Abstract
Astrocytes play an important role in astrocyte-neuron homeostasis. In HIV-1-infected brain, interleukin 1 beta (IL-1β) activation of astrocytes contributes to neurodegeneration. However, the molecular mechanisms underlying IL-1β-activated-astrocytes-induced neurodegeneration in HIV-1-infected brain are largely unknown. We hypothesize that secretory factors from the activated astrocytes affect N-methyl-D-Aspartate (NMDA) receptor, a major pathway implicated in HIV-1-associated neurodegeneration. To test this hypothesis, we studied effects of IL-1β-stimulated astrocyte conditioned medium (ACM+) for its ability to activate NR1a/NR2B receptors expressed on Xenopus oocytes. Astrocytes treated with IL-1β 20 ng/ml for 24 h induced CXCL8, CCL2, MMP1 and MMP7. Pressure ejection of the ACM(+) produced an inward current in NR1a/NR2B-expressing oocytes. The inward current produced by ACM(+) was blocked by NMDA receptor antagonist, APV but not by non-NMDA receptor antagonist, CNQX. These results suggest that IL-1β stimulated astrocytes activate NR1a/NR2B receptors which may have implications in HIV-1-associated neurodegeneration.
Keywords: Astrocyte, IL-1β, HIV-1, NMDA receptor, Xenopus oocytes, voltage clamp
1. Introduction
Human immunodeficiency virus type one (HIV-1)-associated dementia (HAD) is a severe form of HIV-1-associated neurocognitive disorders (HAND) and diagnosed with cognitive impairments, motor disturbances and behavioral abnormalities. Although the incidence of HAD has been decreased by highly active antiretroviral therapy (HAART), it remains a significant complication of HIV-1 infection as patients with acquired immuno-deficient syndrome (AIDS) live longer, antiretroviral drugs remain unable to effectively cross the blood-brain barrier (BBB), and HIV-1 resistance grows due to viral strain mutation. The abundance of macrophages in the brain appears to better correlate with HAD than the extent of brain infection [1-4], implicating that an increase in trafficking of macrophages to the brain may be associated with the development of HAD.
In addition to the macrophages, astrocytes also have important role in the pathogenesis of HAD. Astrocytes are the most abundant cell type within the central nervous system (CNS) and play an important role in CNS homeostasis and function [5, 6]. They are also the target cells for immune mediators and viruses such as HIV-1 that induce reactive astrogliosis, a common feature seen in many neurological disorders including HAD [7-10]. Astrocytes respond to pathological challenges by rapid activation, not only at the site of challenge but also in the surrounding neuropil. Thus, disruption of normal function of the astrocyte by agents including HIV-1-infection leads to neuronal injury [8, 11, 12]. However, mechanism of activated astrocytes resulting neuronal injury is not known.
N-methyl-D-aspartate (NMDA) receptor, formed by the assembly of NR1 and NR2 subunits, is a principal subtype of ion tropic glutamate receptor that plays a central role in synaptic mechanisms of learning and memory [13]. Activation of NMDA receptor contributes in diseases with neurodegeneration and dementia [9, 14-16]. Increasing evidence suggest the involvement of NMDA receptors in HIV-1-associated neurotoxicity[9, 17, 18]. It has been shown that HIV-1-associated neuronal injury/death can be prevented or attenuated by NMDA receptor antagonists [9, 19-22]. However, it is not clear whether the activated astrocytes induce NMDA activation. As IL-1β is a major proinflammatory cytokine exerting a stimulatory action on astrocytes during HIV-1 brain infection [23, 24], we evaluated conditioned media recovered from IL-1β-stimulated human astrocytes for its ability to activate the recombinant NR1a/NR2B receptors expressed in Xenopus oocytes. Our results revealed that IL-1β-stimulated human astrocytes activate NR1a/NR2B receptors.
2. Materials and methods
2.1. Preparation of astrocytes
Human astrocytes were isolated from first and early second trimester abortus obtained from the Birth Defects Laboratory, University of Washington, Seattle, in full compliance with the ethical guidelines of the NIH and the Universities of Washington and Nebraska Medical Center. Specimens were dissected and mechanically dissociated by teasing through a Nitex bag and a 70-mm sieve. The cell suspension was centrifuged, re-suspended in medium and plated at a density of 2 × 107 cells/150 cm2. Non-adherent microglia and oligodendrocytes were removed by gentle agitation. The adherent astrocytes were treated with trypsin, and single cell suspensions were cultured under similar conditions to enhance the purity of replicating astroglial cells. The purity of these astrocytes was >99% as determined by glial fibrillary acid protein (GFAP) staining [6, 25, 26].
