Abstract
Tyrosine phosphatase TpbA in Pseudomonas aeruginosa PA14 is a negative regulator of the diguanylate cyclase TpbB. Inactivation of TpbA caused rugose colony morphology which is related to cell persistence in clinical infections. We show here that TpbA is a dual specific tyrosine phosphatase, that TpbB is phosphorylated, and that TpbA controls phosphorylation of TpbB at both Tyr and Ser/Thr residues in vivo as detected by Western blot analysis. In addition, TpbB is demonstrated to be a substrate of TpbA in vitro using purified enzymes. Thus, TpbA controls the rugose morphology in P. aeruginosa by dephosphorylating TpbB.
Keywords: tyrosine phosphatase, diguanylate cyclase, rugose colony
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen that is responsible for many biofilm infections including those associated with ventilator-associated pneumonia, urinary and peritoneal dialysis catheters, bacterial keratitis, otitis externa, burns, and lungs [1]. Persistence of this bacterium is linked to its ability to form biofilms [2] and rugose small-colony variants (RSCVs) [3] which are characterized by wrinkled, small colonies and an elevated capacity to form biofilms.
In P. aeruginosa PA14, we found previously that tpbA (PA3885) encodes a conserved tyrosine phosphatase (tyrosine phosphatase related to biofilm formation) that when inactivated, converts the smooth wild-type strain into an RSCV, with dramatically increased biofilm formation, attachment, and aggregation along with decreased swimming and no swarming [4]. The mechanism of the RSCV formation in the tpbA mutant is linked to increased 3,5-cyclic diguanylic acid (c-di-GMP) that results in elevated activity of the pel polysaccharide locus [4]; this result was the first link between bacterial tyrosine phosphatase activity and c-di-GMP formation. Furthermore, TpbA increases extracellular DNA production by decreasing c-di-GMP concentrations [5]. c-di-GMP is an ubiquitous intracellular second messenger that acts as a central regulator in bacterial physiology, especially in regulating the transition between motile and sessile states [6]. c-di-GMP is synthesized from two molecules of guanosine-5'-triphosphate (GTP) by diguanylate cyclases (DGCs) that contain GGDEF domains, and degraded by phosphodiesterases (PDEs) that contain EAL or HD-GYP domains [6]. PA14 has 37 putative c-di-GMP related proteins, including 16 proteins with a DGC domain, 5 with a PDE domain, and 16 that contain both domains [7]. Critically, genetic screening indicated that inactivation of tpbB (PA1120, yfiN), which encodes an active DGC [7], suppressed the phenotype observed in the tpbA mutant [4]. This implies that TpbA regulates c-di-GMP concentrations through TpbB. Our original results for the role of TpbB in RSCV morphology formation were recently verified by an independent group that corroborated that TpbB is important for persistence related to cystic fibrosis [8]. However, how TpbA regulates TpbB has not been elucidated yet.
In this study, we demonstrate that TpbA dephosphorylates TpbB in vivo. In addition, we show that TpbB serves as the substrate of TpbA in vitro, providing direct evidence of TpbA/TpbB interactions.
MATERIALS AND METHODS
Cell Culture
Strains used in this study are listed in Table 1. Pseudomonas aeruginosa PA14 (wild-type) and its isogenic mutants were obtained from the Harvard Medical School [9]. P. aeruginosa and Escherichia coli were routinely grown in Luria-Bertani (LB) medium [10] at 37°C unless noted. Gentamicin (15 μg/mL) was used for growth of the P. aeruginosa tpbA transposon mutant, carbenicillin (300 μg/mL) was used to maintain pMQ70-tpbB, and 50 μg/mL kanamycin was used to maintain plasmid pET28b-tpbA.
Table 1.
