Abstract
Background
There is a worldwide need for a pediatric HIV-1 diagnostic test that has a high diagnostic accuracy, is technically simple and cost efficient. The Up24 HIV-1 assay, which requires both the HIV-1 p24 ELISA and the ELAST signal amplification kit, has previously been shown to be a robust tool to diagnose pediatric HIV-1 from dried whole blood spots (DBS) (Cachafeiro et al., JCM 2009;47:459–6213). In order to make the assay more accessible to a resource-limited clinical setting, we eliminated the ELAST system, which simplified the Up24 assay, reduced its cost, and tested the accuracy of the modified assay in a rural Malawian hospital.
Objectives
In this proof of concept study, we tested the ability of a simplified Up24 antigen assay, without ELAST, to detect HIV-1 on DBS obtained via heel prick from 6-week-old Malawian infants.
Study design
A case–control study of DBS collected from 113 HIV-infected and 109 HIV-negative infants, using the HIV-1 DNA PCR assay as the reference standard.
Results
The simplified HIV-1 Up24 assay had a sensitivity and specificity of 84% and 98%, respectively. When HIV-1 prevalence is 15%, the positive- and negative-predictive values are 89% and 97%, respectively.
Conclusion
The simplified Up24 assay has a good positive- and a robust negative-predictive values, is easier to perform and has a reduced cost compared to both HIV DNA PCR and Up24 assays. With additional testing, the simplified Up24 assay has the potential to increase global access to pediatric HIV-1 diagnostics.
Keywords: Human immunodeficiency virus type 1, (HIV-1), Pediatrics, Diagnostics, Malawi, HIV-1 p24 antigen detection assay, Dried blood spot (DBS)
1. Background
Despite the evidence that timely antiretroviral treatment (ART) in HIV-infected infants reduced death by approximately 75%,1 only 35% of the HIV-infected children in sub-Saharan Africa (SSA) were receiving ART.2 The lack of affordable, accessible and accurate HIV-1 infant diagnostic tests is one of the major bottlenecks limiting timely access to ART among children in SSA. In Malawi, it is estimated that 85,488 HIV-infected mothers deliver annually,3 but in 2009, only three public hospitals located in urban districts in Malawi had capacity to perform HIV DNA PCR. However, in an attempt to increase coverage of infant HIV testing, the Early Infant Diagnosis (EID) Programme collects dry blood samples for centralized PCR testing from 41 of the 425 health facilities delivering prevention of HIV mother-to-child transmission (PMTCT) services.3,4 One of the EID testing facilities is based at Queen Elizabeth Central Hospital (QECH) located in the city of Blantyre (population ~660,0005); QECH is the only public health facility in the southern region of Malawi (population ~5.9 million3) with the capacity to conduct HIV DNA PCR. In 2009, QECH performed 8642 tests of which 2297 were from Blantyre city (Hannania Moyo, EID Senior Laboratory Technician, QECH, personal communication). Thus, it is estimated that that only ~11% of the Malawian children have ready access to HIV DNA PCR. This system poses logistical problems and ultimately limits coverage of ART in children.
Several published studies have demonstrated the ability of HIV-1 p24 antigen detection assays to diagnose pediatric HIV-infection in plasma6–12 and dried blood spots from venous blood.13–16 Importantly, the Up24 assay has been validated against HIV-1 subtype C, which comprises >95% of all HIV-infections in Malawi7,11,12,15–19 and it can be easily adapted to a resource-limited setting.20 Up24 assays contain two modules, a p24 ELISA and ELAST (ELISA signal amplification kit), and two reagents comprise the ELAST kit: the Biotynil Tyramide and the ELAST HRP, and collectively, the ELAST reagents increase the sensitivity of the p24 ELISA. Before using the ELAST kit, the Biotynil Tyramide needs a single step dilution, and the ELAST HRP needs a two-step serial dilution, which must be experimentally determined for each lot. As a result, in addition to increasing the cost per test of the Up24 assay, the ELAST module adds technical complexity, labor time, and it increases assay variability.
2. Objective
In this proof of concept study, we tested the ability of a simplified Up24 antigen assay, without ELAST, to detect HIV-1 on DBS obtained via heel prick from 6-week-old Malawian infants.
