Abstract
Rearing in social isolation has profound effects on several aspects of behavior in adult rodents. However, little is known about effects of social stress on social behavior in these animals. In the present study, we examined social recognition in mice of both sexes that were individually housed from 30 days of age until testing at approximately 80 days of age, individually housed from day 30 until day 60, followed by group housing from day 60 until testing at around 80 days of age and in control mice that were group housed throughout experiment. A standard social recognition test was performed with ovariectomized female conspecifics introduced into the home cage of tested mice for 1 minute, eight consecutive times with 9 minute breaks between tests, and in the ninth test, new, unfamiliar females were introduced. The time spent investigating stimulus mice during each of the nine tests was recorded. Group housed male and female mice showed strong pattern of social learning, whereas mice reared in isolation from day 30 until testing did not show evidence of social recognition. Interestingly, mice reared in isolation from 30 until 60 days of age and then group housed again, also showed reduced ability for social learning in comparison to the controls housed in groups through the entire period. These results therefore show that social isolation has a profound effect on social behavior in mice, and that even isolation for a limited period can produce lasting behavioral deficits.
Keywords: Mice, social stress, isolation, social recognition
Introduction
Early life stress can have long lasting deleterious effects on brain development and consequently on behavior in adult life [1]. Rearing in isolation either in infancy or in adolescence can produce severe behavioral consequences in adult life in rodents that are social animals by nature [2]. Such studies provide for better understanding of animal behavior and may have implications for human behavior and psychiatric disorders where social isolation is a contributing factor [1].
Mice and rats are social mammals living in large social groups in natural conditions [3, 4]. In laboratory conditions, however, males are sometimes housed individually to prevent intermale aggression, or unwanted mating if males and females would be housed together. Therefore, males (and even females) that have been used in behavioral testing are sometimes housed individually [5]. Unfortunately, such studies do not account for possible effects of social isolation on the outcome of behavioral testing. This is a potential problem because previous studies have shown that rearing in social isolation during the pubertal period can lead to hyperactivity and reduced habituation, reduced novel object recognition, and reduced floating time in forced swim tests. Interestingly, the effects on anxiety-like behavior are somewhat conflicting since social isolation decreased anxiety-like behavior assessed by elevated plus maze testing, but increased anxiety-like behavior in light-dark field testing in mice; and similar effects of social isolation have been reported for rats [6–9]. Although the molecular mechanisms that lead to these behavioral deficits are mostly unknown, several studies have shown that social isolation directly affects brain development in juvenile rodents. Rearing in isolation has been reported to result in reductions of medial prefrontal cortex volume [10], cytoskeletal alterations in hippocampus [11] and changes in CREB expression and dopamine and serotonin turnover in different parts of the brain [6, 12]
Social recognition is critical for establishing and maintaining social structures in groups of animals living together. Tests for social recognition were first described by Thor and Holloway [13] and are based on monitoring the time that tested animals spend investigating conspecifics introduced multiple times into the cages of tested animals. In rats and mice, the time of investigation normally decreases with exposure to the same animal, and is increased upon exposure to novel unfamiliar animals, usually to the time as observed during the first exposure to the novel animal [5]. Interestingly, social recognition is usually sexually dimorphic with the reduction in time during the test with same animal being more prominent in males than in females [14, 15]. The influence of social isolation on performance in social recognition tests has not been thoroughly investigated. Recently, Zhao et al. reported abnormalities in social recognition in male rats, housed individually [16], but we are not aware of any reports concerning the effect of social isolation on social recognition in mice of both sexes, and particularly whether social isolation for a limited time period could have a lasting effect on social learning. The present study, therefore, examined social recognition in mice of both sexes that were reared in groups or in isolation from day 30 (beginning of puberty) until testing or for a limited period of time to explore whether social isolation could cause long-lasting alterations in murine social behavior.
