Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–41. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schroeder-Gloeckler JM, Rahman SM, Janssen RC, Qiao L, Shao J, Roper M, Fischer SJ, Lowe E, Orlicky DJ, McManaman JL, Palmer C, Gitomer WL, Huang W, O' Doherty RM, Becker TC, Klemm DJ, Jensen DR, Pulawa LK, Eckel RH, Friedman JE. CCAAT/enhancer-binding protein beta deletion reduces adiposity, hepatic steatosis, and diabetes in Lepr(db/db) mice. J Biol Chem. 2007;282:15717–29. doi: 10.1074/jbc.M701329200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Screaton RA, Conkright MD, Katoh Y, Best JL, Canettieri G, Jeffries S, Guzman E, Niessen S, Yates JR, 3rd, Takemori H, Okamoto M, Montminy M. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Shao J, Qiao L, Janssen RC, Pagliassotti M, Friedman JE. Chronic hyperglycemia enhances PEPCK gene expression and hepatocellular glucose production via elevated liver activating protein/liver inhibitory protein ratio. Diabetes. 2005;54:976–84. doi: 10.2337/diabetes.54.4.976. [DOI] [PubMed] [Google Scholar]
- 41.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–6. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verma G, Datta M. IL-1beta induces ER stress in a JNK dependent manner that determines cell death in human pancreatic epithelial MIA PaCa-2 cells. Apoptosis. 2010;15:864–76. doi: 10.1007/s10495-010-0498-4. [DOI] [PubMed] [Google Scholar]
- 43.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–51. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Shao J, Muhlenkamp P, Liu S, Klepcyk P, Ren J, Friedman JE. Increased insulin receptor substrate-1 and enhanced skeletal muscle insulin sensitivity in mice lacking CCAAT/enhancer-binding protein beta. J Biol Chem. 2000;275:14173–81. doi: 10.1074/jbc.m000764200. [DOI] [PubMed] [Google Scholar]
- 45.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. Embo J. 1998;17:5708–17. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;21:21. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yemelyanov A, Czwornog J, Chebotaev D, Karseladze A, Kulevitch E, Yang X, Budunova I. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26:1885–96. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]