Strains and plasmids used in this study. GmR, KmR, CarR, and ApR indicate gentamicin, kanamycin, carbenicillin, and ampicillin resistance, respectively.
| Strains and plasmids | Relevant genotype | Source |
|---|---|---|
| P. aeruginosa | ||
| PA14 | Wild-type strain | [9] |
| tpbA | PA14_13660 Ω Mar2xT7, GmR | [9] |
| E. coli | ||
| BL21(DE3) | F−ompT hsdSB(rB−mB−) gal dcm λ(DE3) Ω placUV5∷ T7 polymerase | Novagen |
| Plasmid | ||
| pMQ70-tpbB | PBAD∷tpbB, complementation plasmid, CarR, ApR | [4] |
| pET28b-tpbA | PT7∷tpbA-cHis+, expression vector for TpbA-cHis, KmR | [4] |
Protein Purification
Recombinant TpbA with a 6X-His tag at the carboxy terminus (TpbA-cHis) was produced in E. coli BL21 (DE3) with plasmid pET28b-tpbA by inducing with 1 mM IPTG and was purified using Ni-NTA resin as described previously [4]. The purified TpbA-cHis was dialyzed against buffer (50 mM Tris-HCl, pH 7.5, 100 mM NaCl, and 10% glycerol) at 4°C overnight and concentrated using a 10 kDa cut-off centrifugal filter unit (Millipore, Billerica, MA).
To purify TpbB, tpbA was transformed with pMQ70-tpbB [4] and used to produce the full length TpbB with a 6X-His tag at the carboxy terminus (TpbB-cHis). After 36 h of incubation from a single colony, one mL was used to inoculate 1 L of LB medium supplemented with 300 μg/mL carbenicillin and 0.05% arabinose; this culture was incubated at 37°C with shaking. Early stationary-phase cells were harvested by centrifugation at 8000 × g for 10 min at 4°C. Cells were resuspended in 20 mL lysis buffer (50 mM Tris-HCl, pH 8.0) with phosSTOP phosphatase inhibitor cocktail (Roche, Indianapolis, IN) and 100 μl protease inhibitor cocktail (Sigma, St. Louis, MO). Cells were disrupted twice by a French Press (Thermo Electron Corporation Waltham, MA). Non-soluble cellular debris was removed by centrifuging twice at 15,000 × g for 30 min. The whole cell lysate was centrifuged at 100,000 × g for 1 h at 4°C to separate the soluble fraction from the total membrane fraction [11]. The membrane fraction was re-suspended in 4 mL purification buffer (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 10% glycerol, 1.5% TX-100 with Roche phosSTOP phosphatase inhibitor cocktail and 10 μl Sigma protease inhibitor cocktail) and incubated on ice overnight. TpbB-cHis was then purified using Ni-NTA agarose resin (Qiagen, Valencia, CA) as described by the manufacturer's protocol. The protein was eluted with purification buffer supplemented with 100 mM imidazole. Protein concentrations were assayed by the BCA assay (Pierce, Rockford, IL).
Western Blot
Two sets of Western blot experiments were performed. The first was to detect phosphorylation of TpbB in vivo. In this experiment, whole membrane TpbB proteins from PA14/pMQ70-tpbB and tpbA/pMQ70-tpbB were isolated from early stationary phase cultures as described in the protein purification section. Then the membrane protein was re-suspended in lysis buffer containing 1.5% TX-100, and the protein concentration was assayed by the BCA assay. The same amount of membrane protein (2 μg) was loaded into each well of a 10% SDS-PAGE gel, then transferred to a PVDF membrane, which was then blocked with 4% BSA in TBST (10 mM Tris pH 7.5, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. The blot was incubated with a 1:1000 dilution of anti-phosphotyrosine antibody 4G10 (Millipore) or anti-phosphoserine/threonine antibody (BD Science, Franklin Lakes, NJ) at 4 °C for overnight. After washing three times with TBST, the membrane was incubated with horseradish peroxidase-conjugated goat anti-mouse IgG antibody (Cell signaling Technology, Danvers, MA) at room temperature for 1 h and detected by Super Signal Pico substrate (Pierce) after washing with TBST. Then the blot was stripped to remove the antibodies, blocked again, and re-probed with 1:2000 dilution of His-tag antibody (Cell Signaling Technology, Danvers, MA) using the same procedure. The other Western blot analysis was performed to detect phosphorylation of purified TpbB samples. In this experiment, 300 ng of purified TpbB were loaded into each well of a SDS-PAGE gel instead of using the whole membrane fraction.