Material and methods
Animals
C57BL/6J mice were bred in standard conditions with 12-12 LD cycle (lights on at 5 am and off at 5 pm) and food (phytoestrogen free diet; Harlan Teklad Diet 2016, Harlan, Milan, Italy) and water ad libitum. C57BL/6J strain of mice was chosen as this is a strain frequently used in genetic analyses of brain and behavior. Mice were weaned at 21 days of age and mice from the same litters were divided into three groups. Mice from the first group (social group) were divided into groups of three mice of same sex after weaning at 3 weeks of age, mice from second group (isolated) were isolated into individual cages at 30 days of age and mice from third group (isolated/social) were isolated into individual cages at 30 days of age and grouped into social groups of three mice (of same sex) at 60 days of age. Group housed mice were housed in 16 cm high cages with floor area 38 × 22 cm and individually housed mice were kept in 13 cm high cages with 35 × 15 cm floor area. For stimulus mice, ovariectomized female mice of the same strain were used. Ovariectomized stimulus female mice were used to prevent any possible influence of estrus cycle on duration of sniffing of tested mice, and to make stimulus mice neutral to both male and female test mice. The goal was to present the stimulus mice as conspecifics but not possible mating partners or aggressive opponents. Stimulus mice were ovariectomized around 60 days of age. Mice were anaesthesized with the mixture of ketamine (Vetoquinol Biowet, Gorzowie, Poland; 100 μg/g BW), acepromazine (Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 μg/g BW) and xylazine (Chanelle Pharmaceuticals Ltd., Loughrea, Ireland; 10 μg/g BW) and both ovaries were excised through small wounds. Wounds were stitched and mice received two injections of butorfanol (Turbogesic, Fort Dodge Animal Health, Fort Dodge, IA, USA; 2 μg/g BW) after surgery to ease any potential pain. Mice were allowed to recover for at least 10 days before being used as stimulus mice. All animal experiments were approved by Veterinary commission of Slovenia (VURS) and were done according to ethical principles and NIH guidelines.
Social recognition test
The ability to recognize familiar conspecifics was tested in 80-day-old mice. Estrous cyclicity was evaluated in experimental female mice by examining vaginal cytology. Vaginal smears were stained with haematoxyline and eosine using standard procedures and examined using brightfield microscopy (Nikon Eclipse 80i). Only female mice in diestrus (identified by the presence of large numbers of leucocytes) were used for behavior testing to control for differences in behavior due to hormonal changes. Diestrus was chosen as a period of the estrus cycle with most reproductive hormones (estradiol, progesterone, LH and FSH) at low levels [17]. All tests were done at the beginning of the dark period under dim red illumination. Prior to testing (24h), socially housed mice were put individually into new cages (small cages 35 × 15 × 13 (L x W x H) with filtertops) with bedding from their old cage; isolated mice were tested in their home cages with at least 3 day-old bedding. On the day of testing, ovariectomized females were put into the cage for 60 seconds and then removed. The same female was put into the cage again after 9 minutes and this was repeated 8 times. After a final 9 minute break, a new, unfamiliar ovariectomized female was put into the cage for the ninth test. During each 1 minute trial, the duration of sniffing by the tested mice was recorded using ‘stopwatch’ software (Center for Behavioral Neurosciences, Atlanta, GA). All tests were performed in the presence of an observer who remained still and quiet during the test to minimize potential observer effects. All tests were done by the same investigator (J.K.) who was blinded to group assignment at the time of testing.
Statistical analyses
All statistical analyses were done using NCSS software (NCSS statistical software, Kaysville, UT). At least nine mice of each sex were tested in each group (social, isolated, isolated/social). To test differences between groups over the trials, repeated measures ANOVA was performed with housing conditions and sex as independent variables followed by post hoc Bonferroni test. To assess differences between test 1 and 8 and tests 8 and 9 within individual groups, repeated measures ANOVA followed by posthoc Bonferroni testing was performed. Differences were considered statistically significant with p < 0.05.