3. Study design
IRB approval was obtained from the Malawi College of Medicine Research Ethics Committee and the Ohio State University. Additional permission was obtained from the Malawi Ministry of Health to use archived, de-identified DBS samples from infants who attended the Thyolo District Hospital. A priori sample size calculations indicated that 136 HIV-infected and 118 HIV-negative samples would give an 80% power at the 0.05 significance level to detect differences from the HIV-1 DNA PCR test when the sensitivity or specificity of the HIV-1 Up24 test is below 90%. Infant DBS samples from the Thyolo District Hospital are currently tested for HIV-1 at Queen Elizabeth Central Hospital (referral hospital in Southern Malawi) with the Roche Amplicor HIV-1 DNA PCR assay when the infant is 6 and 10 weeks old, and again 6 weeks after breastfeeding has stopped. The DBS cards were stored in ziploc bags containing dessicant and a humidity detector card, and the samples were excluded if the humidity detector card showed abnormalities. DBS samples from HIV-exposed 6-week-old infants who had an unequivocal negative or positive DNA PCR result were selected. Three 6 mm punches from a single DBS were collected and transported to Thyolo District Hospital for HIV-1 Up24 antigen testing by a Malawian laboratory technician who was masked to the HIV-1 DNA PCR results. Alliance® HIV-I p24 ELISA kits (Perkin Elmer) were purchased from Separation Scientific SA (Honeydew, South Africa) and Up24 assays were performed as reported by Cachafeiro et al.,13 with the modifications described in Table 1. When the simplified Up24 ELISA and HIV DNA PCR were discordant at any of the cutoff values, a third DBS was sent to the UNC Core Retrovirology lab for an additional HIV-1 DNA PCR assay (Roche HIV-1 DNA PCR v1.5). The tiebreaker results from UNC lab were considered the true HIV-1 status. Sensitivity, specificity, and likelihood ratios22 were determined from contingency tables and positive- and negative-predictive values (PPV and NPV, respectively) for each proposed cutoff were calculated according to the following equations:
where “sen” equals assay sensitivity, “spec” equals assay specificity, and “p” equals the theoretical prevalence of the HIV-1 in the pediatric population.
Table 1.
Differences in assay conditions between this publication and Cachafeiro et al., JCM (2009). DBS: dried blood spot; SDS: sodium dodecyl sulfate; HRP: horse radish peroxidase; ELAST: ELISA amplification system.
| Characteristic | Cachafeiro et al., JCM (2009)13 |
This publication |
|---|---|---|
| Sample | Two disks punched from DBS |
Three disks punched from DBS |
| Specimen preparation buffer |
No SDS | 0.3% SDS |
| Volume of elution buffer |
250μl | 275μl |
| Input volume of sample into wells |
250μl | 270μl |
| Detector antibody incubation |
1h | 2h |
| HRP incubation | 15 min | 90min |
| ELAST (signal amplification) |
Present | Absent |
4. Results
A total of 222 DBS samples from 113 HIV-infected and 109 HIV-negative infants were assayed with the simplified HIV-1 p24 ELISA. The modifications listed in Table 1 were implemented to compensate for the decreased sensitivity of the Up24 assay in the absence of ELAST amplification step. In order to optimize the sensitivity and specificity of the simplified Up24 assay, several assay cutoffs were evaluated, including the following: the average of the negative controls (NC) multiplied by two (2NC); NC plus 0.05 OD units (NC + 0.05); or NC + 2 standard deviations (SD), NC + 3SD, or NC + 5SD. The data in Table 2A indicate that NC + 3SD had the highest sensitivity and lowest specificity, while NC + 5SD had the highest specificity, an intermediate sensitivity, and the highest positive likelihood ratio (Table 2B).
Table 2.
