Abstract
Chlamydia trachomatis causes respiratory and sexually transmitted infections. Here, we tested a vaccine formulated with the recombinant major outer membrane protein from C. trachomatis mouse pneumonitis (CT-MoPn) for its ability to protect mice against an intranasal (i.n.) challenge. The adjuvants CpG and Montanide were used for systemic routes, intramuscular (i.m.) and subcutaneous (s.c.), and cholera toxin for mucosal routes, sublingual (s.l.) and colonic (c.l.). Mucosal immunizations were performed either alone or in combination with systemic routes. Mice inoculated i.n. with 104 inclusion-forming units (IFU) of CT-MoPn served as a positive control and the Neisseria gonorrhoeae recombinant porin B (Ng-rPorB) as the negative antigen control. Immunized animals were challenged i.n. with 104 IFU of CT-MoPn. Following immunization the combination groups showed high chlamydial serum IgG titers (s.l.+i.m.+s.c. 25,600; c.l+i.m.+s.c. 102,400) and the IgG2a/IgG1 ratios indicated a Th1 response. Following the i.n. challenge the s.l./i.m.+s.c. group showed the best protection as demonstrated by an increase in body weight of 0.3% over the 10 day course of infection. A statistically significant difference was found when compared with the Ng-rPorB immunized animals that had lost 20% of their original body weight (P < 0.05). In addition, the repeated measures ANOVA test showed significant difference in body weight change for the combined immunized groups versus their mucosal counterparts and also the systemic immunized group. A statistically significant difference (P < 0.05) was also observed in the median number of IFUs recovered from the lungs when the s.l.+i.m.+s.c. (2.8 × 106 ) and c.l.+i.m.+s.c. (3.4 × 106) groups where compared to their respective mucosal only groups (s.l.: 61.9 × 106 and c.l: 136.2 × 106) and the control Ng-rPorB immunized mice (198.2 × 106) (P < 0.05). In conclusion, a combined systemic plus mucosal vaccination provides better protection against a respiratory challenge with C. trachomatis than either systemic or mucosal immunizations alone.
Keywords: Chlamydia trachomatis, recombinant major outer membrane proteins, systemic and mucosal immunization, mice, respiratory infections, vaccine
1. Introduction
Sexually transmitted diseases are a major health concern worldwide and the search for preventative and therapeutic measures are ongoing. Chlamydia trachomatis is the most common bacterial sexually transmitted pathogen that can produce acute and chronic genital manifestations affecting both females and males [1–3]. In addition, C. trachomatis can cause respiratory, gastrointestinal, ocular infections and other systemic manifestations [1, 4–7]. Early stages of infection can be treated with antibiotics. However, sequelae can develop if this pathogen is not treated adequately or soon after infection [8–10]. Furthermore, most cases are asymptomatic and go untreated [1]. The recent emergence of antibiotic resistant strains and the risk of reinfection in screened and antibiotic treated individuals emphasize the need for an efficacious vaccine [11–13].
Vaccines, using whole inactivated and viable C. trachomatis, were initiated to prevent trachoma [6, 14, 15]. These trials however were abandoned due to short-term protection that in addition was, for most part, serovar specific. Furthermore, in some vaccinated individuals ocular inflammation ocurred following re-exposure [4, 5, 16, 17]. Although the cause of inflammatory reaction has not yet been elucidated, it was attributed to an antigenic component present in Chlamydia [18, 19]. Therefore, the need to develop a subunit vaccine was considered.
The MOMP has been extensively studied as a vaccine candidate [12, 16]. MOMP contributes 60% of total mass of the outer membrane and has B- and T-cell epitopes [16, 20]. In mice, the native MOMP (nMOMP) from Chlamydia can induce strong protection against respiratory and genital challenges and in non-human primates it can protect against an ocular infection [21–24]. Unfortunately, the costs required to produce nMOMP makes this antigen unlikely to be implemented in humans. Hence, as an alternative, recombinant MOMP (rMOMP) preparations have been tested and so far the protection obtained is not as robust as that observed with nMOMP [25]. These differences in protection have been attributed to the structural conformation of MOMP [25]. Although the protection induced by a recombinant preparation may not be as robust as that elicited by the nMOMP this should not be a deterrent for pursuing the formulation of a vaccine with the rMOMP. A computer model has shown that, even a vaccine with limited efficacy, can have a tremendous impact on the epidemiology of this infection [26].
The aim of this study was to compare the protective ability of vaccination protocols combining mucosal and systemic routes for immunization versus their mucosal or systemic only counterparts against an intranasal challenge with C. trachomatis. To our knowledge this is the first study where combined routes of immunization have been tested against a C. trachomatis respiratory challenge.
2. Materials and Methods
2.1 Chlamydia trachomatis stocks
C. trachomatis MoPn strain Nigg II (also called Chlamydia muridarum) was obtained from the American Type Culture Collection (ATCC; Manassas, VA) [27]. HeLa 229 cells were used to grow the organism in Eagle’s minimal medium supplemented with 5% fetal calf serum [28]. Elementary bodies (EB) were purified as described using Renografin (Squibb, Princeton, NJ) and stored at −70°C in sucrose-phosphate-glutamate solution (SPG) [18].
2.2 Cloning of the C. trachomatis rMOMP
Genomic DNA from C. trachomatis MoPn strain Nigg II was extracted using the Wizard genomic DNA Purification Kit (Promega) [25]. The MoPn MOMP gene (GenBank, accession no. AE002272, X63409) was amplified without the leading sequence with Pfu Turbo DNA Polymerase (Stratagene, La Jolla, CA) using the following primers. Forward primer: 5’ ACGCCCATGGCACTGCCTGTGGGGAATCCTGCT 3’, and reverse primer: 5’ AGCGGTCGACTTAGAAACGGAACTGAGCATT 3’. The MOMP DNA was cloned into pET-45b vector (Novagen, Gibbstown, NJ) at the NcoI and SalI sites using T4 DNA ligase (New England Biolabs, Ipswich, MA), and transformed into Escherichia coli TOP10 competent cells. After confirmation of positive clones by sequencing, the plasmid was transformed into E. coli BL21 (DE3) competent cells for expression in the presence of 0.4 mM IPTG. The efficacy of the protein induction was checked by SDS PAGE.
2.3 Cloning of the N. gonorrhoeae recombinant porin B
N. gonorrhoeae strain FA1090 from the ATCC was grown on GC agar plates [25]. Genomic DNA was extracted with the Wizard genomic DNA Purification Kit (Promega, Madison, WI). The recombinant PorB gene (36 kDa; 330 AA) without the leading sequence (GenBank ID: AAW90430) was amplified by the PCR with the following primers: Forward primer, Ngo-F2: 5' TATGCCATGGCCGATGTCACCCTG 3’ and reverse primer, Ngo-R1: 5’ GCGGATCCTTAGAATTTGTGGCGCAG 3’ [25]. The PCR product was cloned into pET 45b vector at NcoI and BamHI sites and transformed into TOP10 cells. The plasmid carrying Ng-rPorB was transformed into BL21 (DE3) competent cells and the protein production was induced by 0.4 mM IPTG.