Results
Differences in time spent sniffing between groups
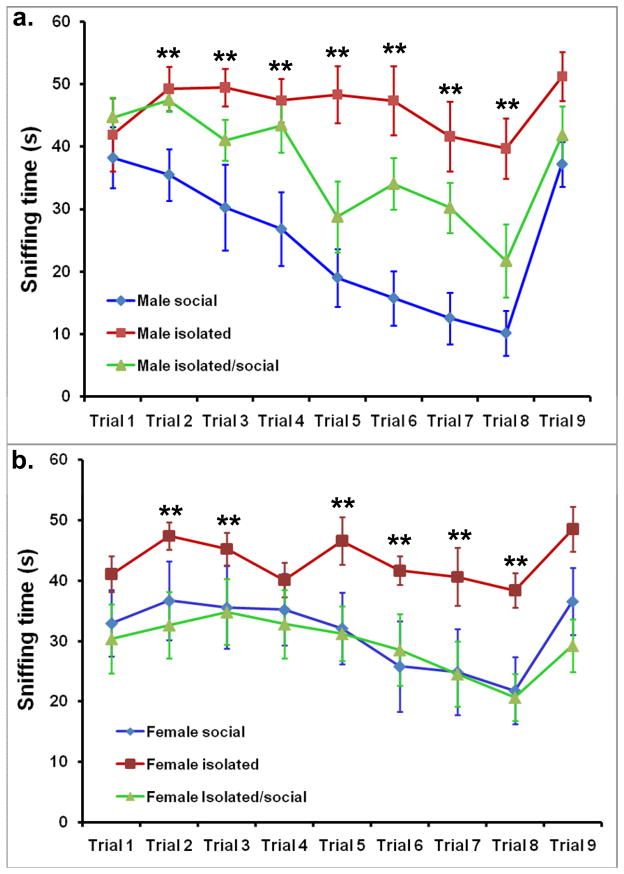
All mice were exposed 8 times for 1 minute to the same stimulus mouse followed by ninth test with a new stimulus mouse. No aggressive (attacks, bites, charges) or sexual behavior (mounting, lordosis) was observed between test mice and stimulus mice during any test. Statistical analysis using repeated measure ANOVA with housing condition and sex as independent variables revealed a significant interaction between housing conditions and test (F (16,51)=5.11, p < 0.01) and between sex and test (F(8,51)=3.55, p < 0.001). As shown in figure 1, the greatest amount of habituation to the repeated presentation of the same stimulus female and the greatest response to a new female was observed in male mice grouped socially throughout the experiment. Time spent sniffing declined significantly with each test (p < 0.001) and recovered to levels observed in test 1 during the ninth test (test with new mice; p < 0.001). In socially housed female mice as well as in male and female mice isolated from day 30 until day 60 (returned to social housing on day 60), a pattern of habituation to the repeated presentation of the same stimulus female was also apparent. Nonetheless, the reduction in time spent sniffing between tests 1 and 8 was much lower in comparison to males housed socially, suggesting reduced habituation or learning in these mice (Figure 1). By contrast, in both male and female mice housed individually from day 30 until testing at around 80 days of age (i.e., isolated), animals failed to habituate to the stimulus female (Figure 1). Even though the time spent sniffing increased from tests 8 to 9 in both males and females, this difference was not statistically significant nor was the minimal reduction in time sniffing between tests 1 and 8, suggesting that these mice did not habituate to the stimulus mice and did not recognize them as familiar during test 8 (or any of the previous tests).
Figure 1.
A pattern of social habituation was observed in the male social group, where time spent sniffing stimulus mice decreased significantly from trials 1 to 8 (a). The pattern of social habituation was also noted with isolated/social males (a) and in female social group and female isolated/social group (b) while social habituation to the same stimulus mice was not noted in male (a) and female (b) mice that were isolated from day 30 until testing (isolated group). Significant difference in sniffing time between three groups of mice was noted in most trials in both sexes (** p < 0.01).