TpbA Phosphatase Assay
To examine whether TpbA is a dual specific tyrosine phosphatase, TpbA activity was checked using the tyrosine phosphopeptide END[pY]INASL (Promega, Madison, WI) and the threonine phosphopeptide KR[pT]IRR (Millipore) using the Tyrosine Phosphatase Assay System (Promega). TpbA (10 μg) was incubated with 50 μM of peptide in 50 μl of reaction buffer (10 mM Tris-Acetate, pH 5.5, 10 mM MgCl2) for 2 h at 37°C. The reaction was quenched by adding 50 μl of a molybdate dye solution and incubating at room temperature for 30 min. Released phosphate was quantified by measuring the absorbance at 630 nm.
To check whether TpbB is the substrate of TpbA, the buffer for purified TpbB was changed to 50 mM Tris-acetate, pH 5.5 with 0.1% TX-100 using a 30 kDa centrifuge filter unit. Then equal amounts of TpbA and TpbB were incubated at 37°C in reaction buffer for 2 h. The reaction was quenched by adding SDS-loading dye and heating at 95°C for 10 min. For the control, TpbB (3 μg) was treated using the same procedure but without adding TpbA.
c-di-GMP Phosphodiesterase Assay
To check whether TpbA is a phosphodiesterase which degrades c-di-GMP, PDE activity was assayed as previously described [12] using HPLC to quantify c-di-GMP concentrations. Purified TpbA (3 μg) was incubated with 0.1 mM c-di-GMP (BIOLOG Life Science Institute, Bremen, Germany) in 50 μl of reaction buffer (50 mM Tris-HCl, pH 9.0 or 50 mM Tris-Acetate, pH 5.5, with 50 mM NaCl, 0.5 mM EDTA, 5 mM DTT, and 5 mM MgCl2) at 37 °C for 1 h, and the reaction was quenched by adding 10 mM CaCl2 followed by heating at 98°C for 10 min. The reaction products were analyzed by HPLC using a C18 reverse-phase column (150*3.9 cm, 4 μm, Nova-Pak, Waters) and nucleotides were detected at a wavelength of 254 nm. Phosphodiesterase YahA from E. coli was used as a positive control and used to generate 5′-phosphoguanylyl-(3′→5′)-guanosine [12] from c-di-GMP. Guanosine monophosphate was obtained from Sigma.
TpbB Diguanylate Cyclase Assay
TpbB diguanylate cyclase activity was assayed by 31P NMR in vitro. The reaction was initiated by the addition of 10 μg of TpbB into a 400 μl reaction mixture (5 mM GTP in 50 mM Tris, pH 8.0, 50 mM NaCl, 5 mM MgCl2, 10 mM TX-100, and 5 mM DTT containing 30% D2O) in the NMR tube. 31P NMR spectra were recorded using a Varian INOVA 400 spectrometer. The spectra were accumulated with proton decoupling, 90° pulses, an acquisition time of 1.6 s, first delay of 1.0 s, and with the temperature maintained at 37°C throughout the experiment. Phosphoric acid (85%) was used as an external standard for the chemical shift at 0 ppm. The reaction mixture was kept in the probe of the instrument during the experiment, while spectra were recorded at various time intervals. As a negative control, the same amount of TpbB was denatured by heating at 95°C for 10 min before adding to the reaction mixture. The product c-di-GMP concentration was calculated as the percentage of the integrated intensity of the substrate peak by comparing the sum of the integrated intensities of the substrate and product peaks in each spectrum.