2.4 Purification of the C. trachomatis rMOMP and the N. gonorrhoeae rPorB from inclusion bodies
E. coli pellets were treated with TEN buffer with 8M urea, 0.1mM PMSF and 0.02 mM DTT as described by Marston [29], to a concentration of 10 mg/ml. The solubilized MOMP was loaded onto a Sephacryl-S-300 column (1 × 50 cm Sigma-Aldrich, St. Louis, IL) that was pre-equilibrated with 100mM Tris-HCl, pH 8.0, 200 mM NaCl, 10 mM EDTA, 0.2 mM DTT, and 0.05% Z3-14 [22, 25, 30, 31]. The collected fractions were concentrated using PEG 8000, and dialyzed extensively against 0.02M PBS, pH 7.4, 150mM NaCl, and 0.05% Z3-14, and stored at −80°C [22, 25].
The Ng-PorB pellet was solubilized in 0.2 M Tris, pH 8.2; 6M guanidine-HCl, 2 mM EDTA, 1 mM PMSF and 20 mM DTT and was centrifuged at 12,000 ×g for 20 min and the supernatant was dialyzed against PBS (pH 7.4) with 0.05% Z3-14, stored at −80ºC and used for immunization [22, 25].
The molecular weight and purity of samples were determined using 10% tricine-SDS-PAGE [32]. The recombinant proteins rMOMP and Ng-rPorB were found to have less than 0.05 EU of LPS/mg of protein using the limulus amoebocyte assay (BioWhittaker, Inc., Walkersville, MD).
2.5 Immunization protocols
Three week old female BALB/c (H-2d) mice were purchased from Charles River Laboratories (Wilmington, MA). Intramuscular (i.m.) immunization was carried out by injecting the antigen in the posterior thigh muscles. The subcutaneous (s.c.) immunization was delivered by injecting under the skin in the back close to the tail. The sublingual (s.l.) immunization was adapted from Song et al. [33]. Mice were anaesthetized with Xylazene/Ketamine and forceps were placed under their tongue. The antigen was administered in intervals of 2 min in 2 μl volumes to avoid being swallowed. Colonic (c.l.) administration of antigen was adapted from Amorij et al. [34] and McConnell et al. [35]. Mice were fasted overnight for food but were allowed to have water ad libitum. The following day the mice were anaesthetized and the vaccine was administered c.l. using a 38 mm × 1.2 mm Teflon feeding needle with a silicon tip (Scanbur BK, Sollentuna, Sweden). The mice were held in recumbent position for 5 min to prevent leakage.
Animals were grouped based on the route of immunization (Table 1). The mice were immunized via mucosal routes alone (s.l. or c.l.) or in combination with systemic routes (s.l.+i.m.+s.c. or c.l.+i.m.+s.c. The total amount of antigen administered per mouse per immunization was 20 μg. When the vaccine was delivered by a combination of i.m.+s.c. with either the s.l. or c.l. route, a total amount of 20 μg/mouse/immunization was maintained.
Table 1.
Experimental groups
| Antigen | Mucosal adjuvant / Route | Systemic adjuvant /Route | Group name | No. of immunizations | Antigen amount / mouse / dose |
|
|---|---|---|---|---|---|---|
| Mucosal route | Systemic route | |||||
| rMOMP | Cholera Toxin / sublingual | -- | rMOMP- s.l. | 3 | 20 μg | |
| rMOMP | Cholera Toxin / sublingual | CpG + Montanide / intramuscular + subcutaneous | rMOMP- s.l.+i.m.+s.c. | 3 | 10 μg | 10 μg |
| rMOMP | Cholera Toxin / colonic | -- | rMOMP- c.l. | 3 | 20 μg | |
| rMOMP | Cholera Toxin / colonic | CpG + Montanide / intramuscular + subcutaneous | rMOMP- c.l.+i.m.+s.c. | 3 | 10 μg | 10 μg |
| rMOMP | -- | CpG + Montanide / intramuscular + subcutaneous | rMOMP- i.m.+s.c. | 3 | 20 μg | |
| Ng-rPorB | -- | CpG + Montanide / intramuscular + subcutaneous | Ng-rPorB- i.m.+s.c. | 3 | 20 μg | |
| MoPn | -- / intranasal | -- | MoPn- i.n. | 1 | 104 IFU | |
The following adjuvants were used. Cholera toxin (CT; Sigma Aldrich, St. Louis, MO) at 1 μg per dose per mouse was administered by the s.l. and c.l. mucosal routes only. For the i.m. and s.c. immunizations, CpG ODN-1826 (5’ TCCATGACGTTCCTGACGTT 3’; Coley Pharmaceutical Group; Ontario, Canada) 10 μg per dose per mouse and Montanide ISA 720 (Seppic, Inc., Fairfield, NJ) at a 70:30 v/v ratio were used [22, 36]. The immunizations were given 3 times at two week intervals. The positive control mice received 104 IFU of C. trachomatis MoPn via the i.n. route at the same time that the other groups received the first immunization. All animal protocols were approved by the University of California, Irvine Animal Use and Committee.
2. 6 Intranasal challenge
After the last immunization the animals were rested for 4 weeks. The day of the challenge the baseline body weight of the mice was measured. The mice were challenged i.n. with 104 IFU of C. trachomatis MoPn under Xylazene/Ketamine anesthesia [28]. For ten consecutive days after the challenge the body weight was monitored. At day 10, the mice were euthanized; their lungs were collected and weighed [28]. A 5ml solution of SPG was added and the lungs were homogenized using a Stomacher (Dynatech Laboratories, Inc., Alexandria, VA). HeLa cells grown in 48-well tissue culture plates were inoculated with 10-fold dilutions of the lung homogenates and incubated for 30 h at 37°C. The monolayers were fixed with methanol and inclusions were stained using a pool of monoclonal antibodies (mAb) to the MOMP, the 60-kDa cysteine-rich protein (crp), the 150-kDa putative outer membrane protein and lipopolysaccharide (LPS) of C. trachomatis MoPn [28]. The limit of detection per pair of lungs/mouse was 50 IFU. This experiment was repeated twice with a total of 14–15 mice per group.
2.7 Sample collection and antibody titers
Blood from the orbital plexus was collected before each immunization and a day before the respiratory challenge. Genital samples were obtained by washing the vagina twice with 20 μl PBS, pH 7.2 [22]. Processed samples were stored at −20°C until used. The samples were pooled per group and used to perform the immunoassays.
The enzyme-linked immunosorbent assay (ELISA) was used to detect Chlamydia-specific antibodies [28]. C. trachomatis EB at 10 μg/ml concentration were coated in a flat-bottom 96-well plate and incubated at 4°C overnight. Goat anti-mouse IgA (Cappel, Aurora, OH), IgG1, IgG2a, IgG2b, IgG3 (Southern Biotechnology Associates, Birmingham, AL) and IgG diluted at a concentration of 1:1000 were used to determine the sub-class or isotype-specific antibodies. ABTS [2, 2’-azino-bis-(3-ethylbenzthiazoline-6-sulfonate)] (Sigma-Aldrich, St. Louis, MO), was used as the substrate and the plates were scanned in an ELISA plate reader at 405nm (Labsystem Multiscan; Helsinki, Finland).