2.2. Measurement of soluble factors in astrocyte-conditioned media (ACM)
Astrocytes were activated with IL-1β 20 ng/ml (R & D Systems) for 2 h. Subsequently, cells were washed with DMEM/F12 supplemented with 10% FBS was added. Twenty-four hour later, both the IL-1β stimulated ACM (ACM+) and non-IL-1β stimulated ACM (ACM-) were collected and protein levels were measured using ELISA (R & D Systems). The collected ACMs were stored at a -80°C freezer and used in the following experiment.
2.3. Preparation of oocytes and injection of NR1a/NR2B
Plasmid cDNA encoding the NR1a subunit was a generous gift from Dr. S. Nakanishi (Kyoto University, Faculty of Medicine, Kyoto, Japan). NR2B was generously provided by Dr. Daniel T. Monaghan (University of Nebraska Medical Center, Omaha, NE). Plasmids were linearized with Not I (NR1a) or Sal I (NR2B) and transcribed in vitro using an RNA polymerase transcription kit (Ambion, Austin, TX)
Adult female Xenopus leaves were obtained from Xenopus One, Ann Arbor, MI. Frogs were anaesthetized by immersion in 2L of 0.17% (w/v) Tricaine (3-aminobenzoic acid ethyl ester methanesuphonate salt, Sigma) for 5 min and then cooled by addition of an equal volume of ice to the Tricaine solution for 10 min. Ovaries were surgically removed from the frogs through a small incision in the lower abdomen. Ovaries and isolated oocytes were kept in a standard oocyte solution (SOS) contained (in mM) NaCl (100.0), KCl (2.0), CaCl2 (2.0), MgCl2 (1.0), Hepes (5.0), adjusted pH to 7.6 with NaOH. BaCl2 (2.5) was used to substitute CaCl2. The SOS was supplemented with 2.5 mM sodium pyruvate (Sigma, St. Louis, MO) and 50 mg/L gentamicin (Gibco/BRL, Grand Island, NY). Oocytes were isolated and defolliculated by a modified method described previously [27]. Briefly, clumps of ovaries were incubated with Type 1A collagenase (20mg/ml) in SOS for 20 - 25 min with gentle shaking. After rinsing several times with supplemented SOS solutions (sSOS), defolliculation was completed by gentle washing with 0.1M potassium phosphate (pH 6.5) over 5 - 10 min.
NMDA receptor subunit complementary RNAs (cRNAs) were dissolved in RNase-free, sterile distilled water. NR1a and NR2B cRNAs were mixed in a molar ratio of either 1:1 or 1:2 to minimize the formation of NR1a homomers. Stage V and VI Xenopus oocytes were injected 6 - 24 h after isolation with the mixtures of cRNAs. A total of 30 - 46 ng of cRNAs were injected into each oocyte. The injected oocytes were placed in 35 mm (in diameter) petri dishes filled with sSOS 210 - 225 mosmol/kg (measured by vapor pressure) and the sSOS was changed daily. All oocytes were kept in an incubator with constant temperature of 18°C.
2.4. Determination of NR2B protein in Xenopus oocytes
Three days after cRNA injection, a membrane fraction was prepared from Xenopus oocytes. Briefly, 100 oocytes were homogenized with a glass-Teflon homogenizer in 1 ml of 7.5 mM phosphate buffer, pH 7.4 including phenylmethylsulfonyl fluoride (PMSF). The homogenate was spun down at 750 × g for 5 min at 4 °C to pellet the yolk and cellular debris. The membranes were then pelleted from the supernatant at 20,000 × g for 30 min at 4 °C. The floating yolk and supernatant were removed, and then the membrane pellets were washed with 7.5 mM phosphate buffer, pH 7.4. The pellets were resuspended in 50 μl of 7.5 mM phosphate buffer, pH 7.4. Total protein amount was measured using BCA Protein Assay Kit (Pierce, Rockford, IL). Each 50 μg of membrane protein sample were run on 7.5% Tris-HCl gel (Bio-Rad, Hercules, CA) and transferred onto polyvinylidene difluoride (PVDF) membrane (Bio-Rad). PVDF-bound proteins were visualized by staining with Ponceau S and the membrane was blocked for 1 hour in 5% non-fat dry milk in TBST (Tris Buffer Saline with 0.5% Tween 20). Subsequently, the membrane was incubated in the same buffer with rabbit anti-NR2B affinity purified polyclonal antibody (1:500, Chemicon, Temecula, CA) overnight at 4 °C. After three washes in TBST, the secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit IgG, 1:10000, Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was applied for 1 h. After three washes in TBST, protein bands were visualized using an enhanced chemiluminescence system (Western Blotting Luminol Reagent, Santa Cruz Biotechnology, Inc).