RESULTS
TpbA is a dual specific tyrosine phosphatase
We initially determined that TpbA is a negative regulator of c-di-GMP [4]. Here we checked whether the increase in c-di-GMP seen upon inactivating TpbA is due to TpbA acting as a phosphodiesterase. Recombinant TpbA with a 6X-His tag at the carboxy terminus was produced in E. coli BL21 (DE3) and purified using a Ni-NTA column as described previously [4]. The purified TpbA was confirmed to be active by using a general phosphatase assay with the substrate p-nitrophenyl phosphate (pNPP). Sequence alignment of TpbA with known PDEs indicates that TpbA does not contain either an EAL motif [12] or HD-GYP motif [13]. Indeed, by assaying for PDE activity with the purified TpbA using high-performance liquid chromatography (HPLC) to quantify c-di-GMP concentrations [12], we found c-di-GMP was not degraded by TpbA while under the same reaction conditions, all the c-di-GMP was converted to 5′-phosphoguanylyl-(3′→5′)-guanosine (5'-pGpG) by E. coli phosphodiesterase YahA [12]. Hence, the increased c-di-GMP level upon inactivating TpbA [4] should be the result of activation of the diguanylate cyclase activity of TpbB, rather than TpbA itself acting as a PDE.
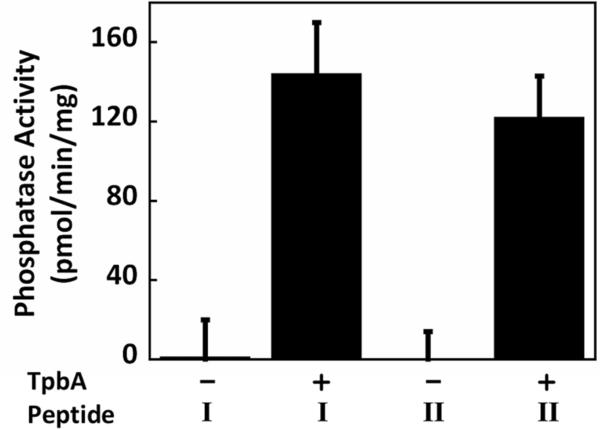
We have previously demonstrated that TpbA has protein tyrosine phosphatase activity toward both pNPP and two phosphotyrosine peptides [4]. We have extended our initial results here to discover that TpbA has phosphatase activity for both phosphorylated Ser/Thr as well as Tyr residues. As shown in Fig. 1, TpbA has phosphatase activity toward both a tyrosine phosphopeptide END(pY)INASL and a threonine phosphopeptide KR(pT)IRR at similar rates, 144 ± 26 pmol/(min·mg) and 122 ± 21 pmol/(min·mg), respectively. Hence, TpbA is a dual specific tyrosine phosphatase, which is consistent with high sequence similarities of TpbA with other dual specific phosphatases [14].
Figure 1.
TpbA phosphatase activity toward synthetic phospho-peptides. Peptide I is phospho-tyrosine peptide END(pY)INASL and Peptide II is phospho-threonine peptide KR(pT)IRR. Reactions were performed with 10 μg of TpbA and 50 μM peptides for 2 h at 37°C. Error bars represent the standard deviation from two replicates.