2.8 In vitro neutralization assay
The method described by Peterson et al. [37] was followed for this assay. In brief, duplicate sets of two-fold serial dilution of serum samples were made with 5% guinea pig serum in Ca+2 and Mg+2 free PBS. The serum was incubated with 1 × 104 IFU of C. trachomatis MoPn for 45 min at 37°C. The mixture was inoculated into HeLa-229 monolayers grown in 15 × 45 mm glass shell vials and centrifuged for an hour at 1000 × g. Media with cycloheximide at 1 μg/ml was added to the cells and incubated for 30 h. The monolayers were then fixed with methanol and the chlamydial inclusions stained as described above. The number of IFU was counted and neutralization was defined as greater than or equal to 50% decrease in the number of IFU, as compared to the control sera from the pre-immunized animals.
2.9 Immunoblots
Tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out for C. trachomatis MoPn EB [32]. EB (50 μg of protein) was loaded on a 7.5 cm wide slab gel. Transfer onto nitrocellulose membrane was followed by blocking non-specific binding sites with BLOTTO (bovine lacto transfer technique optimizer; 5% [wt/vol] nonfat dry milk, 2mM CaCl2, 50mM Tris-HCl [pH 8.0]). Serum samples were diluted and were used to probe the membrane strips by incubating them at 4°C overnight [23, 28]. Detection of antibody binding was performed with the horseradish peroxidase-conjugated goat anti-mouse antibody developed with 0.01% hydrogen peroxide and 4-chloro-1-naphthol. The mAb MoPn-40 was used as a positive control [22].
2.10 In vitro splenic CD4+ T cells response by lymphocyte-proliferation assay (LPA) and cytokine assay
The LPA was performed as described previously with the following modifications [23, 28]. Splenocytes were collected from two spleens per group and CD4+ T lymphocytes were separated using anti-mouse CD4 particles-DM through a positive fraction (BD Biosciences, San Jose, CA). Antigen presenting cells (APCs) were pre-prepared by irradiating (3000 rads, 137Cs) syngenic unseparated splenocytes and incubated in 200 μl in round bottom 96-well plates (Costar, Corning Inc.) at 37ºC for 2 h with MoPn EB at 1:5 ratio. T cells were added to APCs at a ratio of 1:1. The tissue culture medium was prepared by adding 5% FBS, 2% L-glutamine, 5 μg/ml gentamicin and 0.5% β-mercaptoethanol to RPMI 1640 (RPMI-FBS; Mediatech Inc., Manassas, VA). Concavalin A at 5μg/ml concentration served as a positive stimulant and tissue culture medium (RPMI-FBS) as a negative antigen control. Supernatants from three wells were collected 24 h post incubation and stored at −70ºC for cytokine analysis. At the end of 72 h incubation, 1.0 μCi of thymidine [methyl-3H] (47 Ci/mmol; Amersham, Arlington Heights, IL) in 25 μl of culture medium was added to the rest of the wells. After an additional 18 h of incubation, the cells were washed, collected on filter paper (Filter MAT, Skatron Instruments, USA) and scintillation fluid was added. A scintillation counter (Beckman Instruments, Fullerton, CA) was used to measure the incorporation of [3H] in the samples tested.
IFN-γ and IL-4 levels in the supernatants collected from the splenic T cells stimulated were measured using a commercial kit (BD Pharmingen, San Diego, CA) as described previously [23].
2.11 Statistics
The Mann-Whitney U test, Fischer’s Exact test and the unpaired Student’s t test using the Sigma Stat® software program (Systat Sofware Inc.) were utilized for statistical analyses. Repeated measures ANOVA test was performed for the changes in body weight during the 10 post challenge days. GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA) was used to depict data in a figure format. The P value of < 0.05 was considered to be statistically significant.
3. Results
3.1 Humoral immune response
Four weeks after the last immunization blood and vaginal washes were collected to determine the levels of Chlamydia-specific antibodies (Table 2). High Chlamydia-specific total IgG titers were found in the groups vaccinated i.m.+s.c.; s.l.+i.m.+s.c. and c.l.+i.m.+s.c. with rMOMP. Total IgG (102,400) levels were the highest in rMOMP-c.l.+i.m.+s.c. immunized group and the lowest (400) in the s.l. immunized group. The positive control group vaccinated i.n. with 104 IFU of Chlamydia had IgG serum levels of 25,600. The negative control group immunized i.m.+s.c. with Ng-rPorB had no Chlamydia-specific antibody titers.
Table 2.
Antibody titers of immunized mice the day before the i.n. challenge with C. trachomatis MoPn
| Groups | Anti-C. trachomatis MoPn ELISA titer |
Serum Neutralizing titer | ||||||
|---|---|---|---|---|---|---|---|---|
| Serum |
Vaginal wash |
|||||||
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | IgG | IgA | ||
| rMOMP- s.l. | 400 | 400 | <100 | <100 | <100 | <10 | <10 | <50 |
| rMOMP- s.l.+i.m.+s.c. | 25,600 | 12,800 | 51,200 | 51,200 | 12,800 | 40 | 10 | 1,250 |
| rMOMP- c.l. | 3,200 | 800 | 3,200 | 6,400 | 800 | <10 | <10 | 250 |
| rMOMP- c.l.+i.m.+s.c. | 102,400 | 3200 | 294,800 | 102,400 | 51,200 | 160 | 20 | 1,250 |
| rMOMP- i.m.+s.c. | 51,200 | 12,800 | 102,400 | 102,400 | 12,800 | 160 | <10 | 1,250 |
| Ng-rPorB- i.m.+s.c. | <100 | <100 | <100 | <100 | <100 | <10 | <10 | <50 |
| MoPn- i.n. | 25,600 | 3,200 | 25,600 | 25,600 | 12,800 | 20 | 160 | 6,250 |
To establish if the immunization elicited a Th1 or Th2 response the levels of IgG2a and IgG1 were determined. A high IgG2a/IgG1 ratio, indicative of a Th1 response, was observed in the groups vaccinated with combined routes. In particular, the rMOMP-c.l.+i.m.+s.c. immunized group had a high IgG2a/IgG1 ratio of 64 (204,800/3,200). The c.l. immunized group had an IgG2a/IgG1 ratio of 4 (3,200/800), whereas, the s.l. immunized group had a negative ratio (<100/400). The positive control group immunized i.n. with live MoPn had a IgG2a/IgG1 ratio of 8 (25,600/3200).