(A) Properties of the simplified p24 antigen detection assay at different cutoff values. (B) Contingency table of NC + 5SD p24 antigen detection assay and HIV DNA PCR assay. NC: average of negative control wells; SD: standard deviation; CI: confidence interval; PCR: polymerase chain reaction.
| Assay cutoff | Sensitivity (95% CI) | Specificity (95% CI) | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) |
|---|---|---|---|---|
| (A) | ||||
| 2NC | 0.79 (0.70,0.85) | 0.96 (0.91, 0.99) | 21.5 (8.2, 56.4) | 0.22 (0.15, 0.32) |
| NC+ 0.05 | 0.74 (0.66,0.82) | 0.95 (0.90 0.98) | 16.2 (6.8, 38.4) | 0.27 (0.20, 0.37) |
| NC+ 2SD | 0.91 (0.84,0.95) | 0.93 (0.86, 0.96) | 12.4 (6.4, 24.3) | 0.10 (0.05, 0.17) |
| NC+ 3SD | 0.90 (0.83, 0.94) | 0.95 (0.90, 0.98) | 19.7 (8.3, 46.4) | 0.10 (0.06, 0.18) |
| NC+ 5SD | 0.84 (0.76,0.90) | 0.98 (0.94, 1.0) | 45.8 (11.6, 181) | 0.16 (0.11, 0.25) |
| PCR (+) | PCR (−) | |||
|---|---|---|---|---|
| (B) | ||||
| p24 (+) | 95 | 2 | ||
| p24 (−) | 18 | 107 |
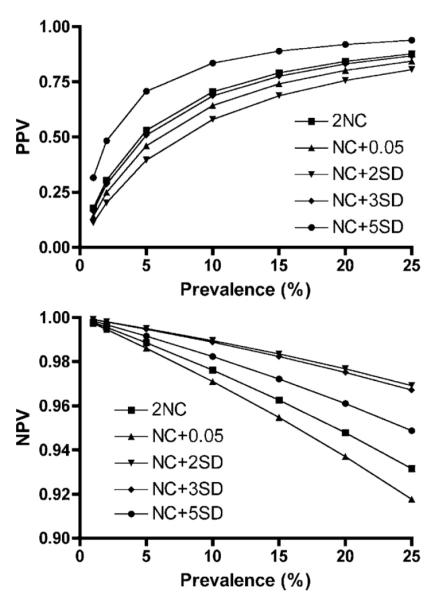
In order to explore the ability of the simplified Up24 assay to correctly diagnose pediatric HIV-1 infection, we calculated the positive- and negative-predictive values for the five p24 assay cutoff-definitions over a plausible range of HIV-1 prevalence values (1–25%). Fig. 1 (top) shows that NC + 5SD had the most robust PPV over the entire prevalence range, with a PPV of 89% at 15% HIV-prevalence. Fig. 1 (bottom) shows that all cutoff values had robust NPVs, with NC + 5SD falling just slightly below the best cutoff values, with a NPV of 97% at 15% HIV-prevalence.
Fig. 1.
Variation in positive-predictive value (PPV, top panel) and negative-predictive value (NPV, bottom panel) of the HIV-1 p24 antigen detection assay over a theoretical range of pediatric HIV-1 prevalence values. PPV and NPV were calculated according to the formulas listed in the text. NC: average of negative controls; SD: standard deviation.
5. Discussion
This study was done in a setting where overall rate of HIV-1 mother-to-child transmission (MTCT) without any preventive intervention was previously estimated at approximately 28%.23,24 With the roll-out of prevention of MTCT programs, the MTCT prevalence is likely to have decreased to approximately 15%.25,26 Using the NC + 5SD cutoff, the PPV was between 84% and 89% at a theoretical HIV-1 prevalence of 10–15%; these data, in combination with the high positive likelihood ratio (45.8), demonstrate that the simplified Up24 antigen detection assay may be acceptable in resource-limited settings. However, a confirmatory test may still be required before starting HIV-infected infants on ART, especially if the estimated HIV-1 prevalence falls below 10%. The high NPV (>96%) at a pediatric HIV-1 prevalence of less than 15% and the low negative likelihood ratio (0.16) demonstrates that a negative result is very good at excluding infant HIV-infection. This means one can confidently use this test to exclude infants who do not need confirmatory HIV-1 tests.