Difference in time spent sniffing within groups
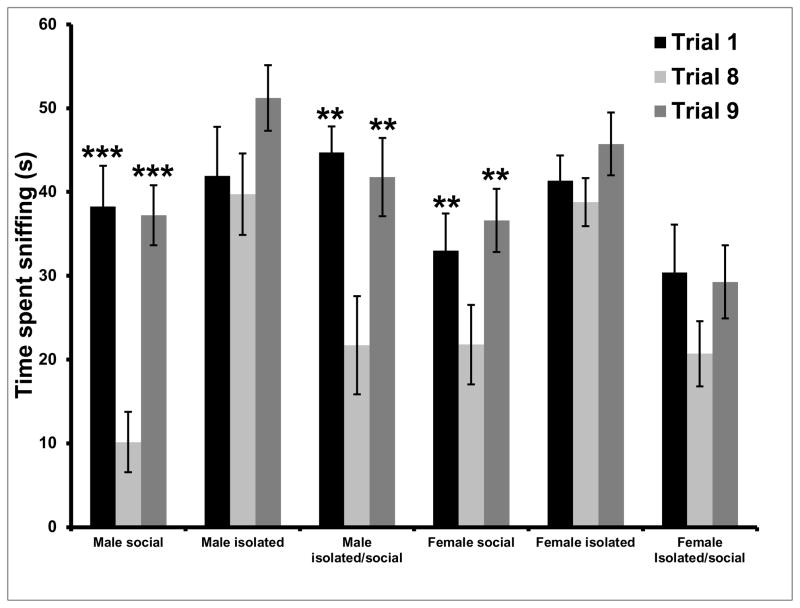
Analysis of the difference in time spent sniffing between trials 1, 8 and 9 within individual groups of mice revealed a significant effect of housing conditions as social recognition was indicated in both groups of socially housed mice. In socially housed mice there was a significant difference between tests 1 and 8 in both males (p < 0.001) and females (p < 0.05) and between tests 8 and 9 in males (p < 0.001) and females (p < 0.01). In isolated groups of mice, only isolated/social males showed habituation with a significant difference between tests 1 and 8 (p < 0.01) and tests 8 and 9 (p < 0.01) while time spent sniffing the stimulus mice in the other three groups of mice (isolated males, isolated females, isolated/social females) did not differ significantly between tests 1, 8 and 9 (Figure 2).
Figure 2.
Time spent sniffing stimulus mice differed significantly between tests 1 and 8 and tests 8 and 9 in group housed male mice, group housed females and isolated/social males while there was no significant difference between tests 1 and 8 and tests 8 and 9 in male and female mice reared in isolation and in isolated/social females, suggesting that in these three groups of mice, social isolation affected their ability to differentiate between familiar and unfamiliar conspecifics (** p < 0.01; *** p < 0.001; all different from test 8 within the same group).
Discussion
Previous studies have shown that rearing in social isolation during development leads to long lasting changes in brain structure or gene expression [6, 10–12], and causes behavioral changes in adult life such as increased locomotor activity, decreased anxiety-like behavior (elevated plus maze), impaired novel object recognition and increased aggression [6, 7, 12, 18]. However, with the exception of one recent study [16], we are not aware of other reports concerning the effect of prolonged social isolation on social learning in rodents. Therefore, the results of the present study provide data indicating that either long-term or transient social isolation impacts social recognition in mice, particularly in males.