TpbA dephosphorylates TpbB directly or indirectly
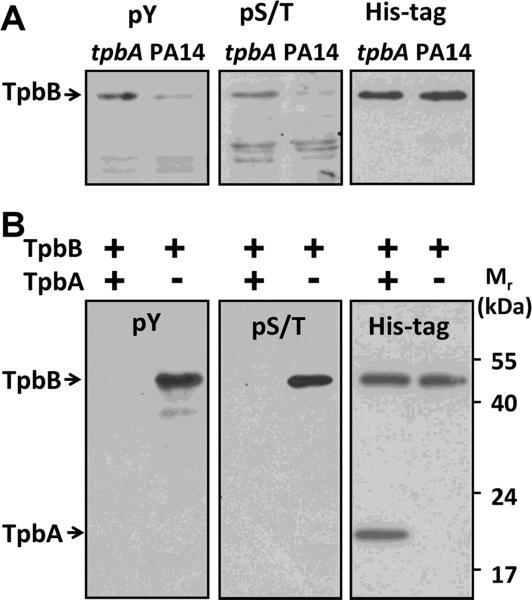
We next addressed how TpbB activity is negatively regulated by the protein phosphatase TpbA by examining the level of TpbB phosphorylation in the presence and absence of TpbA. To examine the level of TpbB phosphorylation in whole cells, we compared the phosphorylation state of TpbB in a PA14 wild-type host vs. its phosphorylation state with TpbA inactivated via the tpbA mutation using a Western blot analysis with antibodies specific for phosphor-Tyr or phosphor-Ser/Thr residues. Since TpbB is an integral membrane protein, only the membrane protein portion of the whole cell lysate was used to reduce the complexity of the Western analysis. As shown in Fig. 2A, TpbB was found to be phosphorylated at both Tyr residues and Ser/Thr residues; this is the first report of the phosphorylation of TpbB. In addition, there was greater Ser/Thr phosphorylation as well as greater Tyr phosphorylation for PA14 cells with inactivated tpbA compared to wild-type cells. These results indicate that TpbA regulates the in vivo activity of TpbB either directly or indirectly through the level of TpbB phosphorylation; inactivation of the phosphatase TpbA results in greater phosphorylation of TpbB and thus higher DGC activities with increased c-di-GMP concentrations.
Figure 2.
Western blot analysis of TpbB phosphorylation in vivo and in vitro. (A) Whole membrane proteins were isolated from PA14 wild-type cells and from the tpbA mutant. TpbB with a C-terminal His-tag was produced in these cells by the pMQ70-tpbB plasmid. Proteins in the blot were detected by a phospho-Tyr antibody 4G10 (pY) or a phospho-Ser/Thr antibody (pS/T). The same membrane was then stripped and proteins were detected by a His-tag antibody to confirm the same amount of TpbB was produced and loaded for each sample as shown in the right blot.
(B) TpbB was incubated with or without TpbA at 37°C for 30 min; the reaction was quenched by adding SDS-loading dye and incubating at 95°C for 10 min. The reaction product was resolved by 10% SDS-PAGE and detected using the same antibodies as (A). Each experiment was performed twice, and a representative blot is shown.
TpbB is a substrate of TpbA
To determine if TpbA directly regulates the TpbB phosphorylation state, we utilized purified TpbA and TpbB. Since TpbA is a dual specific tyrosine phosphatase, it is possible that TpbB is phosphorylated at both Tyr and Ser/Thr residues so both types of residues could be substrates for TpbA. Full-length, integral membrane protein TpbB which carries a C-terminal His-tag was purified from the tpbA mutant so that it would be phosphorylated (Roche phosSTOP phosphatase inhibitor cocktail was used throughout the purification procedures to prevent dephosphorylation). After incubation of this TpbB with purified TpbA, TpbB was no longer phosphorylated at both Tyr and Ser/Thr sites as shown in Fig. 2B. Together, these in vivo and in vitro results demonstrate that TpbA dephosphorylates TpbB at Ser/Thr and Tyr sites.
