Abstract
Despite the extensive data on dG-AAF, the major DNA adduct derived from the model carcinogen 2-acetylaminofluorene, little is known with respect to its solution structures. Here we provide NMR/CD evidence for three conformers of dG-AAF in duplex DNA: major groove (B), base-displaced stacked (S), and minor groove wedge (W). The S/B/W-conformational heterogeneities were found to be sensitive to the nature of the flanking DNA sequence contexts and pH.
Arylamines and their nitro derivatives are a major group of environmental mutagens, and have been implicated in the etiology of human cancers (1). In vivo metabolic activation of the prototype carcinogens 2-acetylaminofluorene (2) and 2-nitrofluorene (3) results in production of dG-AF1 and dG-AAF as major stable adducts (Fig. 1a) (4). Despite their structural similarities, the two DNA adducts exhibit distinct mutation and repair activities. dG-AF has been shown to adopt multiple conformers, giving rise to unique sequence-dependent mutation and repair outcomes (5, 6). However, very little is known about the structure-function relationships of dG-AAF, which is remarkable given the extensive in vivo and in vitro data that have accumulated related to AAF (3,277 items on PubMed Search for “acetylaminofluorene”).
Figure 1.
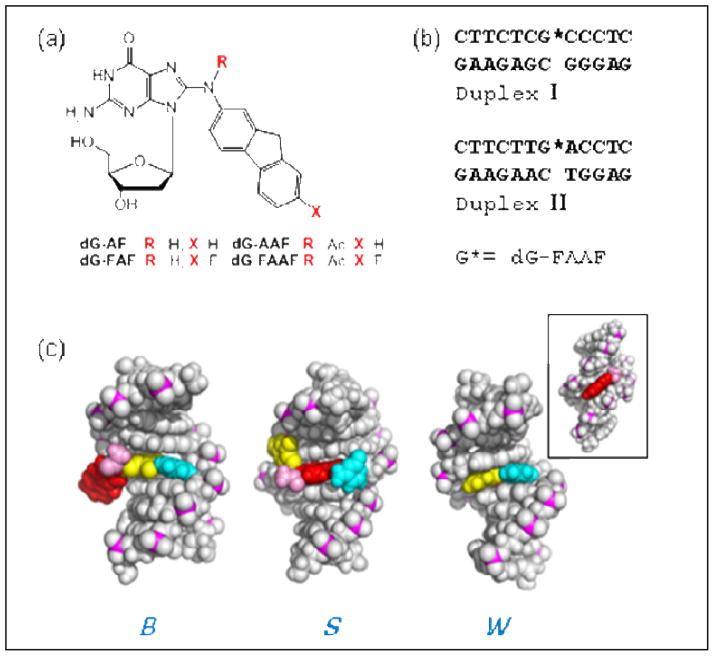
(a) Structures of dG-AF, dG-AAF and their fluoro models, dG-FAF, dG-FAAF; (b) 12-mer duplexes I and II used in the present study; (c) Views from the major-grooves of a duplex for three FAAF-induced conformational motifs: B, S, and W-conformers. Color code: modified G (yellow) and complementary C (cyan) at the lesion site; fluorene (red) and N-acetyl (pink). Inset shows the minor groove view of W-conformer.
In 1993, O’Handley et al. (7) reported that ~70% of an AAF-modified 9-mer duplex in the CG[AAF]C sequence context adopts a base-displaced S-conformer with stacking of the fluorene moiety into a double helix (Fig. 1c). The structure of the remaining 30% of the AAF-modified duplex has not been elucidated. Using an 19F NMR approach (8), we showed that a 12-mer duplex in the TG*A sequence context (Duplex II, G*=FAAF, Fig. 1b) exhibits ~40:60% 19F signals with an exclusive presence of the S-conformer, differing only in the N-acetyl group’s cis and trans conformation (9). This result contrasts with data from AF and FAF (Fig. 1a), which consistently adopt an S/B conformational heterogeneity (6, 10).