The total IgG and IgA titers from vaginal washes are also shown in Table 2. The rMOMP-c.l.+i.m.+s.c. group and rMOMP-i.m.+s.c. group of animals had IgG titers at 160 in vaginal washes. Detectable levels of IgA were only found in the vaginal washes for the combined immunized groups. The MoPn immunized animals had high IgA (160) and low IgG (20) titers in the vaginal washes.
The neutralizing antibody titers are indicated in Table 2. High titers (1,250) were observed in groups of mice that received rMOMP only systemically or in combination with a mucosal route. The positive control group immunized i.n. with EB had the highest neutralizing titer (6,250). No detectable levels of neutralizing antibodies were found in the rMOMP-s.l. immunized group.
Analysis of serum samples collected the day before the i.n. challenge was carried out by Western blot (Fig. 1). Serum samples obtained from the rMOMP vaccinated groups reacted only with the MOMP band (40 kDa). Sera from the control animals immunized with EB reacted with bands with molecular masses higher than 100 kDa, the 60 kDa crp, the 60 kDa hsp and the 28 kDa proteins and LPS. Negative control group animals, immunized with Ng-rPorB did not have Chlamydia-specific antibodies. The mAb MoPn-40 to the MOMP band was used as a reference.
Fig 1.
Western Blot of serum samples from immunized mice the day before the i.n. challenge with C. trachomatis MoPn. Lane 1) molecular weight standards. Lanes 2–8) serum samples from mice immunized with: lane 2) rMOMP- s.l.; lane 3) rMOMP- s.l.+i.m.+s.c.; lane 4) rMOMP- c.l.; lane 5) rMOMP- c.l+i.m.+s.c.; lane 6) rMOMP- i.m. +s.c.; lane 7) Ng-rPorB- i.m.+s.c.; lane 8) MoPn- i.n. Lane 9) MAb MoPn-40 to C. trachomatis MoPn MOMP used as a control.
3.2 Splenic CD4+ T cell mediated immune response (CMI)
The splenic CD4+ T cell mediated immune responses for each group of mice evaluated by an in vitro LPA and cytokine assays the day before the i.n. challenge are summarized in Table 3. Significant proliferative responses were elicited by EB to splenic CD4+ T lymphocytes for all groups of mice vaccinated with rMOMP (s.l., 4.6 ± 2.1 × 103 cpm; s.l.+i.m.+s.c., 7.6 ± 2.2 × 103 cpm; c.l., 8.5 ± 3.8 × 103 cpm; c.l.+i.m.+s.c., 5.8 ± 3.1 × 103 cpm; i.m.+s.c., 10.8 ± 6.1 × 103 cpm) in comparison with the control group immunized with Ng-rPorB (1.6 ± 0.5 × 103 cpm, P < 0.05). As expected, the mice immunized i.n. with MoPn exhibited the strongest CD4+ T lymphocyte proliferative response (18.7 ± 5.5 × 103 cpm).
Table 3.
CD4+ T-cell responses of immunized mice the day before the i.n. challenge with C. trachomatis MoPna
| Groups | CD4+ cell proliferative response to EBb (103 × Δcpm) |
In vitro cytokine production in response to EB (pg/ml) |
|
|---|---|---|---|
| IFN-γ | IL-4 | ||
| rMOMP- s.l. | 4.6 ± 2.1c | 73 ± 2c,d | < 4 |
| rMOMP- s.l.+i.m.+s.c. | 7.6 ± 2.2c | 93 ± 0c,e,f,g | < 4 |
| rMOMP- c.l. | 8.5 ± 3.8c | 48 ± 1c | < 4 |
| rMOMP- c.l.+i.m.+s.c. | 5.8 ± 3.1c | 78 ± 6c,i | < 4 |
| rMOMP- i.m.+s.c. | 10.8 ± 6.1c | 83 ± 2c | < 4 |
| Ng-rPorB- i.m.+s.c. | 1.6 ± 0.5 | < 31 | < 4 |
| MoPn- i.n. | 18.7 ± 5.5c | 1,659 ± 110c | < 4 |
Values are mean ± 1SD.
C. trachomatis MoPn EB were added at a ratio of EB : APC+CD4+ cells of 1:5.
P < 0.05 by the Student’s t test in comparison with Ng-rPorB immunized group.
P <0.05 by the Student’s t test by comparing rMOMP- s.l. group with rMOMP- c.l. group.
P <0.05 by the Student’s t test by comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- i.m.+s.c. group.
P < 0.05 by the Student’s t test by comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- s.l. group.
P <0.05 by the Student’s t test by comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- c.l.+i.m.+s.c. group.
P < 0.05 by the Student’s t test by comparing rMOMP- c.l.+i.m.+s.c. group with rMOMP- c.l. group.
The levels of IFN-γ and IL-4 secreted from EB-stimulated CD4+ T cells were measured by ELISA (Table 3). Interestingly, the IFN-γ level was significantly higher in the mice immunized by the s.l.+i.m.+s.c. routes (93 ± 0.0 pg/ml) compared with the mice immunized s.l. alone (73 ± 2 pg/ml, P < 0.05), or the i.m.+s.c. (83 ± 2 pg/ml; P < 0.05) routes. Similarly, the level of IFN-γ from the CD4+ T cells of mice immunized by the c.l.+i.m.+s.c. route (78 ± 6 pg/ml) was significantly higher than that of the mice immunized by the c.l. route alone (48 ± 1 pg/ml, P < 0.05), but not statistically different to that of the mice immunized by i.m.+s.c. (P > 0.05). The level of IFN-γ detected in the animals immunized s.l.+i.m.+s.c. was significantly higher than that of the mice immunized with rMOMP by any other route (P < 0.05, respectively). The highest level of IFN-γ was detected in the mice immunized i.n. with MoPn EB (1,659 ± 110 pg/ml). In the Ng-rPorB immunized group IFN-γ was below the level of detection. IL-4 was below the level of detection for all groups.
3.3 Changes in body weight following the intranasal challenge
Four weeks after the last immunization all animals were challenged i.n. with 104 IFU of C. trachomatis MoPn and the weight of each mouse was monitored for 10 days (Fig. 2A and Table 4). The mean body weight change for each group was used as a parameter of the general disease caused by C. trachomatis. The body weight of the animals in the positive control group, immunized with and challenged with 104 IFU C. trachomatis MoPn, decreased slightly (1%) the first four days and came back up. By day 10 they had gained 3.3% of their original body weight. The Ng-rPorB group, which served as the negative control, lost almost 20% of their initial body weight by day 10 p.i. The rMOMP-s.l. immunized group lost 13% of their body weight by day 4. These animals recovered 3% of their weight by day 6. By day 10, the rMOMP- s.l. group had lost 10.8% of their original body weight. The rMOMP- c.l. group kept losing weight, and by day 8 their body weight dropped by 17%. They recovered 2% weight over the next two days, making an overall loss at 15% of their original body weight. The rMOMP- i.m.+s.c. immunized group lost 8% of their initial body weight by day 4, then recovered 2% by day 6. By day 10 their body weight loss was at 6.8%. The rMOMP- c.l.+i.m.+s.c. immunized group lost of 7% of their body weight by day 4 and by day 10 they had a 3% body loss from their initial weight. The rMOMP- s.l.+i.m.+s.c. vaccinated animals initially lost 5% of their body weight by day 4 but by day 10, they had gained 0.3% of their original body weight. The repeated measures ANOVA test showed a significant difference in body weight loss in all animals vaccinated with rMOMP, except for the group immunized c.l. (P = 0.697) compared to the Ng-rPorB immunized group. Significant difference was seen by the ANOVA between the MoPn group of animals in comparison to the rMOMP immunized groups. Statistically, differences were also observed when repeated measures ANOVA was applied to the i.m.+s.c. group versus the groups immunized s.l.+i.m.+s.c. and c.l.+i.m.+s.c. respectively (P < 0.05).