2.5. Electrophysiological Recording of the oocytes
For electrophysiological recordings, oocytes were transferred into a recording chamber (Warner Instruments, Hamden, CT) and perfused continuously with either Ba2+- or Ca2+-containing SOS at a rate of 1.5 -1.8ml/min. Whole-cell currents were measured using a two-electrode voltage-clamp technique at day 3 - 6 post-injection. Current-passing and voltage-measuring electrodes were filled with 2M KCl and had a resistance of 0.5 -1.0 MΩ. Electrical signals were amplified using Geneclamp 500 amplifier (Axon Instruments, Union City, CA), filtered at 1kHz and digitized at 2.5 kHz through a Digidata 1320A digitizer. pCLAMP 8 software was used to collect (gap-free configuration) and analyze data. Since there was no significant difference in current amplitudes recorded 3 - 6 days after cRNA injection or recorded in oocytes perfused with either Ba2+- or Ca2+-containing SOS, the data were pooled.
2.6. Statistical analysis
Statistical analyses were made by two-tailed t-tests or one-way analysis of variance (ANOVA). Differences were considered significant if p < 0.05. All data were expressed as the mean ± S.D. unless otherwise indicated.
3. Results
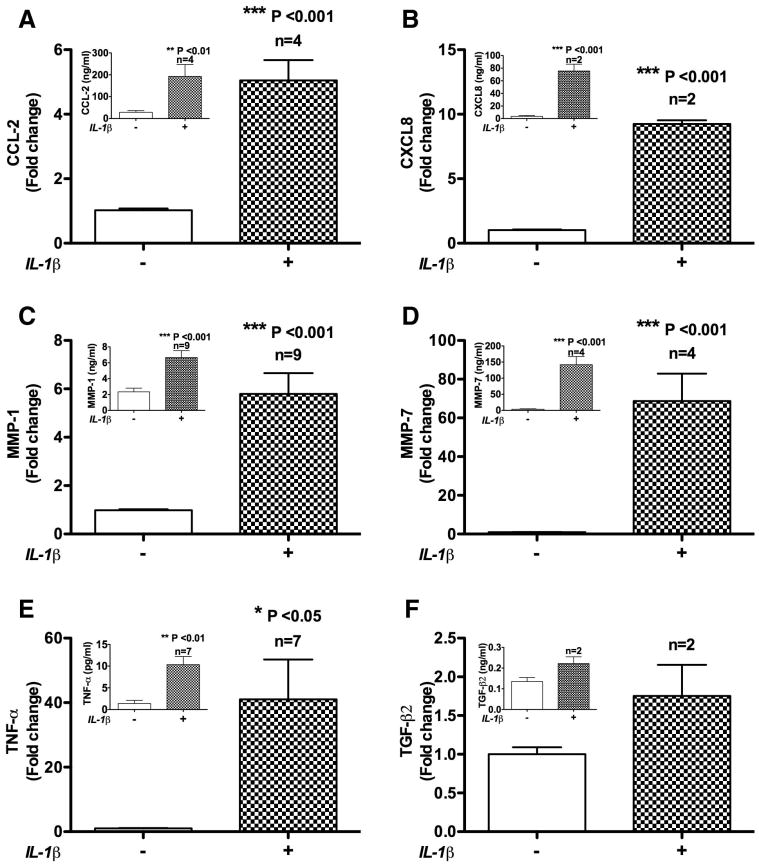
To understand IL-1β role on astrocytes in disease conditions, astrocytes were treated with IL-1β 20ng/ml for 2 h and the medium was analyzed for soluble factors using ELISA. Astrocytes treated with IL-1β induced CCL2 four-fold higher than that detected in non-treated control. The control mean value was 1.371ng/ml (p < 0.001; Fig 1A). IL-1β-induced cytokines interleukin-8 (CXCL8/IL-8) 9.24 fold (p < 0.001; Fig 1B), tumor necrosis factor alpha (TNF-α) 41 fold (p < 0.05; Fig 1E) and transforming growth factor beta 2 (TGF-β2) 1.75 fold (Fig 1F) compared to their respective controls. The control mean values of IL-8, TNF-α and TGF-β2 were 3.52 ng/ml, 1.37 pg/ml and 0.135 ng/ml respectively. Astrocytes treated with IL-1β of the above dose also induced matrix metalloproteinase-1 (MMP-1) 5.78 fold (p < 0.001) (Fig 1C) and matrix metalloproteinase-7 (MMP-7) secretion 68.75 fold (p < 0.001) (Fig 1D) compared to their respective controls. The control mean values of MMP-1 and MMP-7 were 2.33 ng/ml and 3.71 ng/ml respectively.