Integral membrane protein TpbB is a DGC
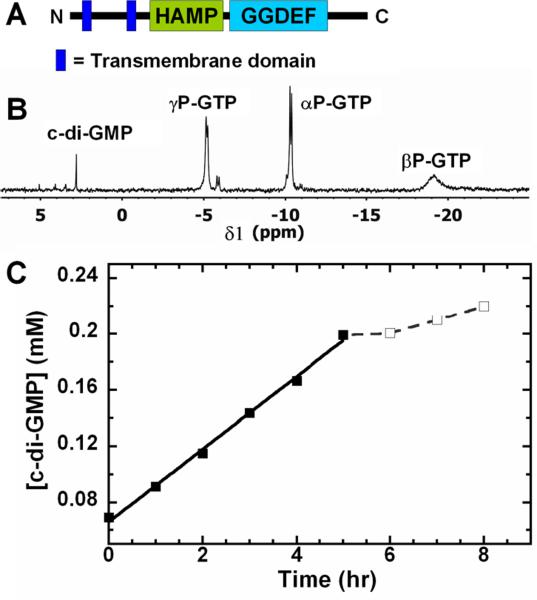
TpbB is an integral membrane protein with two transmembrane domains as shown in Fig. 3A. TpbB activity as a DGC was initially shown using HPLC analysis with P. aeruginosa whole cells that overproduced TpbB [7]. Also, a truncated TpbB without the transmembrane and periplasmic regions was purified from E. coli and shown to be active as a DGC [8]. In our experiment, the full-length integral membrane protein was purified from PA14, and we used 31P NMR to quantify the production of c-di-GMP by TpbB (Fig. 3B & C). Since TpbB contains a conserved allosteric product inhibition site (I-site) [8] similar to many DGCs [15], high levels of c-di-GMP should decrease TpbB activity. As expected, we saw this inhibition for c-di-GMP concentrations greater than 0.2 mM (Fig. 3C); hence, we used concentrations lower than 0.2 mM to avoid feedback inhibition of c-di-GMP on TpbB for the enzyme specific activity calculation. The full length TpbB was active as a DGC and had a c-di-GMP production rate of 17.1 ± 0.7 pmol/(min·mg).
Figure 3.
TpbB enzyme activity as a DGC. (A) Domain organization of TpbB. Two transmembrane domains, the HAMP domain and the GGDEF domain are indicated. (B) Representative 31P NMR spectrum after converting GTP to c-di-GMP by TpbB. (C) Production of c-di-GMP by TpbB from 5 mM GTP; the c-di-GMP concentration was calculated according to the peak integration value in the 31P NMR spectrum. The experiment was performed twice, and the dashed line indicates feedback inhibition for c-di-GMP concentrations higher than 0.2 mM.
DISCUSSION
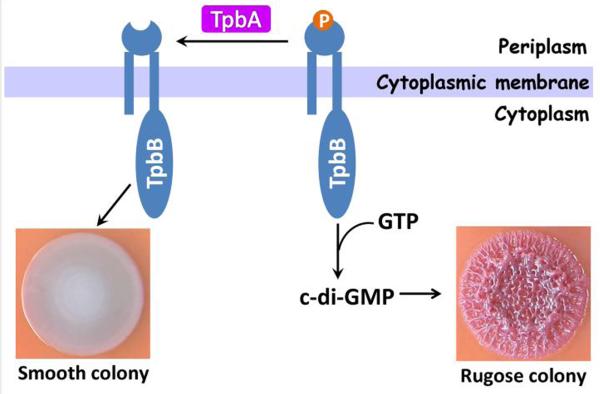
We demonstrated in this study that (i) TpbA is a dual specific tyrosine phosphatase rather than a c-di-GMP PDE, (ii) TpbB is phosphorylated at Ser/Thr and Tyr residues, (iii) TpbA regulates the phosphorylation level of the DGC TpbB in vivo by Western analysis, (iv) TpbB is a substrate of TpbA by using purified TpbA and TpbB, and (v) purified full-length TpbB is active as a DGC that is subject to feedback inhibition. Therefore, we conclude that the protein tyrosine phosphatase TpbA reduces the RSCV morphology of PA14 by dephosphorylating the DGC TpbB which serves to decrease c-di-GMP formation by TpbB as illustrated in Fig. 4.