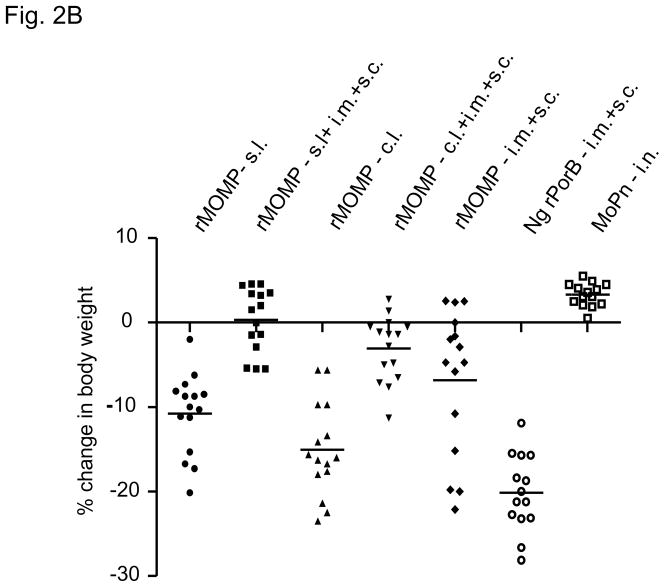
Fig 2.
Fig 2A. Percentage change in mean body weight after the i.n. challenge with C. trachomatis MoPn.
Fig 2B. Percentage change in body weight of individual mice at day 10 after the i.n. challenge. Each symbol represents an individual mouse and the horizontal bar indicates the mean.
Table 4.
Disease burden and yields of C. trachomatis from lungs
| Groups | % change in mean body weight at day 10a | Weight of lungs (g)b | Median IFU recovered from lungs (range) × 106 |
|---|---|---|---|
| rMOMP- s.l. | −10.8 ± 1.2c,d | 0.35 ± 0.01 | 61.9 (7.2 – 1,672.6)c |
| rMOMP- s.l.+i.m.+s.c. | 0.3 ± 1.0c,e,f,g | 0.25 ± 0.01c,e | 2.8 (0.02 – 128.0)c,e |
| rMOMP- c.l. | −15.0 ± 1.4c | 0.36 ± 0.01 | 136.2 (6.4 – 3,572.4) |
| rMOMP- c.l.+i.m.+s.c. | −3.0 ± 1.0c,h | 0.29 ± 0.01c,h | 3.4 (0.002 – 23.9)c,h |
| rMOMP- i.m.+s.c. | −6.8 ± 2.2c | 0.27 ± 0.01c | 8.0 (0.03 – 1,115.1)c |
| Ng-rPorB- i.m.+s.c. | −19.9 ± 1.1 | 0.34 ± 0.01 | 198.2 (22.3 – 38,286) |
| MoPn- i.n. | 3.3 ± 0.4c | 0.21 ± 0.01c | 0.00005c |
Percent change in mean body weight at day 10 ± 1 SE.
Weight of lungs, mean ± 1 SE.
P < 0.05 by the Student’s t test (columns 2 and 3) and Mann-Whitney U test (column 4) compared with Ng-rPorB immunized group
P <0.05 by the Student’s t test comparing rMOMP- s.l. group with rMOMP- c.l. group.
P < 0.05 by the Student’s t test (columns 2 and 3) and Mann-Whitney U test (column 4) by comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- s.l. group.
P < 0.05 by the Student’s t test comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- c.l.+i.m.+s.c. group.
P < 0.05 by the Student’s t test comparing rMOMP- s.l.+i.m.+s.c. group with rMOMP- i.m.+s.c. group.
P < 0.05 by the Student’s t test (columns 2 and 3) and Mann-Whitney U test (column 4) by comparing rMOMP- c.l.+i.m.+s.c. group with rMOMP- c.l. group.
The body weight change of individual mouse at day 10 is shown in Fig. 2B. Other than the positive control immunized with live EB, the rMOMP- s.l.+i.m.+s.c. animals showed the best recovery in body weight. Their body weight at day 10 p.i. was 0.3% higher than the initial body weight. All other rMOMP vaccinated groups showed a drop from their original body weights. The combined vaccination groups had less change in body weight when compared to their respective mucosal groups. Statistically there was a significant difference in body weight loss at day 10 between the i.m.+s.c. group and the group immunized s.l.+i.m.+s.c. both of which were immunized with rMOMP (P < 0.05). Significant differences in body weight were seen for all the rMOMP immunized groups when compared to the Ng-rPorB immunized control group (P < 0.05). The MoPn group of animals which were immunized i.n. and also challenge i.n. with 104 IFU showed the best recovery.
3.4 Lung weight
The lung weights, an indication of the IFU load and the inflammatory response, are reported in Fig. 3 and Table 4. The mean lung weight was 0.25 g for the s.l.+i.m.+s.c. group, 0.27 g for i.m.+s.c. group and 0.29 g for the c.l.+i.m.+s.c. immunized group. Significant differences were observed for these groups when compared to the negative control group immunized with Ng-rPorB (mean lung weight 0.34 g). On the contrary, the rMOMP- s.l. (0.35 g) and rMOMP- c.l. (0.36 g) immunized groups had no statistical significance when compared to the negative control. These groups, when compared to their respective combination group, showed significant difference in the weight of their lungs (P < 0.001). The combination groups (s.l.+i.m.+s.c. vs c.l.+i.m.+s.c.) had no significant difference between themselves (P = 0.097). No significant differences were observed for the i.m.+s.c. group vs the groups immunized s.l.+i.m.+s.c. (P = 0.433) or c.l.+i.m.+s.c. (P = 0.329). The control MoPn immunized group had the lowest mean weight of lungs of 0.21 g.
Fig 3.
Weight of the lungs (in grams) collected at day 10 after the i.n. challenge. Each symbol represents an individual mouse and the horizontal bar indicates the mean.