Fig. 1.
IL-1β simulated astrocytes release soluble factors in vitro. A. astrocytes treated with IL-1β 20 ng/ml for 2 h induced CCL2 secretion 5.04 fold compared to the control (p<0.001). B. IL-1β treatment induced Interleukin-8 (CXCL8/IL-8) 9.24 fold (p<0.001), C. matrix metalloproteinase-1 (MMP-1) 5.78 fold (p<0.001), D. matrix metalloproteinase-7 (MMP-7) 68.75 fold (p<0.001), E. tumor necrosis factor alpha (TNF-α) 41 fold (p<0.05) and F. transforming growth factor beta 2 (TGF-β2) secretion 1.75 fold compared to their respective controls. Insets in each panel show data from individual representative donor. Values for n represent the number of individual astrocyte donors used for each measurement and calculation of fold changes.
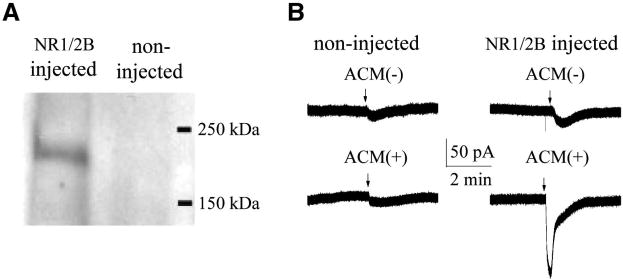
The expression of NR1a/NR2B NMDA receptors on Xenopus oocytes were confirmed by Western blot. Using anti-NR2B polyclonal antibody we detected a clear band with molecular weight of ∼180 kDa in oocytes injected NR1a/NR2B cRNAs, but not in non-injected oocytes (Fig. 2A). The functional expression of NR1a/NR2B was determined by pressure ejection of a mixture containing NMDA (800μM) and glycine (40μM), making a final concentration in the bath chamber of NMDA 200 μM and glycine 10 μM. As observed previously, ejection of NMDA/glycine produced inward currents in oocytes injected with cRNAs of NR1a/NR2B receptors, but not in non-injected (control) oocytes [28]. The average inward current conducted by NR1a/NR2B receptors were 172.5 ± 69.3nA (n = 8). The NMDA/glycine-induced inward current was blocked by bath application of 2-amino-5-phosphnovalerate (APV; 50μM, n = 3), a specific NMDA receptor antagonist, but not by a non-NMDA receptor antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX; 20μM, n=3), indicating that the NMDA/glycine-induced inward current was mediated through NR1a/NR2B receptors (data not shown).
Fig. 2.
Expression of NR1a/NR2B receptors in Xenopus oocytes and activation of NR1a/NR2B receptors by ACM(+). A. Expression of NR1/NR2B on Xenopus oocyte membranes after microinjection of a mixture of NR1 and NR2B cRNAs. Left lane: NR2B subunit expression in the NR1a/NR2B mRNA-injected oocytes. Note a clear band with an approximate molecular weight of 180 kD in the left lane. Result at right lane was from non-injected control oocytes, showing that no recognizable band was detected. B. An example of inward currents induced by IL-1β-stimulated ACM (ACM+). The results on the left column were from two non-injected oocytes and the data on the right column were from two different oocytes injected with NR1a/NR2B cRNAs. Note that ACM(+) produced an inward current in NR1a/NR2B-expressing oocytes, but not in non-NR1a/NR2B-expressing oocytes (non-NR1a/NR2B injected). Application of ACM(-) had no apparent effects in producing inward current. These results suggest that IL-1β-stimulated astrocytes secrete NMDA receptor agonist-like substances, resulting in activation of NR1a/NR2B receptors expressed in Xenopus oocytes.