Figure 4.
Schematic showing how TpbA controls rugose morphology in P. aeruginosa by dephosphorylating TpbB.
The molecular mechanism of how phosphorylation regulates TpbB c-di-GMP synthesis activity requires further study. Regulating DGC activity by phosphorylation is not unique for TpbB; for example, PleD [16], a cytosolic protein from C. crescentus and WspR [17], a cytosolic protein from P. aeruginosa, are both activated in vivo by phosphorylation at the Asp residue of their N-terminal response regulator CheY domains. However, in vitro experiments indicate that phosphorylation is not required for enzyme activity of purified PleD [16] and WspR [18]. For PleD, it was proposed that phosphorylation increases enzyme activity by facilitating protein dimerization [19]. For WspR, a different mechanism was proposed, that phosphorylation promotes the formation of an intermediate tetramer which is required for the activation of WspR [20]. It is possible that phosphorylation of TpbB, similar to other DGCs, also promotes enzyme activity by mediating oligomerization. However, what is unique about TpbB is that it is a membrane protein and the likely phosphorylation sites are located in the periplasmic region since TpbA is a periplasmic protein [4]. How this periplasmic phosphorylation signal is transduced to regulate the cytosolic GGDEF domain activity needs to be elucidated. There also appear to be multiple inputs that regulate TpbB DGC activity. This is seen in other DGCs in that WspR activity is regulated by c-di-GMP concentrations (for feedback inhibition) and by protein concentrations (higher protein concentration promotes protein oligomerization) [21]. For TpbB, along with TpbA and c-di-GMP feedback inhibition, another periplasmic protein, YfiR, is a negative regulator of TpbB activity in vivo although the mechanism has not been elucidated yet [8].
TpbB is also unique in that it is phosphorylated at Tyr and Ser/Thr residues. Tyrosine phosphorylation was once thought to exist exclusively in eukaryotes, and bacterial tyrosine phosphorylation emerged only a decade ago [22]. Compared to the classical two-component systems characterized by phosphorylated His and Asp residues, Tyr and Ser/Thr phosphorylation in P. aeruginosa is not well-studied. Therefore, our work is helping to shape a new paradigm of gene regulation in P. aeruginosa that affects virulence by linking tyrosine phosphatase activity, c-di-GMP synthesis, and biofilm formation.
ACKNOWLEDGEMENTS
This research was supported by the NIH (R01 GM089999). T.W. is the T. Michael O'Connor Endowed Professor at Texas A & M University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Mace C, Seyer D, Chemani C, Cosette P, Di-Martino P, Guery B, Filloux A, Fontaine M, Molle V, Junter GA, Jouenne T. Identification of biofilm-associated cluster (bac) in Pseudomonas aeruginosa involved in biofilm formation and virulence. PLoS One. 2008;3:e3897. doi: 10.1371/journal.pone.0003897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ryder C, Byrd M, Wozniak DJ. Role of polysaccharides in Pseudomonas aeruginosa biofilm development. Curr Opin Microbiol. 2007;10:644–8. doi: 10.1016/j.mib.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Drenkard E, Ausubel FM. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature. 2002;416:740–3. doi: 10.1038/416740a. [DOI] [PubMed] [Google Scholar]
- [4].Ueda A, Wood TK. Connecting quorum sensing, c-di-GMP, pel polysaccharide, and biofilm formation in Pseudomonas aeruginosa through tyrosine phosphatase TpbA (PA3885) PLoS Pathog. 2009;5:e1000483. doi: 10.1371/journal.ppat.1000483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ueda A, Wood TK. Tyrosine phosphatase TpbA of Pseudomonas aeruginosa controls extracellular DNA via cyclic diguanylic acid concentrations. Environmental Microbiology Reports. 2010;2:449–455. doi: 10.1111/j.1758-2229.2010.00171.x. [DOI] [PubMed] [Google Scholar]