3.5 Yields of C. trachomatis IFU from the lungs
Yields of the C. trachomatis IFU recovered from the lungs are shown in Fig. 4 and Table 4. In comparison with the negative control Ng-rPorB group (median IFU of 198.2 × 106), significant protection was observed in animals vaccinated with rMOMP- s.l.+i.m.+s.c. (median 2.8 × 106 IFU), rMOMP-c.l.+i.m.+s.c. (median 3.4 × 106 IFU) and rMOMP-i.m.+s.c. (median 8 × 106 IFU). However, no significant differences were seen between Ng-rPorB immunized group (median IFU 198.2 × 106) vs rMOMP-s.l. (median 61.9 × 106 IFU) or rMOMP-c.l. (median 136.2 × 106 IFU) vaccinated animals (P > 0.05). No significant differences were seen between the i.m.+s.c. group and the groups immunized s.l.+i.m.+s.c. (P = 0.158) or c.l.+i.m.+s.c. (P = 0.263). The MoPn immunized group, used as the gold standard, had IFU below the level of detection ( <50).
Fig 4.
Number of IFU recovered from the lungs of individual animals ten days after the i.n. challenge. Each symbol represents an individual mouse and the horizontal bar indicates the median. The limit of detection is 50 IFU. In the MoPn- i.n. group no inclusions were found, hence a value of 49 IFU per individual mouse was assigned.
4. Discussion
The goal of this study was to compare systemic, mucosal and combined routes of immunization for their ability to elicit a protective response against a chlamydial challenge. Our results show enhanced protection against a C. trachomatis respiratory challenge by vaccinating mice using combined systemic plus mucosal routes. To our knowledge, this is the first report of a vaccination protocol using combination of systemic and mucosal routes of immunization to protect against a Chlamydia infection.
The route of administration of a vaccine influences the strength and nature of immune responses [38, 39]. The i.m. route is the one most commonly used to vaccinate humans against pathogenic organisms independent of their site of entry. For example, the human papillomavirus (HPV), influenza and pneumococcal vaccines are delivered by an i.m. injection to protect against mucosal pathogens [40–42]. The same route is also used to deliver vaccines against Clostridium tetani and hepatitis B virus, two systemic pathogens [43–45]. The portal of entry of Chlamydia can involve several organ systems including the conjunctiva and the respiratory, gastrointestinal and genitourinary tracts [7, 46] Therefore, identification of routes of immunization that can elicit strong immune responses at various mucosal sites is critical if we want to formulate an efficacious vaccine against this pathogen. This is particularly important because due to the compartmentalization of the mucosal immune system, stimulation of the various mucosal inductive sites results in an uneven distribution of immune responses at the various effector sites [39, 47, 48]. Overall, the most effective way to induce an immune response at a specific effector site is to locally administer the immunization, or perhaps, stimulate sites with related lymph drainage [39, 49, 50].
Up to now, i.n., immunization with live and subunit chlamydial vaccines, has been shown to be the best approach for inducing protection against respiratory and genital challenges [28, 51]. Unfortunately, the use of the i.n. route for immunization can result in significant negative secondary effects. For example, cases of facial (Bell's) paralysis were reported following the i.n. delivery of the influenza vaccine [52]. The immediate proximity of the olfactory epithelium, located in the middle of the roof of the nasal cavity, to the central nervous system is therefore, a significant concern for delivering vaccines by the i.n. route. For these reasons, here, we decided to explore the s.l. and c.l. routes, two recently described mucosal routes for immunization [33, 35, 53, 54].
Cuburu et al. [54] explored the s.l. route of immunization and found that, using ovalbumin and cholera toxin as a mucosal adjuvant, they were able to induce in mice broad-based systemic and mucosal immune responses. Song et al. [33] have reported that s.l. vaccination with inactivated influenza virus induces both systemic and mucosal immune responses and confers protection against a lethal i.n. challenge. Recently, Cuburu et al. [53] immunized mice by the s.l. route with HPV-like particles, using cholera toxin as the adjuvant, and observed protection against a genital challenge with HPV pseudovirions. Therefore, vaccination by the s.l. route appears to induce a strong immune response in the respiratory and the genitourinary tracts two organs systems affected by Chlamydia.
In these studies, vaccination of mice with the MOMP and cholera toxin using the s.l. route, elicited very low Chlamydia-specific antibody titers in serum and no measurable antibody response in the vagina. The CMI and the levels of IFN-γ in the supernatants from the stimulated splenocytes were significant. Following the i.n. challenge the mice lost significantly less body weight than the negative control group immunized with Ng-rPorB. Similarly, the number of chlamydial IFU recovered from the lungs was low in comparison with the negative control group. However, neither the immune response nor the protection observed in the mice immunized only by the s.l. route was as robust as that obtained in the animals vaccinated by combining the s.l. and the i.m.+s.c. routes. Furthermore, combining the s.l. and i.m.+s.c. routes for immunization, resulted in a more robust protection than vaccinating only by the i.m.+s.c. routes.
In addition to the s.l. route we also tested the c.l. route for immunization. McConnell et al. [35] compared in mice the oral and c.l. routes for vaccination. Prior to their study, only Ogra and Karzon had explored the c.l. route for immunization [55]. McConnell et al. [35], using ovalbumin adjuvanted with CT, found that c.l. administration induces significantly higher levels of colonic and vaginal IgA and serum IgG than the oral route. Based on their findings, McConnell et al. [35] suggested that the c.l. route might be an appropriate route for vaccinating against sexually transmitted disease.
Here, we decided to explore this route for immunization since Chlamydia can infect both the respiratory and genital tracts and therefore a vaccine should ideally protect at both portals of entry. Overall, the systemic and mucosal immune responses elicited by the c.l. route were weak [35]. This was unexpected since, based on the results reported by McConnell et al. [35], we anticipated a strong antibody response in the vagina. Furthermore the protection elicited against the respiratory challenge was minimal. However, like in the case of s.l. immunization, combining the c.l. and i.m.+s.c. routes elicited strong antibody response in serum and vagina. Interestingly, combination of the c.l. and the i.m. + s.c. routes resulted in a protective response against the respiratory challenge as robust as that obtained by combining the s.l. with the i.m. + s.c. routes.
Our results parallel recently reported findings with other pathogens showing that, combining systemic and mucosal routes for vaccination, elicits a more robust immune response than using a single route. For example, Vajdy et al. [56] immunized BALB/c female mice with two Helicobacter pylori antigens, cytotoxin-associated gene A (CagA) and neutrophil-activating protein, using a combination of mucosal (oral or i.n.) and/or systemic (i.m.) routes. Their results showed that mucosal, followed by systemic immunization, elicited stronger systemic and mucosal antibody responses, than mucosal alone, parenteral alone or systemic followed by mucosal routes for vaccination. Similarly, Barnett et al. [57] tested several combinations of systemic (i.m.) and mucosal (i.n.) routes in adult female rhesus macaques (Macaca mulata) using the HIV-1 envelope protein as the antigen. Mucosal, followed by systemic vaccination, induced the highest levels of serum IgA, vaginal, nasal and saliva IgG as well as peripheral IFN-γ ELISPOT responses, in comparison with i.n. or i.m. alone or systemic followed by mucosal immunizations.