We here present new data that provides further insights on the conformers that contribute to the conformational mix of dG-AAF in solution. Specifically, our results are consistent with adoption by dG-AAF of a major groove B-type (B) and a minor groove “wedge” (W) conformer, in addition to the S-conformer elucidated by O’Handley et al. (7) (Fig. 1c), and that sequence context determines the population balance between the states. The B-type conformer has a glycosyl bond conformation that is anti, while S and W are syn. The representative torsion angles of the S-, B-, and W-conformers are summarized in Supplementary Table 1. Similar to dG-AF (6), the B-type dG-AAF maintains Watson-Crick hydrogen bonds, thereby placing the fluorenyl and the acetyl moieties in the major groove (Fig. 1c). Molecular dynamics simulations have shown that the B-type dG-AAF conformer can readily be accommodated in the active sites of bypass polymerases Dpo4 (11) and Pol iota (12). As observed in the S-conformer, the AAF-modified dG adopts the syn conformation; however, instead of having disrupted hydrogen bonding, the modified dG can pair with the complementary dC utilizing its Hoogsteen edge; this places the acetylamino moiety in the narrow minor groove (13)(Fig. 1c). Although this “wedge” W-conformer led to severe distortion of the DNA binding area in the active site of Pol iota (12), it is a distinct possibility in polymerase-free duplex DNA (13).
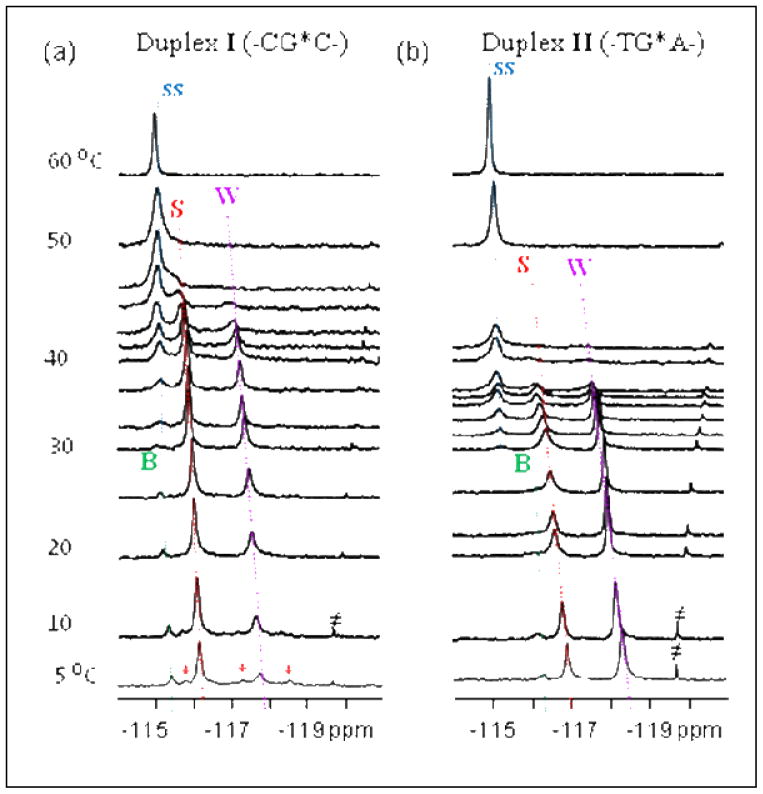
In the present study, we conducted spectroscopic studies to investigate the AAF-induced S/B/W-heterogeneity and sequence effects. The utility of 19F over 1H NMR for probing muti-conformeric DNA has been well documented (6,8,9,10). The imino proton spectra of the FAAF-modified 12-mer DNA duplexes I (CG*C) and II (TG*A)(Supplementary Fig. S2) exhibited at least 14 resolvable imino protons with varying degrees of intensity, indicating the presence of multiple conformations. In line with this interpretation, the 19F NMR spectrum of duplex I revealed prominent 19F signals at −115.45, −116.15, and −117.75 ppm at 5°C (Fig. 2a). Their intensity ratio was 5:65:30, which is in close agreement with the ~70%:30% ratios reported in the 1H NMR study cited above in the same CG*C sequence context (7). Accordingly, the major signal at −116.15 ppm was assigned to that arising from the S-conformer. Duplex II (Fig. 2b) exhibited three signals at −116.35, −116.95, and −118.35 ppm in a 5:30:65 ratios at 5°C. The conformational similarity of the two duplexes is evident from the temperature dependence of their 1D spectra (Fig. 2). While the three 19F signals in each duplex were in slow exchange at 5°C, they became exchange broadened, giving rise to coalescent signals at 50°C and 42°C for I (−115.0 ppm) and II (−115.1 ppm), respectively. The strong temperature dependence of off-diagonal peaks in the contour plots of EXSY spectra (Supplementary Fig. S4) confirmed that these signals arise via chemical exchange from three slowly exchanging conformations. This contrasts with the lack of chemical exchange reported previously for the same FAAF-duplex, which led to the proposition that the exclusive S-conformer FAAF molecules may differ only in their relative acetyl group orientations, cis (γ′~180°) or trans (γ′~0°)(9).
