Rewarding solutions and food have been used since the beginning of animal research and are commonly used in the context of cognitive-based behavioral assays, such as training animals to press a lever or select the proper instrument. In the study of many drugs, sweet solutions are commonly used to deliver drugs to test for addictive capacity and potential for abuse. Chronic administration of oral sucrose has been shown to alter pain perception in a range of reflexive tests via multiple effects on the activity of the endogenous opioid system. Unfortunately these studies have demonstrated one of three possible outcomes: ad libitum exposure to a sucrose solution may induce analgesia [10, 15, 18–20], have no effect on analgesia [17], or even induce hyperalgesia [1, 13] in reflexive-based testing paradigms. To date, several studies [1, 6, 9, 19] have examined the relationship of variable concentrations of non-caloric and caloric solutions on performance in operant testing systems and have focused on bar-pressing tasks. Our study advances these ideas by examining the potential of the reward solution to alter response in a facial operant testing system and its implications when testing animal models of pain or drugs for their analgesic potential.
The goal of the current study was to determine the effect of caloric vs. non-caloric reward on operant pain behavior. In this set of experiments we examined the influence of caloric (sucrose) and non-caloric (saccharin) liquid rewards on the performance of a recently developed thermal facial operant test at neutral (37°C) and noxious (48°C) temperatures. Historically, our laboratory has utilized a 33% sweetened condensed milk solution as the reward in our reward/conflict paradigm testing system [12, 14, 16]. In the current study, we used an operant orofacial assay to measure the effect of various concentrations of a caloric (sucrose, 5–50%) sweet reward solution at two temperatures: neutral (37°C) and noxious (48°C). In addition, we compared the effect of two sweet rewards: caloric (sucrose, 5–50%) and non-caloric (saccharin, 0.01–1%) at 37°C.
Thermal sensitivity was evaluated using an operant orofacial pain assay [12, 14, 16]. Seven-week old male hairless Sprague-Dawley rats (250–300g, Charles River, Raleigh, NC) were housed in a standard environment. The sucrose experiments used animals as such: 37°C (5% n=8, 10% n=16, 20% n=24, 30% n=8, 40% n=8, 50% n=8) and at 48°C (5% n=8, 10% n=4, 20% n=4, 30% n=8, 40% n=8, 50% n=8). The milk experiments used animals as such: 37°C (1% n=8, 10% n=8, 15% n=8, 25% n=6, 33% n=16, 50% n=8) and at 48°C (1% n=4, 10% n=4, 15% n=4, 25% n=6, 33% n=4, 50% n=4).. All rats had ad libitum access to food and water between testing sessions, and their weights were monitored weekly. Rats were food fasted for 16 h prior to each testing session and trained to drink sweetened condensed milk while making facial contact with a thermal probe, set at 37°C over a 2 week period. Rats were then tested at either 37°C or 48°C and two behavioral outcome measures were recorded for each 20 min testing session: licks, (number of contacts with the sipper tube, which cannot be made without contacting the thermal probe) and stimulus contacts (number of contacts with the thermal probe) which were used to calculate the success ratio (licks/stimulus contact). Data was converted to a percent baseline using our traditional reward solution of 33% milk at 37°C to standardize the data [12, 14, 16]. A one-way analysis of variance with Dunnett’s post-test was performed to determine difference in concentration at each temperature. The control group for the Dunnetts post-hoc test was 33% milk which has been used historically in our studies using thermal facial operant testing [12–14, 16]. A two-way analysis of variance was used to compare the effect of temperature. A 37°C/48°C ratio was calculated and analyzed with a one-way analysis of variance with Dunnett’s post-test with the 37°C/48°C ratio from 33% sweetened condensed milk used as the control group. All experimental procedures were approved by the University of Florida Institutional Animal Care and Use Committee. All statistical evaluations were made using GraphPad Prism (v. 4.02, GraphPad Software, San Diego, CA). Significance was set at p<0.05 and indicated as follows: Concentration Analysis: # p < 0.05, ## p < 0.01; Temperature Analysis: ** p < 0.01, *** p < 0.001; 37°C/48°C ratio: § p < 0.05, §§ p < 0.01.