In conclusion, we report that vaccination with rMOMP combining mucosal and systemic routes provides enhanced protection against a C. trachomatis respiratory challenge when compared with protocols using only mucosal or systemic routes for immunization. Our results support findings from studies using combined systemic and mucosal combined routes of immunization [38, 56, 58, 59]. It will now be important to test the effectiveness of combining systemic and mucosal routes of immunization to provide protection, against ocular and genitourinary and gastrointestinal tract chlamydial infections using the same vaccine formulation tested here.
Acknowledgments
This work was supported by the Public Health Service grant AI 067888 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miller WC, Ford CA, Morris M, Handcock MS, Schmitz JL, Hobbs MM, et al. Prevalence of chlamydial and gonococcal infections among young adults in the United States. Jama. 2004 May 12;291(18):2229–36. doi: 10.1001/jama.291.18.2229. [DOI] [PubMed] [Google Scholar]
- 2.Chlamydia screening among sexually active young female enrollees of health plans--United States, 2000–2007. MMWR Morb Mortal Wkly Rep. 2009 Apr 17;58(14):362–5. [PubMed] [Google Scholar]
- 3.Darville T. Chlamydia trachomatis genital infection in adolescents and young adults. Adv Exp Med Biol. 2006;582:85–100. doi: 10.1007/0-387-33026-7_8. [DOI] [PubMed] [Google Scholar]
- 4.Grayston JT, Wang SP. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978 Apr–Jun;5(2):73–7. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Schachter J, Dawson CR. Human chlamydial infections. Chicago: Publishing Science Group; 1978. [Google Scholar]
- 6.Schachter J, Dawson C. Human chlamydial infections. Littleton: PSG Publishing Co; 1978. [Google Scholar]
- 7.Stamm W. Chlamydia trachomatis infections of the adult. In: Holmes KKPS, Stamm WE, Piot P, Wasserheit JW, Corey L, Cohen MS, Watts DH, editors. Sexually transmitted diseases. New York: McGrawHill Book Co; 2008. pp. 575–93. [Google Scholar]
- 8.Westrom L, Joesoef R, Reynolds G, Hagdu A, Thompson SE. Pelvic inflammatory disease and fertility. A cohort study of 1,844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex Transm Dis. 1992 Jul–Aug;19(4):185–92. [PubMed] [Google Scholar]
- 9.Ness RB, Soper DE, Holley RL, Peipert J, Randall H, Sweet RL, et al. Effectiveness of inpatient and outpatient treatment strategies for women with pelvic inflammatory disease: results from the Pelvic Inflammatory Disease Evaluation and Clinical Health (PEACH) Randomized Trial. Am J Obstet Gynecol. 2002 May;186(5):929–37. doi: 10.1067/mob.2002.121625. [DOI] [PubMed] [Google Scholar]
- 10.Ness RB, Smith KJ, Chang CC, Schisterman EF, Bass DC. Prediction of pelvic inflammatory disease among young, single, sexually active women. Sex Transm Dis. 2006 Mar;33(3):137–42. doi: 10.1097/01.olq.0000187205.67390.d1. [DOI] [PubMed] [Google Scholar]
- 11.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis. 2005 Nov 15;192(10):1836–44. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 12.Hickey DK, Aldwell FE, Tan ZY, Bao S, Beagley KW. Transcutaneous immunization with novel lipid-based adjuvants induces protection against gastric Helicobacter pylori infection. Vaccine. 2009 Nov 23;27(50):6983–90. doi: 10.1016/j.vaccine.2009.09.078. [DOI] [PubMed] [Google Scholar]
- 13.Somani J, Bhullar VB, Workowski KA, Farshy CE, Black CM. Multiple drug-resistant Chlamydia trachomatis associated with clinical treatment failure. J Infect Dis. 2000 Apr;181(4):1421–7. doi: 10.1086/315372. [DOI] [PubMed] [Google Scholar]
- 14.Wang SP, Grayston JT, Alexander ER. Trachoma vaccine studies in monkeys. Am J Ophthalmol. 1967 May;63(5 Suppl):1615–30. doi: 10.1016/0002-9394(67)94155-4. [DOI] [PubMed] [Google Scholar]
- 15.Grayston JT, Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- 16.Brunham RC, Rey-Ladino J. Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol. 2005 Feb;5(2):149–61. doi: 10.1038/nri1551. [DOI] [PubMed] [Google Scholar]
- 17.Grayston JT, Wang SP, Yeh LJ, Kuo CC. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985 Nov–Dec;7(6):717–25. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 18.Caldwell HD, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1981 Mar;31(3):1161–76. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrison SG, Su H, Caldwell HD, Morrison RP. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4(+) T cells but not CD8(+) T cells. Infect Immun. 2000 Dec;68(12):6979–87. doi: 10.1128/iai.68.12.6979-6987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brunham RC, Peeling RW. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994 Oct;3(5):218–33. [PubMed] [Google Scholar]
- 21.Cheng C, Bettahi I, Cruz-Fisher MI, Pal S, Jain P, Jia Z, et al. Induction of protective immunity by vaccination against Chlamydia trachomatis using the major outer membrane protein adjuvanted with CpG oligodeoxynucleotide coupled to the nontoxic B subunit of cholera toxin. Vaccine. 2009 Oct 19;27(44):6239–46. doi: 10.1016/j.vaccine.2009.07.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pal S, Peterson EM, de la Maza LM. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect Immun. 2005 Dec;73(12):8153–60. doi: 10.1128/IAI.73.12.8153-8160.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal S, Theodor I, Peterson EM, de la Maza LM. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect Immun. 2001 Oct;69(10):6240–7. doi: 10.1128/IAI.69.10.6240-6247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009 Jun 15;182(12):8063–70. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun G, Pal S, Weiland J, Peterson EM, de la Maza LM. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine. 2009 Aug 6;27(36):5020–5. doi: 10.1016/j.vaccine.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de la Maza MA, de la Maza LM. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine. 1995 Jan;13(1):119–27. doi: 10.1016/0264-410x(95)80022-6. [DOI] [PubMed] [Google Scholar]
- 27.Nigg C. An Unidentified Virus Which Produces Pneumonia and Systemic Infection in Mice. Science. 1942 Jan 9;95(2454):49–50. doi: 10.1126/science.95.2454.49-a. [DOI] [PubMed] [Google Scholar]