Figure 2.
Temperature dependence of 1D 19F NMR (−114 ~ −121 ppm) spectra of the FAAF-modified duplex (a) I and (b) II recorded in 10% H2O buffer at pH 6.8. ss (single strand, blue), B (green), S (red), W (purple) conformers. ≠ impurity.
Owing to the similarity in flanking sequence and population contexts, we previously assigned the major center signal at −116.15 ppm in the spectrum of duplex I as that of the S-conformer (Fig. 2a). Consequently, the similar signal in the spectrum of the TG*A duplex II in Figure 2b could likewise be assigned to the S-conformer. It was observed that the chemical shift range of the upfield signals (at −177.8 and −118.3 ppm, respectively for I and II) falls into the range (−118 ~ −119 ppm) commonly observed for dA-mismatched W-conformer FAF-duplexes (14). As with FAF, the FAAF-induced S/W equilibrium is expected to occur readily since both conformers maintain very close χ, α′, and β′ torsion angles. The EXSY data in Supplementary Fig. S4 revealed a facile exchange between the S- and W-conformers. Taken together, the upfield signals were assigned as arising from the W-conformation (see below for further elaboration). Finally, the minor, but persistent, downfield signal (at −115.45 and −116.35 ppm, respectively for duplex I and II) was assigned to the B-conformer. The modest downfield shift (−0.80 ppm) relative to the S-conformer can be attributed to the lack of ring-current effects in the major groove (8). Also noted is the signal’s close proximity in chemical shift to the denatured FAAF-modified single strand 12-mer oligonucleotide (i.e., at 60°C)(6). We were unable to assign other minor conformers (marked as ‘*’, particularly in Duplex I, Fig. 2a) detected at 5°C. In principle, the dG-AAF adduct can adopt additional conformations depending on the flexible rotation of the fluorenyl-nitrogen (β′) and amide (γ′) bonds (13). However, the available data did not allow us to differentiate them into specific β′ and γ′ rotamers.
The W-conformer described above adopts a syn-dG[FAAF]:anti-dC alignment at the lesion site using the Hoogstein edge of the dG. As shown in Figure 3b, such a scenario might involve hydrogen bonds of the O6 of guanine with either the N3-protonated cytosine alone, or jointly with one of the exocyclic amino protons, or the cytosine amino proton alone. If the N3 protonation of cytosine were required, the solution pH would alter the stability of the W-conformer and thus its relative population. The pKa of cytosine has been shown to increase to as high as 8 in certain double and triple helices containing Hoogsteen type base pairs (15). Norman et al. (16) have proposed a stable syn-dG[AF]:anti-dA+ alignment in acidic pHs, which provides a basis for G→T mutations observed in vivo.
Figure 3.