In the current study, the strongest pattern of social recognition was noted in male mice that were housed socially in groups of three. For this group of mice, time spent investigating familiar ovariectomized female mice during 8 tests linearly decreased to reach the lowest levels during the eighth test, but reverted to the initial time during the ninth test with novel females. This suggests that male mice, reared in groups, have strong ability for social learning and successfully discriminated between familiar and unfamiliar ovariectomized female mice. However, in male mice that were housed individually from day 30 until testing around day 80, there was little reduction in time spent sniffing the same mice during the 8 tests and no significant difference between tests 8 and 9, suggesting that these mice did not distinguish familiar female from the unfamiliar one (presented in test 9). This is in agreement with the results by Zhao et al. [16], where similar deficits in social recognition were reported in male rats housed individually from day 30 until testing in adulthood, suggesting that social isolation affects both mice and rats similarly with respect to this behavior. The current study contains an important additional group; mice that were reared in isolation from day 30 until day 60 and then were housed in groups of three (same sex) until testing around day 80 (isolated/social group). Interestingly, in this group of male mice, there was a social recognition pattern of behavior. The reduction in time sniffing familiar females, however, was significantly lower than in male mice housed in groups continuously. This might suggest that social isolation for a transient period caused changes that were evident as a behavioral deficit in a form of reduced habituation later in adult life, after mice were resocialized. This reduction was evident even though isolated/social male mice were still able to distinguish familiar from unfamiliar females as demonstrated by the significant increase in times spent sniffing stimulus mice between tests 8 and 9.
As expected, social recognition was also observed in female mice, although the reduction in time during 8 tests was much smaller in comparison to male mice, in agreement with previously published data for rats [19, 20]. Nevertheless, socially housed female mice showed significant differences in time spent sniffing between tests 8 and 9 suggesting that they could distinguish familiar from unfamiliar females. Similar to male mice reared in isolation, female mice reared in isolation from day 30 until testing did not show evidence of social habituation or recognition. Interestingly, however, the time spent sniffing stimulus mice did not change between test 8 and 9 also in isolated/social females suggesting that in female mice, transient isolation was sufficient to cause an effect on their ability to distinguish familiar and unfamiliar conspecifics even some time after they were resocialized.
The influence of sex hormones on the ability to recognize conspecific animals has been shown previously [14, 15]. Therefore, the finding of a sex difference in the social recognition in social group of mice in the current study was not surprising. However, all females were tested in the diestrus phase of the estrous cycle when hormone levels are lowest. Therefore, this finding does not necessarily mean that social recognition and/or habituation are worse in female than in male mice at all times. It is quite possible that during estrus phase, when more estradiol is in the female circulation (perhaps similar in action to males circulating testosterone), social recognition/habituation would be more comparable to males. Although a previous study [19] did not find differences in social recognition between proestrus and estrus female rats, they only looked at two phases (proestrus and estrus).
Social recognition is a form of learning and previous studies have shown that some kinds of learning behaviors (novel object recognition, fear conditioning) are affected by social isolation while others like water maze performance are not [7]. This could imply that similar mechanisms as reported in previous studies could be responsible for reduced social recognition observed in the current study. However, the learning ability of mice in other learning paradigms such as novel object recognition, water maze and others were not addressed in the current study. The social learning process is connected with both short and long term memory and interestingly, previous studies have shown that social isolation could induce changes in hippocampal development [11]. Although lesions of central hippocampus do not produce deficit in social recognition [21], the hippocampus in general plays important roles in the formation of short term memory [22] that are important for the learning process, and lesions of areas around the hippocampus produce some deficits in social recognition [23].
Social recognition is an important behavioral feature that is needed in social groups to differentiate individuals belonging to the same social group from unknown, potentially harmful individuals. Many studies have implicated vasopressin and oxytocin in the regulation of different social behaviors including social recognition (reviewed in [24]. Vasopressin acting through its receptor V1a in lateral septum is thought to be particularly important since V1aR knockout mice do not show social recognition, but this deficit could be rescued by replacing the V1aR gene into the lateral septum alone [25]. Interestingly, however, vasopressin may be more important for social recognition in males than females, since a vasopressin antagonist blocked social learning in male but not in female rats [14]. This is probably due to much higher levels of vasopressin peptide in the lateral septum that is dependent upon exposure to testosterone. Social-learning in females that have much less vasopressin in the lateral septum may rely more on other neurohormones or neuropeptides, perhaps oxytocin, which has also been shown to be important in this behavior [26, 27]. Several studies have shown that oxytocin influences social recognition in mice [28, 29] and this is most likely regulated by estrogens [28, 30, 31]. In the present study, all female mice were therefore tested in the diestrus part of the estrous cycle with the lowest levels of gonadal estrogens to minimize their possible effects, and importantly minimize confusion due to changing levels of estrogens at different cycle stages. In a preliminary experiment using immunocytochemical detection of vasopressin and oxytocin (data not shown) there were no obvious differences in either immunoreactive vasopressin in the lateral septum or oxytocin in the paraventricular nucleus of the hypothalamus between groups of mice in different housing regimes. At the same time, as expected, there was a robust sex difference in immunoreactive vasopressin in fibers of the lateral septum in all experimental groups. Sex differences in vasopressin content in the lateral septum might help explain sex differences in social recognition [32]. However, it may be difficult to relate the differences in behavior to a simple static immunocytochemical view of the vasopressin system as opposed to determining the more active measures of peptide release or turnover.