Our study had several limitations. First, we did not assess the diagnostic accuracy and PPV/NPV of the simplified HIV-1 Up24 antigen assay in different hospital settings, which was beyond the objectives of the study. Thus, before it could be considered for widespread use, the simplified Up24 assay should be piloted in several district hospitals. Second, we included only DBS samples with strongly negative or positive HIV-1 DNA PCR results, which may not reflect a real-life situation, and the simplified Up24 assay should be explored on a set of randomly selected DBS samples.
In summary, although the simplified HIV-1 Up24 antigen detection assay is less sensitive than other HIV-1 diagnostics, this shortcoming is leveraged by the numerous advantages of the simplified p24 antigen assay, which include the following: less expensive test costs (in Malawi, the p24 assay described above is at least two- to three-fold cheaper per test), simpler technology and equipment required for the assays, and the potential to decentralize the assay. Assay decentralization would in turn reduce the costs associated with transporting the samples to the central laboratory, reduce the logistical burden of delivering the results from the laboratory back to the hospital, and most importantly, reduce the time between sample acquisition and HIV-1 diagnosis.
Acknowledgments
We are grateful to the study participants, their infants; Ms. Naomi Sibale, Ms. Liness Mphuka, and the staff at the Thyolo District Hospital; Mr. Hannania Moyo, Early Infant Diagnosis Programme, Queen Elizabeth Central Hospital; and to Ms. Debbie Knight for her logistical support. This project was supported in part by an Ohio State University Public Health Preparedness for Infectious Diseases Pilot Grant (JJK); a travel award from the Ohio State University Office of International Affairs (JJK); NIH grants R00HD056586 (JJK), 5R01TW007305 (VM), and the UNC Center For AIDS Research P30 AI50410 (SAF). The content of this article is solely the responsibility of the authors and it does not necessarily represent the official views of the funding agencies.
Footnotes
Conflict of interest None for all authors.
References
- 1.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359(21):2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization, UNAIDS UNICEF Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. 2010 http://www.who.int/hiv/pub/tuapr_2009_en.pdf.
- 3.National AIDS Commission . Malawi HIV and AIDS monitoring and evaluation report: 2008–2009. UNGASS Country Progress Report; Lilongwe: 2010. [Google Scholar]
- 4.ITAD . Final report of the independent review of Malawi national response of HIV and AIDS for fiscal year 2008–2009. ITAD; Lilongwe: 2009. [Google Scholar]
- 5.National Statistics Office [on 10th July 2010];Malawi population and housing census. 2008 accessed from http://www.nso.malawi.net/
- 6.Fiscus SA, Wiener J, Abrams EJ, Bulterys M, Cachafeiro A, Respess RA. Ultrasensitive p24 antigen assay for diagnosis of perinatal human immunodeficiency virus type 1 infection. J Clin Microbiol. 2007;45(7):2274–7. doi: 10.1128/JCM.00813-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyamuya E, Bredberg-Raden U, Massawe A, Urassa E, Kawo G, Msemo G, et al. Performance of a modified HIV-1 p24 antigen assay for early diagnosis of HIV-1 infection in infants and prediction of mother-to-infant transmission of HIV-1 in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr Hum Retrovirol. 1996;12(4):421–6. doi: 10.1097/00042560-199608010-00014. [DOI] [PubMed] [Google Scholar]
- 8.Nadal D, Boni J, Kind C, Varnier OE, Steiner F, Tomasik Z, et al. Prospective evaluation of amplification-boosted ELISA for heat-denatured p24 antigen for diagnosis and monitoring of pediatric human immunodeficiency virus type 1 infection. J Infect Dis. 1999;180(4):1089–95. doi: 10.1086/315012. [DOI] [PubMed] [Google Scholar]
- 9.Nouhin J, Nguyen M. Evaluation of a boosted-p24 antigen assay for the early diagnosis of pediatric HIV-1 infection in Cambodia. Am J Trop Med Hyg. 2006;75(6):1103–5. [PubMed] [Google Scholar]