- 28.Pal S, Fielder TJ, Peterson EM, de la Maza LM. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect Immun. 1994 Aug;62(8):3354–62. doi: 10.1128/iai.62.8.3354-3362.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marston FA. The purification of eukaryotic polypeptides synthesized in Escherichia coli. Biochem J. 1986 Nov 15;240(1):1–12. doi: 10.1042/bj2400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blake MS, Gotschlich EC. Purification and partial characterization of the opacity-associated proteins of Neisseria gonorrhoeae. J Exp Med. 1984 Feb 1;159(2):452–62. doi: 10.1084/jem.159.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi HL, Tai JY, Blake MS. Expression of large amounts of neisserial porin proteins in Escherichia coli and refolding of the proteins into native trimers. Infect Immun. 1994 Jun;62(6):2432–9. doi: 10.1128/iai.62.6.2432-2439.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 33.Song JH, Nguyen HH, Cuburu N, Horimoto T, Ko SY, Park SH, et al. Sublingual vaccination with influenza virus protects mice against lethal viral infection. Proc Natl Acad Sci U S A. 2008 Feb 5;105(5):1644–9. doi: 10.1073/pnas.0708684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amorij JP, Westra TA, Hinrichs WL, Huckriede A, Frijlink HW. Towards an oral influenza vaccine: comparison between intragastric and intracolonic delivery of influenza subunit vaccine in a murine model. Vaccine. 2007 Dec 21;26(1):67–76. doi: 10.1016/j.vaccine.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 35.McConnell EL, Basit AW, Murdan S. Colonic antigen administration induces significantly higher humoral levels of colonic and vaginal IgA, and serum IgG compared to oral administration. Vaccine. 2008 Jan 30;26(5):639–46. doi: 10.1016/j.vaccine.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 36.Weeratna RD, McCluskie MJ, Xu Y, Davis HL. CpG DNA induces stronger immune responses with less toxicity than other adjuvants. Vaccine. 2000 Mar 6;18(17):1755–62. doi: 10.1016/s0264-410x(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 37.Peterson EM, Zhong GM, Carlson E, de la Maza LM. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect Immun. 1988 Apr;56(4):885–91. doi: 10.1128/iai.56.4.885-891.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srivastava I, Goodsell A, Zhou F, Sun Y, Burke B, Barnett S, et al. Dynamics of acute and memory mucosal and systemic immune responses against HIV-1 envelope following immunizations through single or combinations of mucosal and systemic routes. Vaccine. 2008 May 23;26(22):2796–806. doi: 10.1016/j.vaccine.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 39.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006 Feb;6(2):148–58. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 40.Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007 Apr 20;25(16):3001–6. doi: 10.1016/j.vaccine.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Riddiough MA, Sisk JE, Bell JC. Influenza vaccination. JAMA. 1983 Jun 17;249(23):3189–95. [PubMed] [Google Scholar]
- 42.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N Engl J Med. 2003 May 1;348(18):1737–46. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
- 43.Walker M, WS, CES, PR Genetics of anti-HBs responsiveness, I. HLA-DR7 and nonresponsiveness to hepatitis vaccination. Transfusion. 1981;21:601. [Google Scholar]
- 44.Lang J, Kamga-Fotso L, Peyrieux JC, Blondeau C, Lutsch C, Forrat R. Safety and immunogenicity of a new equine tetanus immunoglobulin associated with tetanus-diphtheria vaccine. Am J Trop Med Hyg. 2000 Nov–Dec;63(5–6):298–305. [PubMed] [Google Scholar]
- 45.Lin HH, Liao HW, Lin SK, Wang LY. HLA and response to booster hepatitis B vaccination in anti-HBs-seronegative adolescents who had received primary infantile vaccination. Vaccine. 2008 Jun 25;26(27–28):3414–20. doi: 10.1016/j.vaccine.2008.04.038. [DOI] [PubMed] [Google Scholar]
- 46.Schachter J. Infection and disease epidemiology. In: Stephens R, editor. Chlamydia: intracellular biology, pathogenesis and immunity. Washington: ASM; 1999. pp. 139–70. [Google Scholar]
- 47.Mestecky J, Moldoveanu Z, Smith PD, Hel Z, Alexander RC. Mucosal immunology of the genital and gastrointestinal tracts and HIV-1 infection. J Reprod Immunol. 2009 Dec;83(1–2):196–200. doi: 10.1016/j.jri.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mestecky J, Russell MW. Induction of mucosal immune responses in the human genital tract. FEMS Immunol Med Microbiol. 2000 Apr;27(4):351–5. doi: 10.1111/j.1574-695X.2000.tb01449.x. [DOI] [PubMed] [Google Scholar]
- 49.Mestecky J, Elson CO. Peyer's patches as the inductive site for IgA responses. J Immunol. 2008 Feb 1;180(3):1293–4. doi: 10.4049/jimmunol.180.3.1293. [DOI] [PubMed] [Google Scholar]
- 50.Kelly KA, Chan AM, Butch A, Darville T. Two Different Homing Pathways Involving Integrin beta7 and E-selectin Significantly Influence Trafficking of CD4 Cells to the Genital Tract Following Chlamydia muridarum Infection. Am J Reprod Immunol. 2009 Apr 22; doi: 10.1111/j.1600-0897.2009.00704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pal S, Peterson EM, de la Maza LM. Intranasal immunization induces long-term protection in mice against a Chlamydia trachomatis genital challenge. Infect Immun. 1996 Dec;64(12):5341–8. doi: 10.1128/iai.64.12.5341-5348.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004 Feb 26;350(9):896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 53.Cuburu N, Kweon MN, Hervouet C, Cha HR, Pang YY, Holmgren J, et al. Sublingual immunization with nonreplicating antigens induces antibody-forming cells and cytotoxic T cells in the female genital tract mucosa and protects against genital papillomavirus infection. J Immunol. 2009 Dec 15;183(12):7851–9. doi: 10.4049/jimmunol.0803740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuburu N, Kweon MN, Song JH, Hervouet C, Luci C, Sun JB, et al. Sublingual immunization induces broad-based systemic and mucosal immune responses in mice. Vaccine. 2007 Dec 12;25(51):8598–610. doi: 10.1016/j.vaccine.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 55.Ogra PL, Karzon DT. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–30. [PubMed] [Google Scholar]
- 56.Vajdy M, Singh M, Ugozzoli M, Briones M, Soenawan E, Cuadra L, et al. Enhanced mucosal and systemic immune responses to Helicobacter pylori antigens through mucosal priming followed by systemic boosting immunizations. Immunology. 2003 Sep;110(1):86–94. doi: 10.1046/j.1365-2567.2003.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnett SW, Srivastava IK, Kan E, Zhou F, Goodsell A, Cristillo AD, et al. Protection of macaques against vaginal SHIV challenge by systemic or mucosal and systemic vaccinations with HIV-envelope. Aids. 2008 Jan 30;22(3):339–48. doi: 10.1097/QAD.0b013e3282f3ca57. [DOI] [PubMed] [Google Scholar]
- 58.Vajdy M, Baudner B, Del Giudice G, O'Hagan D. A vaccination strategy to enhance mucosal and systemic antibody and T cell responses against influenza. Clin Immunol. 2007 May;123(2):166–75. doi: 10.1016/j.clim.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 59.Vajdy M, Singh M, Kazzaz J, Soenawan E, Ugozzoli M, Zhou F, et al. Mucosal and systemic anti-HIV responses in rhesus macaques following combinations of intranasal and parenteral immunizations. AIDS Res Hum Retroviruses. 2004 Nov;20(11):1269–81. doi: 10.1089/aid.2004.20.1269. [DOI] [PubMed] [Google Scholar]