The lasting effect of transient social isolation observed in the current study is particularly interesting since it suggests that brain circuitry regulating social recognition has been altered in a lasting fashion. Previous studies have shown that social isolation could induce changes in hippocampal development [11], but beside structural changes, long lasting effects on behavior could be due to epigenetic regulation of certain genes, achieved either by histone modifications and/or DNA methylation [33]. In this context, it might be useful to examine potential epigenetic regulation of vasopressin/oxytocin systems in future studies.
In conclusion, the current study shows that social isolation strongly influences subsequent social behavior in mice and that even transient social isolation resulted in lasting effects on social behavior. Results of this study contribute to our understanding that social isolation, which is often used in breeding of laboratory mice, causes long-lasting changes in social behavior and should be taken into account when interpreting results from such studies.
Acknowledgments
Authors are thankful to Emilie F. Rissman for reading an early version of the manuscript and discussion related to behavioral and statistical analyses. This study was supported by NIH grant MH61376 (S.A.T. and G.M.), ICGEB grant CRP SLO 06/02, ARRS (Slovenian research agency) grants P4-0053 and J7-2093 (G.M.) and Neza Grgurevic is supported by ARRS doctoral fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 2.Fone KC, Porkess MV. Behavioural and neurochemical effects of post-weaning social isolation in rodents-relevance to developmental neuropsychiatric disorders. Neurosci Biobehav Rev. 2008;32:1087–102. doi: 10.1016/j.neubiorev.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Classen W. Behavior, Neurology and Electrophysiology. In: Krinke GJ, editor. The laboratory rat. London, San Diego: Academic press; 2001. pp. 419–36. [Google Scholar]
- 4.Dixon AK. The social behavior of mouse and its sensory control. In: Hedrich H, editor. The laboratory mouse. London, San Diego: Academic press; 2004. pp. 287–300. [Google Scholar]
- 5.Crawley JN. What’s wrong with my mouse? New York: Wiley-Liss; 2000. [Google Scholar]
- 6.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, et al. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 7.Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–52. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- 8.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 9.Wongwitdecha N, Marsden CA. Social isolation increases aggressive behaviour and alters the effects of diazepam in the rat social interaction test. Behav Brain Res. 1996;75:27–32. doi: 10.1016/0166-4328(96)00181-7. [DOI] [PubMed] [Google Scholar]
- 10.Schubert MI, Porkess MV, Dashdorj N, Fone KC, Auer DP. Effects of social isolation rearing on the limbic brain: a combined behavioral and magnetic resonance imaging volumetry study in rats. Neuroscience. 2009;159:21–30. doi: 10.1016/j.neuroscience.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 11.Bianchi M, Fone KF, Azmi N, Heidbreder CA, Hagan JJ, Marsden CA. Isolation rearing induces recognition memory deficits accompanied by cytoskeletal alterations in rat hippocampus. Eur J Neurosci. 2006;24:2894–902. doi: 10.1111/j.1460-9568.2006.05170.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iniguez SD, et al. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–9. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thor DH, Wainwright KL, Holloway WR. Persistence of attention to a novel conspecific: some developmental variables in laboratory rats. Dev Psychobiol. 1982;15:1–8. doi: 10.1002/dev.420150102. [DOI] [PubMed] [Google Scholar]