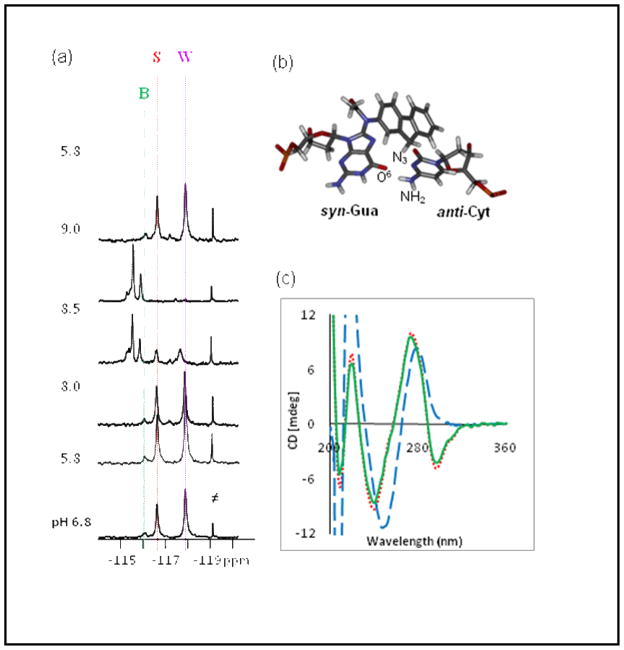
pH dependence of (a) fluorine NMR (5°C, ≠ impurity) and (b) CD spectra (20°C)[pH 7.0 (red); pH 7.0→9.0 (blue); pH 7.0→9.0 →7.0 (green)] of the FAAF-modified duplex II. c) a Hoogsteen-type syn-dG[FAAF]:anti-dC alignment.
To gain further insight into the structure, we performed 1H and 19F NMR assessments of FAAF-duplex II as function of pH. As shown in Supplementary Fig. S5a, there was no change in the imino spectrum between pH 6.7 and 5.8. At pH 8.0, base-catalysis caused fast exchange of the imino protons at or near the lesion site as well as frayed terminal ends. The exchange was almost complete at pH 9.0. However, the original imino protons reappeared after the solution was titrated back to pH 5.8. In line with the imino case, the 19F signal pattern was stable in the pH range of 5.8 to 8.0 (Fig. 3a). However, a new set of signals appeared in the −115 ~ −116 ppm region at the expense of the original B/S/W signals seen at neutral pH. The conformational transition was nearly complete at pH 9.0. As in the imino spectra, the original 19F NMR pattern signals could be titrated back at pH 5.8. Our NMR data seem to argue against the presence of any of the Hoogsteen-type hydrogen bonding scenarios depicted in Figure 3b. First, there were no proton signals above the 15.0 ppm and 9.0–11.0 ppm range, where the N3-protonated cytosines and the exocyclic amino protons involved in base pairing are supposed to appear (17). Second, we observed neither a significant change in imino spectral patterns nor the S/W population ratios as the pH was shifted from 5.8 to 8.0. Based on these results, we conclude that dG-FAAF may exist in a Hoogsteen type alignment with anti-dC, but not involving any hydrogen bonds.
Figure 3c shows the pH dependence of the CD spectra of FAAF-duplex II. At neutral pH (7.0), the duplex exhibited major positive and negative ellipticities around 275 and 240 nm, respectively, which is indicative of a typical B-form DNA duplex. The major negative dip in the 280–320 nm range presumably arises from the unique interaction between the acetylaminofluorene chromophore and DNA. We previously reported similarly induced CD data (ICD290–350nm) for dG-AF and dG-FAF adducts: positive for an S- or W-type conformation and negative for a B-type conformation (18). At pH 9.0, the negative ICD280–320 nm pattern disappeared while the S-shape CDs at 240 nm and 275 nm were shifted to longer wavelengths by 11 and 5 nm, respectively. These red shifts indicate that there has been a substantial disruption of the regular B-form double helix and appear to be in good agreement with the appearance of a new set of conformational heterogeneities observed in the 19F NMR spectrum at the same pH (Fig. 3a). Although we do not know the structural details of this new set of conformers, they clearly originated from the FAAF-S/W/B conformers and are stable even with the Watson-Crick imino protons fully exchanged. As in the NMR experiments, the FAAF-induced conformational heterogeneity was fully titratable in CD experiments.