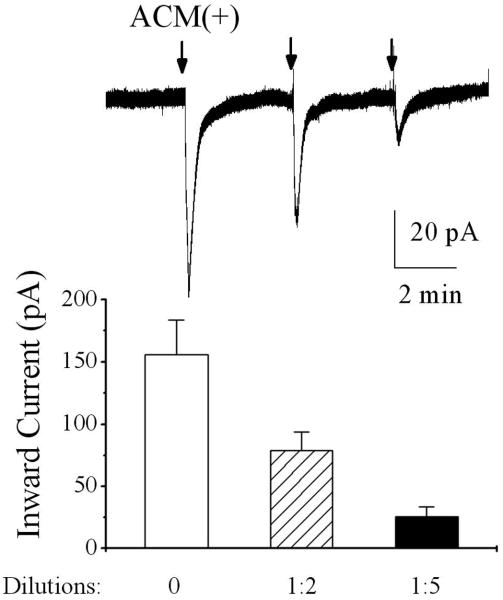
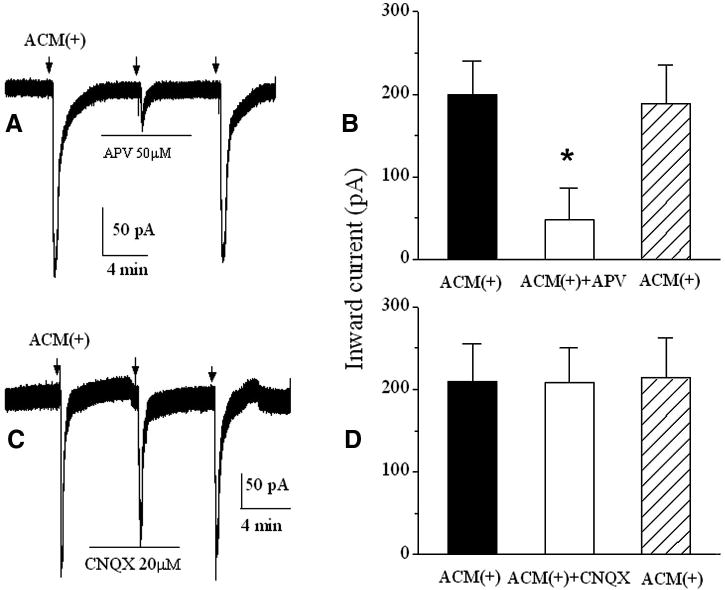
After confirmation of functional expression of NR1a/NR2B receptors in Xenopus oocytes, we tested the conditioned media recovered from human astrocytes with or without IL-1β stimulation on oocytes expressing NR1a/NR2B receptors. Pressure ejection of raw ACM(+) (undiluted, 300μl per ejection operated manually through a syringe system) produced an inward current in oocytes injected with mixed cRNAs of NR1a/NR2B receptors (Fig. 2B lower right, n = 10), but not in non-injected oocytes (Fig. 2B lower left, n = 6). Application of raw ACM(-)(undiluted) did not produce significant inward currents in both non-NR1a/NR2B-expressing (control, Fig. 2B upper left, n = 6) and NR1a/NR2B-expressing oocytes (Fig. 2B, upper right, n=10). These results suggest that the IL-1β-stimulated astrocytes activate NR1a/NR2B receptors expressed on Xenoups oocytes. When applied without dilution (raw ACM) or at the dilution ratios of 1:2 and 1:5, the corresponding inward current amplitudes were 186.1 ± 27.7pA; 78.7 ± 15.1pA and 25.7 ± 7.9 pA (n=8), respectively (Fig. 3). The ACM-induced inward currents were blocked by bath application of APV, indicating that ACM produces inward current via NR1a/NR2B receptors (Fig. 4, n = 14). In contrast, non-NMDA receptor antagonist, CNQX (20 μM), had no significant effect on ACM-induced currents (Fig. 4, n = 16). These results suggest that ACM-induced inward currents were conducted through NR1a/NR2B receptors expressed on Xenopus oocytes.
Fig. 3.
The ACM-induced inward currents were dilution (concentration) dependent. The upper trace shows inward currents induced by pressure ejection of the ACM(+) recovered from IL-1β-stimulated astrocytes at different dilutions which was recorded in a NR1a/NR2B-expressing oocyte. The lower is a bar graph showing the average inward currents induced by ACM(+) in NR1a/NR2B expressing oocytes at different concentrations (dilutions).
Fig. 4.
Blockade of the ACM(+)-induced inward currents by bath application of APV (50μM), a specific NMDA receptor antagonist, but not by CNQX, a non NMDA receptor antagonist. Arrows indicate the time when the pressure ejections were delivered. Horizontal bars illustrate bath perfusion of APV or CNQX.
An important feature for a neurodegenerative disorder is its chronic disease progression involving astroglial activation. To evaluate the potency of acute- and chronic-activated astrocytes on NMDA receptor activation we compared the effects of the ACMs recovered from “acute” and “chronic” IL-1β-stimulated astrocytes on NR1a/NR2B receptors. Pressure ejection of acute ACM and chronic ACM produced inward currents of 155.5 ± 21.2pA and 153.7 ± 20.5pA, respectively in NR1a/NR2B-expressing oocytes. The difference has no statistic significance (p > 0.05, n=33), indicating that there is no significant difference between the concentrations of NMDA receptor agonist-like substances released from acute (2 h) IL-1β-stimulated astrocytes and chronic (24 h) IL-1β-stimulated astrocytes.