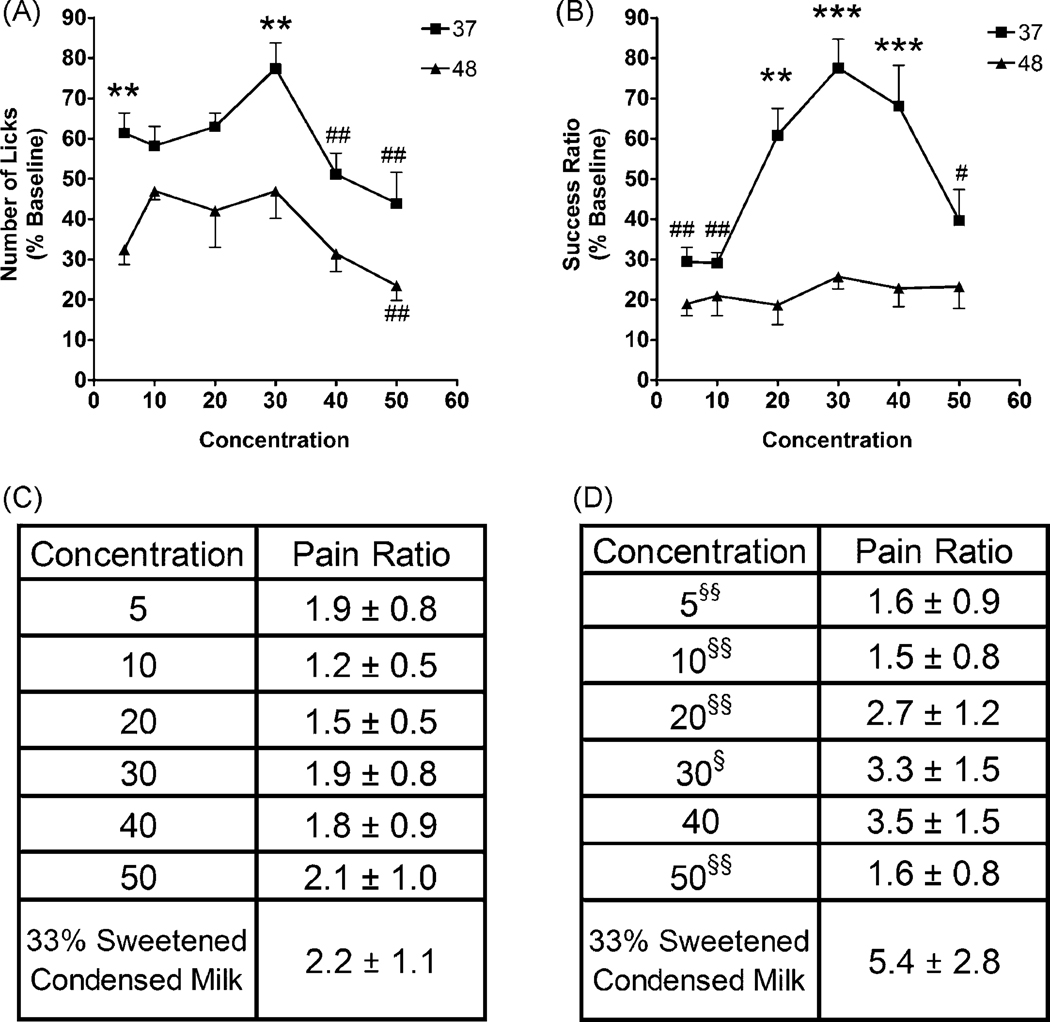
The first outcome evaluated was reward licking contacts which provide a measure of the palatability of the reward. The ingestion of a 40% and 50% sucrose solution produced a significantly reduced number of licks compared to control when tested at 37°C and at 50% sucrose when tested at 48°C (Fig. 1A). The number of licks was consistently higher at 37°C vs. 48°C but only demonstrated a statistically significant increase at 5% and 30% (Fig. 1A). Additionally, we calculated a 37°C/48°C ratio for each concentration (Fig. 1C) to provide a measure of the temperature effect which demonstrated no statistically significant change among sucrose concentrations suggesting the difference in reward licking contacts is related to temperature and not the reward potential of the solution.
Figure 1.
Differential effect of temperature and sucrose concentrations on the number of licks and success ratio. A) At 37°C the number of licks was significantly decreased at 40% and 50% sucrose compared to our historical solution of 33% sweetened condensed milk and at 48°C the number of licks was significantly decreased at 50% (Sucrose: 37°C, F5,66=3.532; 48°C, F5,34=3.415 Milk: 37°C, F5,48=16.93; 48°C, F5,20=4.565). The number of licks was significantly increased at 5% and 30% sucrose at 37°C versus 48°C (Sucrose: 37°C, F5,66=7.776; 48°C, F5,34=0.3962 Milk: 37°C, F5,48=8.408; 48°C, F5,20=0.8738). B) The success ratio at 5%, 10%, and 50% sucrose was significantly decreased at 37C when compared with our historical solution of 33% sweetened condensed milk and there was no difference in concentration at 48°C. The success ratios at 20%, 30%, and 40% sucrose were significantly elevated at 37°C versus 48°C (Sucrose: 37°C, F5,66=7.776; 48°C, F5,34=0.3962 Milk: 37°C, F5,48=8.408; 48°C, F5,20=0.8738). C) The 37°C/48°C ratio calculated from the number of licks (F6,48=1.521). D) The 37°C/48°C ratio calculated from the success ratio. The increased ratio at 40% is statistically significant compare to 5%, 10%, and 50% (F6,48=6.572). Graphs are mean ± s.e.m. Significance was set at p<0.05 and indicated as follows: Concentration Analysis: # p < 0.05, ## p < 0.01; Temperature Analysis: ** p < 0.01, *** p < 0.001; 37°C/48°C ratio: § p < 0.05, §§ p < 0.01.