In conclusion, we have provided 19F NMR/ICD evidence showing that the dG-AAF adduct in duplex DNA exists in an equilibrium of S-, B-, and W-conformers with their population ratios varying significantly depending on the flanking sequence context. Our data also revealed that AAF-induced heterogeneity is unaltered from a pH of ~5.8 to a pH of ~8.0, but transforms into a new set of conformations in a highly basic pH (8.5 ~ 9.0). These new findings concerning the conformers that contribute to the structural heterogeneity adopted by dG-AAF should be useful in interpreting nucleotide excision repair data (19) which shows that sequence context plays a role in determining the relative excision susceptibility (20).
Supplementary Material
Acknowledgments
This research was supported by NIH grant CA098296 and the RI-INBRE Research Core Facility NIH/P20 RR016457). We thank Dr. Paul M. Chiarelli for providing mass spectral characterization of modified oligonucleotides.
Footnotes
ABBREVIATIONS: dG-AAF, [N-(2′-deoxyguanosin-8-yl)-2-acetylaminofluorene]; dG-AF, [N-(2′-deoxyguanosin-8-yl)-2-aminofluorene]; dG-FAAF, [N-(2′-deoxyguanosin-8-yl)-7-fluoro-2-acetyl-aminofluorene]; dG-FAF, [N-(2′-deoxyguanosin-8-yl)-7-fluoro-2-aminofluorene]; EXSY, exchange correlation spectroscopy; ICD, induced circular dichroism.
Supporting Information Available. Experimental details of synthesis and spectroscopic measurements; imino proton spectra, UV-melting curves. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Neumann HG. Aromatic amines in experimental cancer research: tissue-specific effects, an old problem and new solutions. Crit Rev Toxicol. 2007;37:211–236. doi: 10.1080/10408440601028603. [DOI] [PubMed] [Google Scholar]
- 2.Heflich RH, Neft RE. Genetic toxicity of 2-acetylaminofluorene, 2-aminofluorene and some of their metabolites and model metabolites. Mutation Research/Reviews in Genetic Toxicology. 1994;318:73–174. doi: 10.1016/0165-1110(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 3.Cui XS, Eriksson LC, Moller L. Formation and persistence of DNA adducts during and after a long-term administration of 2-nitrofluorene. Mutat Res. 1999;442:9–18. doi: 10.1016/s1383-5718(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 4.Beland FA, Kadlubar FF. Handbook of Experimental Pharmacology. Spring-Verlag; Heidelberg: 1990. Metabolic activation and DNA adducts of aromatic amines and nitroaromatic hydocarbons; pp. 267–325. [Google Scholar]
- 5.Shibutani S, Suzuki N, Tan X, Johnson F, Grollman AP. Influence of Flanking Sequence Context on the Mutagenicity of Acetylaminofluorene-Derived DNA Adducts in Mammalian Cells. Biochemistry. 2001;40:3717–3722. doi: 10.1021/bi0027581. [DOI] [PubMed] [Google Scholar]
- 6.Meneni SR, Shell SM, Gao L, Jurecka P, Lee W, Sponer J, Zou Y, Chiarelli MP, Cho BP. Spectroscopic and theoretical insights into sequence effects of aminofluorene-induced conformational heterogeneity and nucleotide excision repair. Biochemistry. 2007;46:11263–11278. doi: 10.1021/bi700858s. [DOI] [PubMed] [Google Scholar]
- 7.O’Handley SF, Sanford DG, Xu R, Lester CC, Hingerty BE, Broyde S, Krugh TR. Structural characterization of an N-acetyl-2-aminofluorene (AAF) modified DNA oligomer by NMR, energy minimization, and molecular dynamics. Biochemistry. 1993;32:2481–2497. doi: 10.1021/bi00061a005. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Rajabzadeh M, Traficante DD, Cho BP. Conformational Heterogeneity of Arylamine-Modified DNA: 19F NMR Evidence. J Am Chem Soc. 1997;119:5384–5389. [Google Scholar]