4. Discussion
Previous studies have shown that neurodegeneration could be attenuated by NMDA receptor antagonists [9, 19-22]. Since IL-1β activation of astrocytes contributes to neurodegeneration during HIV infection, we have examined the effects of ACM recovered from IL-1β-stimulated human astrocytes on NR1a/NR2B receptors expressed on Xenopus oocytes. Our results showed that pressure ejection of the ACM(+) produced an inward current in NR1a/NR2B-expressing oocytes, but not in non-NR1a/NR2B-expressing oocytes. The ACM(+)-induced inward current was concentration-dependent and blocked by NMDA receptor antagonist APV, but not by AMPA receptor antagonist CNQX. These results suggest that the IL-1β-stimulated astrocytes release soluble NMDAR agonist-like substances, leading to activation of the NR1a/NR2B receptors expressed on Xenopus oocytes and resultant inward current.
Astrocytes are essential for brain homeostasis and play crucial roles in neuronal function and survival. They are also cellular reservoirs and important participants in the pathogenesis of HAND/HAD [8, 29, 30]. In the course of HIV-1 brain infection, astrocytes are among the first cells to respond directly to brain injury and participate in disease process largely through their capacity of releasing neurotoxic factors. These astrocyte-derived neurotoxic factors including, but are not limited to, chemokines such as CCL2, cytokines (especially TNF-α), quinolic acid, platelet activating factor, nitrogen oxide and glutamate. These are thought to be important mediators of HIV-1-induced neuronal damage [8, 31]. Nevertheless, the mechanisms underlying astrocyte/HIV-associated neuronal damage have not been completely elucidated. Previous studies have shown that the detrimental effects of various candidate astrocyte/HIV-associated neurotoxic factors can be blocked in vitro by NMDA receptor antagonists, indicating that activation of NMDA receptors is involved in HIV-1-induced neuronal injury [32-35]. These neurotoxic factors may activate NMDA receptors by direct binding or by impairing glutamate uptake functions of astrocytes and thus increase extracellular concentrations of glutamate. Up-to-date, few studies have shown direct activation of NMDA receptors by astrocyte/HIV-associated neurotoxic factors. We found that pressure ejection of IL-1β-stimulated ACM produced an inward current in Xenopus oocytes expressing NR1a/NR2B NMDA receptors, demonstrating a possible direct action on NMDA receptors by astrocyte/HIV-associated neurotoxic factors.
Numerous studies have shown that the levels of many cytokines increase dramatically following brain insult such as HIV-1 infection. These cytokines may directly interact with neuronal NMDA receptors, or stimulate astrocytes to release soluble factors acting indirectly on neuronal NMDA receptors and resulting in neuronal injury. Among the astrocyte-secreted factors is IL-1β, a cytokine which can recurrently stimulate astrocyte production of more soluble factors including IL-1β. Since IL-1β is a key mediator of inflammation and neuronal death in acute CNS injuries, such as stroke and brain trauma, it has been considered as HAD-relevant pro-inflammatory cytokine and implicated in neurodegenerative diseases such as HAD [35]. We hypothesize that factors from IL-1β-stimulated astrocytes induces neuronal injury via NMDA receptors. This hypothesis is supported by the experimental results that IL-1β-stimulated ACM activate NMDA receptors expressed on Xenopus oocytes and induced neuronal injury when added to rat primary human neuronal cultures [35].
It is well-known that activation of an NMDA receptor opens a channel permeable to Ca2+ ions and a sustained increase of intracellular Ca2+ has been shown to cause neuronal injury in numerous pathophysiological processes [34]. As HIV-1-associated neurodegeneration is a chronic disease progression process involving astroglial activation and resultant production of NMDA receptor agonist-like substances, it is most likely that the elevated levels of intracellular Ca2+, triggered by astrocyte-associated NMDA receptor agonist-like substances, are at least in part involved in the pathogenesis of HIV disease. This postulation is supported by research findings showing that HIV-1-associated neuronal injury can be attenuated or blocked by NMDA receptor antagonists both in vitro and in vivo.