The second outcome we evaluated was the success ratio (number of licks/number of face contacts) which provides a measure of facial pain. The success ratio was significantly decreased at 5%, 10%, and 50% sucrose among animals tested at 37°C and showed no difference between 37°C and 48°C at those concentrations (Fig. 1B). However, at 20%, 30%, and 40% the success ratio was significantly elevated at 37°C versus 48°C (Fig. 1B). There were no statistically significant differences in success ratio at any concentration tested at 48°C. These data suggest that at a neutral temperature (37°C), low concentrations of sucrose do not provide enough reward to maintain contact with the thermode. The decreased success ratio at 50% sucrose (37°C) suggests that the solution may either be unpalatable or the rats may be satiating as suggested by the decreased number of licks. The similarity of the success ratios at all concentrations of sucrose at the noxious temperature (48°C) suggests that in a thermal facial operant test a sweet reward solution does not induce analgesia to a level which significantly reduces the painful thermal stimulus. When we evaluated the 37°C/48°C ratio for each concentration (Fig. 1D) the ratio was significantly decreased compared to 33% sweetened condensed milk at 5%, 10%, 20%, 30%, and 50% sucrose. These data suggest that the success ratio provides a better analysis of thermal facial operant data because it detects changes in facial contacts and provides a measure of thermal sensitivity.
When we investigated the non-caloric agent saccharin, we found that there were a very low number of licks at 37°C at all tested concentrations (0.01%, 0.1%, and 1%) (Fig. 2). The maximal number of licks was induced at a concentration of 0.1% (109±39.94) where as the maximal licks at 37°C for sucrose (30%, 2039±470.8) was almost 20-fold higher. There are two possible explanations for such a low performance when testing saccharin: 1) it suggests that caloric content of the reward solution is important in the performance of thermal facial operant testing or 2) the taste of saccharin is in itself aversive [2, 4, 7].
Figure 2.
Effect of saccharin concentration on the number of licks at 37°C. Saccharin concentration did not alter the number of licks at 37°C. Graphs are mean ± s.e.m.
Previous studies of the effect of sweet reward on behavior have focused on the potential of these solutions to induce analgesia in reflexive testing paradigms such as Hargreaves thermal testing or Von Frey filament mechanical testing. These studies have demonstrated that the production of analgesia is independent of the caloric content of the reward and that it is the sweet taste which is required [3, 5, 8, 20]. However, our data suggests that performance of an operant test requires the reward to be caloric. Additionally, our data demonstrate the complexity of evaluating pain data. For example low success ratios at 5%, 10%, and 50% could mistakenly be interpreted as a painful response when these concentrations may instead represent reduced palatability to the animal.