- 9.Cho BP, Zhou L. Probing the conformational heterogeneity of the acetylaminofluorene-modified 2′-deoxyguanosine and DNA by 19F NMR spectroscopy. Biochemistry. 1999;38:7572–7583. doi: 10.1021/bi990182d. [DOI] [PubMed] [Google Scholar]
- 10.Cho B. In: Structure-function characteristics of aromatic amine-DNA adducts in The Chemical Biology of DNA Damage. Geacintov NA, Broyde S, editors. Wiley; 2010. pp. 217–238. [Google Scholar]
- 11.Wang L, Broyde S. A new anti conformation for N-(deoxyguanosin-8-yl)-2-acetylaminofluorene (AAF-dG) allows Watson-Crick pairing in the Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4) Nucleic Acids Res. 2006;34:785–795. doi: 10.1093/nar/gkj479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donny-Clark K, Shapiro R, Broyde S. Accommodation of an N-(deoxyguanosin-8-yl)-2-acetylaminofluorene adduct in the active site of human DNA polymerase iota: Hoogsteen or Watson-Crick base pairing? Biochemistry. 2009;48:7–18. doi: 10.1021/bi801283d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shapiro R, Hingerty BE, Broyde S. Minor-groove binding models for acetylaminofluorene modified DNA. J Biomol Struct Dyn. 1989;7:493–513. doi: 10.1080/07391102.1989.10508506. [DOI] [PubMed] [Google Scholar]
- 14.Jain N, Meneni S, Jain V, Cho BP. Influence of flanking sequence context on the conformational flexibility of aminofluorene-modified dG adduct in dA mismatch DNA duplexes. Nucleic Acids Res. 2009;37:1628–1637. doi: 10.1093/nar/gkn1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sugimoto N, Wu P, Hara H, Kawamoto Y. pH and cation effects on the properties of parallel pyrimidine motif DNA triplexes. Biochemistry. 2001;40:9396–9405. doi: 10.1021/bi010666l. [DOI] [PubMed] [Google Scholar]
- 16.Norman D, Abuaf P, Hingerty BE, Live D, Grunberger D, Broyde S, Patel DJ. NMR and computational characterization of the N-(deoxyguanosin-8-yl)aminofluorene adduct [(AF)G] opposite adenosine in DNA: (AF)G[syn]. A[anti] pair formation and its pH dependence. Biochemistry. 1989;28:7462–7476. doi: 10.1021/bi00444a046. [DOI] [PubMed] [Google Scholar]
- 17.Roll C, Ketterl C, Fazakerley GV, Boulard Y. Solution structures of a duplex containing an adenine opposite a gap (absence of one nucleotide). An NMR study and molecular dynamic simulations with explicit water molecules. Eur J Biochem. 1999;264:120–131. doi: 10.1046/j.1432-1327.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 18.Liang F, Meneni S, Cho BP. Induced circular dichroism characteristics as conformational probes for carcinogenic aminofluorene-DNA adducts. Chem Res Toxicol. 2006;19:1040–1043. doi: 10.1021/tx0601253. [DOI] [PubMed] [Google Scholar]
- 19.Luo C, Krishnasamy R, Basu AK, Zou Y. Recognition and incision of site-specifically modified C8 guanine adducts formed by 2-aminofluorene, N-acetyl-2-aminofluorene and 1-nitropyrene by UvrABC nuclease. Nucleic Acids Res. 2000;28:3719–3724. doi: 10.1093/nar/28.19.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu D, Bertrand-Burggraf Huang J-C, Fuchs RPP, Sancar A. Human and E. coli excinucleases are affected differently by the sequence context of acetylaminofluorene-guanine adduct. Nucleic Acids Res. 1994;22:4869–4871. doi: 10.1093/nar/22.23.4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.