Astrocytes treated with IL-1β induced CCL2, TNF-α, TGF-β2 and MMP observed in the present study suggests that IL-1β induce potential soluble factors secretion in astrocytes. IL-1β-stimulated and/or virus-infected astrocytes release many factors including cytokines (especially TNF-α), quinolinic acid, platelet activating factor, nitrogen oxide glutamate and arachidonic acid [8]. Upon release, these factors may act alone or in combination on NR1a/NR2B NMDA receptors, producing inward current. It is worth to point out that we did not dissect out the active factor(s) for the activation of NR1a/NR2B receptors observed in this study. The focus of the work, however, was to explore whether the IL-1β-stimulated ACM activates NR1a/NR2B receptors. Nevertheless, our results showed that IL-1β-stimulated ACM directly activate NMDA receptors expressed on Xenopus oocytes, implicating a potential role for IL-1β-stimulated astrocytes in neuronal injury/dysfunction.
In summary, the present study demonstrated that IL-1β-stimulated astrocytes activate NMDA receptors in vitro. Although the active components remain to be determined, the activation of NR1/NR2B receptors by the IL-1β-stimulated ACM may have implications for the pathogenesis of neurodegenerative disorders including HAND/HAD.
Research highlights.
IL-1β-stimulated astrocyes produce soluble factors,
Astrocyte-released soluble factors activate NR2B-containing NMDA receptors,
Astrocyte-induced NMDA receptor activation has implications for neurodegeneration
Acknowledgments
This work was supported by NIH grants R01 NS063878 and NS 041862 (HX) and R01 NS43113 and NS48837 (AG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–711. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 3.Erichsen D, Lopez AL, Peng H, Niemann D, Williams C, Bauer M, Morgello S, Cotter RL, Ryan LA, Ghorpade A, Gendelman HE, Zheng J. Neuronal injury regulates fractalkine: relevance for HIV-1 associated dementia. J Neuroimmunol. 2003;138:144–155. doi: 10.1016/s0165-5728(03)00117-6. [DOI] [PubMed] [Google Scholar]
- 4.Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, Giovanelli A, Shapshak P. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehav HIV Med. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner J, Borgmann K, Deshpande MS, Dhar A, Wu L, Persidsky R, Ghorpade A. Potential mechanisms for astrocyte-TIMP-1 downregulation in chronic inflammatory diseases. J Neurosci Res. 2006;83:1281–1292. doi: 10.1002/jnr.20823. [DOI] [PubMed] [Google Scholar]
- 6.Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restriced HIV-1 infection of astrocytes in postmortem pediatric central nervous tissue. Neuorol. 1994;44f:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 8.Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. Aids. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- 9.Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 10.Kou W, Banerjee S, Eudy J, Smith LM, Persidsky R, Borgmann K, Wu L, Sakhuja N, Deshpande MS, Walseth TF, Ghorpade A. CD38 regulation in activated astrocytes: implications for neuroinflammation and HIV-1 brain infection. J Neurosci Res. 2009;87:2326–2339. doi: 10.1002/jnr.22060. [DOI] [PubMed] [Google Scholar]
- 11.Boven LA, Vergnolle N, Henry SD, Silva C, Imai Y, Holden J, Warren K, Hollenberg MD, Power C. Up-regulation of proteinase-activated receptor 1 expression in astrocytes during HIV encephalitis. J Immunol. 2003;170:2638–2646. doi: 10.4049/jimmunol.170.5.2638. [DOI] [PubMed] [Google Scholar]
- 12.Dou H, Morehead J, Bradley J, Gorantla S, Ellison B, Kingsley J, Smith LM, Chao W, Bentsman G, Volsky DJ, Gendelman HE. Neuropathologic and neuroinflammatory activities of HIV-1-infected human astrocytes in murine brain. Glia. 2006;54:81–93. doi: 10.1002/glia.20358. [DOI] [PubMed] [Google Scholar]
- 13.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 14.Stys PK, Lipton SA. White matter NMDA receptors: an unexpected new therapeutic target? Trends Pharmacol Sci. 2007;28:561–566. doi: 10.1016/j.tips.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. 41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R, Sibley D. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton SA. Ca2+, N-methyl-D-aspartate receptors, and AIDS-related neuronal injury. Int Rev Neurobiol. 1994;36:1–27. doi: 10.1016/s0074-7742(08)60301-3. [DOI] [PubMed] [Google Scholar]