In conclusion, our results suggest that caloric rewards are preferred in the performance of the operant testing paradigm. The effect of reward is significantly diminished noxious temperatures suggesting that the relative nutritive value does not induce analgesia in this paradigm. Altered sucrose concentration produced the largest changes in success ratio at neutral temperatures; this provides provocative evidence for future studies which will utilize sucrose as a reward solution when testing analgesic drugs or animal models of pain. Our study demonstrated that 5%, 10%, and 50% sucrose solutions produce a success ratio which could mistakenly be labeled allodynic if this had been a pain study when in fact the solutions may just be unpalatable. Our study demonstrates the importance of choosing the correct reward solution and therefore has implication in the evaluation of animal models of pain behavior and subsequent translation to the clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ackroff K, Sclafani A. Fructose-conditioned flavor preferences in male and female rats: effects of sweet taste and sugar concentration. Appetite. 2004;42:287–297. doi: 10.1016/j.appet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Bartoshuk LM. Bitter taste of saccharin related to the genetic ability to taste the bitter substance 6-n-propylthiouracil. Science. 1979;205:934–935. doi: 10.1126/science.472717. [DOI] [PubMed] [Google Scholar]
- 3.D'Anci KE, Kanarek RB, Marks-Kaufman R. Beyond sweet taste: saccharin, sucrose, and polycose differ in their effects upon morphine-induced analgesia. Pharmacology Biochemistry and Behavior. 1997;56:341–345. doi: 10.1016/s0091-3057(96)00227-4. [DOI] [PubMed] [Google Scholar]
- 4.Dess NK. Saccharin's aversive taste in rats: evidence and implications. Neuroscience and Biobehavioral Reviews. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 5.Foo H, Mason P. Analgesia accompanying food consumption requires ingestion of hedonic foods. Journal of Neuroscience. 2009;29:13053–13062. doi: 10.1523/JNEUROSCI.3514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guttman N. Operant conditioning, extinction, and periodic reinforcement in relation to concentration of sucrose used as reinforcing agent. Journal of Experimental Psychology. 1953;46:213–224. doi: 10.1037/h0061893. [DOI] [PubMed] [Google Scholar]
- 7.Horne J, Lawless HT, Speirs W, Sposato D. Bitter taste of saccharin and acesulfame-K. Chemical Senses. 2002;27:31–38. doi: 10.1093/chemse/27.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Kanarek RB, White ES, Biegen MT, Marks-Kaufman R. Dietary influences on morphine-induced analgesia in rats. Pharmacology Biochemistry and Behavior. 1991;38:681–684. doi: 10.1016/0091-3057(91)90034-y. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy JM, Baldwin BA. Taste preferences in pigs for nutritive and non-nutritive sweet solutions. Animal Behavior. 1972;20:706–718. doi: 10.1016/s0003-3472(72)80142-8. [DOI] [PubMed] [Google Scholar]
- 10.Klein SP, Green KF. Tolerance to morphine analgesia from brief exposure to a palatable solution. Brain Research Bulletin. 1988;21:963–965. doi: 10.1016/0361-9230(88)90035-4. [DOI] [PubMed] [Google Scholar]
- 11.Mukherjee K, Mathur R, Nayar U. Hyperalgesic response in rats fed sucrose from weaning to adulthood: role of VMH. Pharmacology Biochemistry and Behavior. 2002;73:601–610. doi: 10.1016/s0091-3057(02)00837-7. [DOI] [PubMed] [Google Scholar]
- 12.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 13.Neubert JK, Mannes AJ, Keller J, Wexel M, Iadarola MJ, Caudle RM. Peripheral targeting of the trigeminal ganglion via the infraorbital foramen as a therapeutic strategy. Brain Research: Brain Research Protocols. 2005;15:119–126. doi: 10.1016/j.brainresprot.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular Pain. 2008;4:43. doi: 10.1186/1744-8069-4-43. doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raz I, Hasdai D, Seltzer Z, Melmed RN. Effect of hyperglycemia on pain perception and on efficacy of morphine analgesia in rats. Diabetes. 1988;37:1253–1259. doi: 10.2337/diab.37.9.1253. [DOI] [PubMed] [Google Scholar]
- 16.Rossi HL, Vierck CJ, Jr, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Molecular Pain. 2006;2:37. doi: 10.1186/1744-8069-2-37. doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoenbaum GM, Martin RJ, Roane DS. Discontinuation of sustained sucrose-feeding aggravates morphine withdrawal. Brain Research Bulletin. 1990;24:565–568. doi: 10.1016/0361-9230(90)90160-2. [DOI] [PubMed] [Google Scholar]
- 18.Schoenbaum GM, Martin RJ, Roane DS. Relationships between sustained sucrose-feeding and opioid tolerance and withdrawal. Pharmacology Biochemistry and Behavior. 1989;34:911–914. doi: 10.1016/0091-3057(89)90293-1. [DOI] [PubMed] [Google Scholar]
- 19.Segato EN, Reboucas EC, Freitas RL, Caires MP, Cardoso AV, Resende GC, Shimizu-Bassi G, Elias-Filho DH, Coimbra NC. Effect of chronic intake of sweet substance on nociceptive thresholds and feeding behavior of Rattus norvegicus (Rodentia, Muridae) Nutritional Neuroscience. 2005;8:129–140. doi: 10.1080/10284150500069413. [DOI] [PubMed] [Google Scholar]
- 20.Segato FN, Castro-Souza C, Segato EN, Morato S, Coimbra NC. Sucrose ingestion causes opioid analgesia. Brazilian Journal of Medical and Biological Research. 1997;20:981–984. doi: 10.1590/s0100-879x1997000800011. [DOI] [PubMed] [Google Scholar]