- 14.Bluthe RM, Gheusi G, Dantzer R. Gonadal steroids influence the involvement of arginine vasopressin in social recognition in mice. Psychoneuroendocrinology. 1993;18:323–35. doi: 10.1016/0306-4530(93)90028-j. [DOI] [PubMed] [Google Scholar]
- 15.Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–59. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Sun L, Jia H, Meng Q, Wu S, Li N, et al. Isolation rearing induces social and emotional function abnormalities and alters glutamate and neurodevelopment-related gene expression in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1173–7. doi: 10.1016/j.pnpbp.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Freeman ME. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill J, editors. The physiology of reproduction. 2. New york: Raven press; 1994. pp. 613–58. [Google Scholar]
- 18.Einon DF, Humphreys AP, Chivers SM, Field S, Naylor V. Isolation has permanent effects upon the behavior of the rat, but not the mouse, gerbil, or guinea pig. Dev Psychobiol. 1981;14:343–55. doi: 10.1002/dev.420140407. [DOI] [PubMed] [Google Scholar]
- 19.Markham JA, Juraska JM. Social recognition memory: influence of age, sex, and ovarian hormonal status. Physiol Behav. 2007;92:881–8. doi: 10.1016/j.physbeh.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reeb BC, Tang AC. Sex difference in temporal patterns of social interaction and its dependence upon neonatal novelty exposure. Behav Brain Res. 2005;158:359–65. doi: 10.1016/j.bbr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Bannerman DM, Lemaire M, Beggs S, Rawlins JN, Iversen SD. Cytotoxic lesions of the hippocampus increase social investigation but do not impair social-recognition memory. Exp Brain Res. 2001;138:100–9. doi: 10.1007/s002210100687. [DOI] [PubMed] [Google Scholar]
- 22.Barco A, Bailey CH, Kandel ER. Common molecular mechanisms in explicit and implicit memory. J Neurochem. 2006;97:1520–33. doi: 10.1111/j.1471-4159.2006.03870.x. [DOI] [PubMed] [Google Scholar]
- 23.Bannerman DM, Lemaire M, Yee BK, Iversen SD, Oswald CJ, Good MA, et al. Selective cytotoxic lesions of the retrohippocampal region produce a mild deficit in social recognition memory. Exp Brain Res. 2002;142:395–401. doi: 10.1007/s00221-001-0938-z. [DOI] [PubMed] [Google Scholar]
- 24.Storm EE, Tecott LH. Social circuits: peptidergic regulation of mammalian social behavior. Neuron. 2005;47:483–6. doi: 10.1016/j.neuron.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–13. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–63. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popik P, Vetulani J, van Ree JM. Low doses of oxytocin facilitate social recognition in rats. Psychopharmacology (Berl) 1992;106:71–4. doi: 10.1007/BF02253591. [DOI] [PubMed] [Google Scholar]
- 28.Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci U S A. 2003;100:6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, et al. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–39. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 30.de Kloet ER, Voorhuis DA, Boschma Y, Elands J. Estradiol modulates density of putative ‘oxytocin receptors’ in discrete rat brain regions. Neuroendocrinology. 1986;44:415–21. doi: 10.1159/000124680. [DOI] [PubMed] [Google Scholar]
- 31.Hlinak Z. Social recognition in ovariectomized and estradiol-treated female rats. Horm Behav. 1993;27:159–66. doi: 10.1006/hbeh.1993.1012. [DOI] [PubMed] [Google Scholar]
- 32.de Vries GJ. Sex differences in vasopressin and oxytocin innervation of the brain. Prog Brain Res. 2008;170:17–27. doi: 10.1016/S0079-6123(08)00402-0. [DOI] [PubMed] [Google Scholar]
- 33.Crews D. Epigenetics and its implications for behavioral neuroendocrinology. Front Neuroendocrinol. 2008;29:344–57. doi: 10.1016/j.yfrne.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]