- 18.Lipton SA. Similarity of neuronal cell injury and death in AIDS dementia and focal cerebral ischemia: potential treatment with NMDA open-channel blockers and nitric oxide-related species. Brain Pathol. 1996;6:507–517. doi: 10.1111/j.1750-3639.1996.tb00879.x. [DOI] [PubMed] [Google Scholar]
- 19.Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- 20.Ushijima H, Nishio O, Klocking R, Perovic S, Muller WE. Exposure to gp120 of HIV-1 induces an increased release of arachidonic acid in rat primary neuronal cell culture followed by NMDA receptor- mediated neurotoxicity. Eur J Neurosci. 1995;7:1353–1359. doi: 10.1111/j.1460-9568.1995.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 21.Sas AR, Bimonte-Nelson HA, Tyor WR. Cognitive dysfunction in HIV encephalitic SCID mice correlates with levels of Interferon-alpha in the brain. Aids. 2007;21:2151–2159. doi: 10.1097/QAD.0b013e3282f08c2f. [DOI] [PubMed] [Google Scholar]
- 22.Muller WE, Pergande G, Ushijima H, Schleger C, Kelve M, Perovic S. Neurotoxicity in rat cortical cells caused by N-methyl-D-aspartate (NMDA) and gp120 of HIV-1: induction and pharmacological intervention. Prog Mol Subcell Biol. 1996;16:44–57. doi: 10.1007/978-3-642-79850-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Janabi N, Di Stefano M, Wallon C, Hery C, Chiodi F, Tardieu M. Induction of human immunodeficiency virus type 1 replication in human glial cells after proinflammatory cytokines stimulation: effect of IFNgamma, IL1beta, and TNFalpha on differentiation and chemokine production in glial cells. Glia. 1998;23:304–315. [PubMed] [Google Scholar]
- 24.Peng H, Erdmann N, Whitney N, Dou H, Gorantla S, Gendelman HE, Ghorpade A, Zheng J. HIV-1-infected and/or immune activated macrophages regulate astrocyte SDF-1 production through IL-1beta. Glia. 2006;54:619–629. doi: 10.1002/glia.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canki M, Thai JN, Chao W, Ghorpade A, Potash MJ, Volsky DJ. Highly productive infection with pseudotyped human immunodeficiency virus type 1 (HIV-1) indicates no intracellular restrictions to HIV-1 replication in primary human astrocytes. J Virol. 2001;75:7925–7933. doi: 10.1128/JVI.75.17.7925-7933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghorpade A, Holter S, Borgmann K, Persidsky R, Wu L. HIV-1 and IL-1 beta regulate Fas ligand expression in human astrocytes through the NF-kappa B pathway. J Neuroimmunol. 2003;141:141–149. doi: 10.1016/s0165-5728(03)00222-4. [DOI] [PubMed] [Google Scholar]
- 27.Bear CE, Duguay F, Naismith AL, Kartner N, Hanrahan JW, Riordan JR. Cl-channel activity in Xenopus oocytes expressing the cystic fibrosis gene. J Biol Chem. 1991;266:19142–19145. [PubMed] [Google Scholar]
- 28.Xiong H, McCabe L, Skifter D, Monaghan DT, Gendelman HE. Activation of NR1a/NR2B receptors by monocyte-derived macrophage secretory products: implications for human immunodeficiency virus type one-associated dementia. Neurosci Lett. 2003;341:246–250. doi: 10.1016/s0304-3940(03)00194-0. [DOI] [PubMed] [Google Scholar]
- 29.Keblesh JP, Reiner BC, Liu J, Xiong H. Pathogenesis of Human Immunodeficiency Virus Type-1 (HIV-1)-Associated Dementia: Role of Voltage-Gated Potassium Channels. Retrovirology. 2008;2:1–10. [PMC free article] [PubMed] [Google Scholar]
- 30.Reiner BC, Keblesh JP, Xiong H. Methamphetamine abuse, HIV infection, and neurotoxicity. Int J Physiol Pathophysiol Pharmacol. 2009;1:162–179. [PMC free article] [PubMed] [Google Scholar]
- 31.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 32.Lipton SA. AIDS-related dementia and calcium homeostasis. Ann N Y Acad Sci. 1994;747:205–224. doi: 10.1111/j.1749-6632.1994.tb44411.x. [DOI] [PubMed] [Google Scholar]
- 33.Ahlemeyer B, Kolker S, Zhu Y, Hoffmann GF, Krieglstein J. Increase in glutamate-induced neurotoxicity by activated astrocytes involves stimulation of protein kinase C. J Neurochem. 2002;82:504–515. doi: 10.1046/j.1471-4159.2002.00994.x. [DOI] [PubMed] [Google Scholar]
- 34.Choi DW, Koh JY, Peters S. Pharmacology of glutamate neurotoxicity in cortical cell culture: attenuation by NMDA antagonists. J Neurosci. 1988;8:185–196. doi: 10.1523/JNEUROSCI.08-01-00185.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deshpande M, Zheng J, Borgmann K, Persidsky R, Wu L, Schellpeper C, Ghorpade A. Role of activated astrocytes in neuronal damage: potential links to HIV-1-associated dementia. Neurotox Res. 2005;7:183–192. doi: 10.1007/BF03036448. [DOI] [PubMed] [Google Scholar]