- [6].Hengge R. Principles of c-di-GMP signalling in bacteria. Nat Rev Microbiol. 2009;7:263–73. doi: 10.1038/nrmicro2109. [DOI] [PubMed] [Google Scholar]
- [7].Kulasakara H, Lee V, Brencic A, Liberati N, Urbach J, Miyata S, Lee DG, Neely AN, Hyodo M, Hayakawa Y, Ausubel FM, Lory S. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3'–5')-cyclic-GMP in virulence. Proc Natl Acad Sci U S A. 2006;103:2839–44. doi: 10.1073/pnas.0511090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Malone JG, Jaeger T, Spangler C, Ritz D, Spang A, Arrieumerlou C, Kaever V, Landmann R, Jenal U. YfiBNR mediates cyclic di-GMP dependent small colony variant formation and persistence in Pseudomonas aeruginosa. PLoS Pathog. 2010;6:e1000804. doi: 10.1371/journal.ppat.1000804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A. 2006;103:2833–8. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sambrook J, Fritsch EF, Maniatis T, editors. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- [11].Gotoh N, Wakebe H, Nishino T, Tanino T. Rapid extraction method for detection of outer membrane protein F of Pseudomonas aeruginosa. Journal of Microbiological Methods. 1987;6:265–271. [Google Scholar]
- [12].Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 2005;187:4774–81. doi: 10.1128/JB.187.14.4774-4781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dow JM, Fouhy Y, Lucey JF, Ryan RP. The HD-GYP domain, cyclic di-GMP signaling, and bacterial virulence to plants. Mol Plant Microbe Interact. 2006;19:1378–84. doi: 10.1094/MPMI-19-1378. [DOI] [PubMed] [Google Scholar]
- [14].Yuvaniyama J, Denu JM, Dixon JE, Saper MA. Crystal structure of the dual specificity protein phosphatase VHR. Science. 1996;272:1328–31. doi: 10.1126/science.272.5266.1328. [DOI] [PubMed] [Google Scholar]
- [15].Christen B, Christen M, Paul R, Schmid F, Folcher M, Jenoe P, Meuwly M, Jenal U. Allosteric control of cyclic di-GMP signaling. J Biol Chem. 2006;281:32015–24. doi: 10.1074/jbc.M603589200. [DOI] [PubMed] [Google Scholar]
- [16].Paul R, Weiser S, Amiot NC, Chan C, Schirmer T, Giese B, Jenal U. Cell cycle-dependent dynamic localization of a bacterial response regulator with a novel di-guanylate cyclase output domain. Genes Dev. 2004;18:715–27. doi: 10.1101/gad.289504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Guvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2007;66:1459–73. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hickman JW, Tifrea DF, Harwood CS. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci U S A. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wassmann P, Chan C, Paul R, Beck A, Heerklotz H, Jenal U, Schirmer T. Structure of BeF3- -modified response regulator PleD: implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure. 2007;15:915–27. doi: 10.1016/j.str.2007.06.016. [DOI] [PubMed] [Google Scholar]
- [20].De N, Navarro MV, Raghavan RV, Sondermann H. Determinants for the activation and autoinhibition of the diguanylate cyclase response regulator WspR. J Mol Biol. 2009;393:619–33. doi: 10.1016/j.jmb.2009.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].De N, Pirruccello M, Krasteva PV, Bae N, Raghavan RV, Sondermann H. Phosphorylation-independent regulation of the diguanylate cyclase WspR. PLoS Biol. 2008;6:e67. doi: 10.1371/journal.pbio.0060067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grangeasse C, Cozzone AJ, Deutscher J, Mijakovic I. Tyrosine phosphorylation: an emerging regulatory device of bacterial physiology. Trends Biochem Sci. 2007;32:86–94. doi: 10.1016/j.tibs.2006.12.004. [DOI] [PubMed] [Google Scholar]