- 10.Schupbach J, Boni J, Tomasik Z, Jendis J, Seger R, Kind C. Sensitive detection and early prognostic significance of p24 antigen in heat-denatured plasma of human immunodeficiency virus type 1-infected infants. Swiss Neonatal HIV Study Group. J Infect Dis. 1994;170(2):318–24. doi: 10.1093/infdis/170.2.318. [DOI] [PubMed] [Google Scholar]
- 11.Sherman GG, Stevens G, Stevens WS. Affordable diagnosis of human immunodeficiency virus infection in infants by p24 antigen detection. Pediatr Infect Dis J. 2004;23(2):173–6. doi: 10.1097/01.inf.0000109332.83246.1a. [DOI] [PubMed] [Google Scholar]
- 12.Zijenah LS, Tobaiwa O, Rusakaniko S, Nathoo KJ, Nhembe M, Matibe P, et al. Signal-boosted qualitative ultrasensitive p24 antigen assay for diagnosis of sub-type C HIV-1 infection in infants under the age of 2 years. J Acquir Immune Defic Syndr. 2005;39(4):391–4. doi: 10.1097/01.qai.0000158401.59047.84. [DOI] [PubMed] [Google Scholar]
- 13.Cachafeiro A, Sherman GG, Sohn AH, Beck-Sague C, Fiscus SA. Diagnosis of human immunodeficiency virus type 1 infection in infants by use of dried blood spots and an ultrasensitive p24 antigen assay. J Clin Microbiol. 2009;47(2):459–62. doi: 10.1128/JCM.01181-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knuchel MC, Jullu B, Shah C, Tomasik Z, Stoeckle MP, Speck RF, et al. Adaptation of the ultrasensitive HIV-1 p24 antigen assay to dried blood spot testing. J Acquir Immune Defic Syndr. 2007;44(3):247–53. doi: 10.1097/QAI.0b013e31802c3e67. [DOI] [PubMed] [Google Scholar]
- 15.Patton JC, Coovadia AH, Meyers TM, Sherman GG. Evaluation of the ultrasensitive human immunodeficiency virus (HIV)-1 p24 antigen assay on dried blood spots (DBS) for infant diagnosis. Clin Vaccine Immunol. 2007;15(2):388–91. doi: 10.1128/CVI.00265-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patton JC, Sherman GG, Coovadia AH, Stevens WS, Meyers TM. Ultrasensitive human immunodeficiency virus type 1 p24 antigen assay modified for use on dried whole-blood spots as a reliable, affordable test for infant diagnosis. Clin Vaccine Immunol. 2006;13(1):152–5. doi: 10.1128/CVI.13.1.152-155.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgisser P, Vernazza P, Flepp M, Boni J, Tomasik Z, Hummel U, et al. Performance of five different assays for the quantification of viral load in persons infected with various subtypes of HIV-1. Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2000;23(2):138–44. doi: 10.1097/00126334-200002010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Goldschmidt PL, Devillechabrolle A, Ait-Arkoub Z, Aubin JT. Comparison of an amplified enzyme-linked immunosorbent assay with procedures based on molecular biology for assessing human immunodeficiency virus type 1 viral load. Clin Diagn Lab Immunol. 1998;5(4):513–8. doi: 10.1128/cdli.5.4.513-518.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patton JC, Akkers E, Coovadia AH, Meyers TM, Stevens WS, Sherman GG. Evaluation of dried whole blood spots obtained by heel or finger stick as an alternative to venous blood for diagnosis of human immunodeficiency virus type 1 infection in vertically exposed infants in the routine diagnostic laboratory. Clin Vaccine Immunol. 2007;14(2):201–3. doi: 10.1128/CVI.00223-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George E, Beauharnais CA, Brignoli E, Noel F, Bois G, De Matteis Rouzier P, et al. Potential of a simplified p24 assay for the early diagnosis of infant HIV-1 infection in Haiti. J Clin Microbiol. 2007;45(10):3416–8. doi: 10.1128/JCM.01314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44(8):763–70. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 23.Biggar RJ, Miotti PG, Taha TE, Mtimavalye L, Broadhead R, Justesen A, et al. Perinatal intervention trial in Africa: effect of a birth canal cleansing intervention to prevent HIV transmission. Lancet. 1996;347(9016):1647–50. doi: 10.1016/s0140-6736(96)91486-5. [DOI] [PubMed] [Google Scholar]
- 24.Semba RD, Kumwenda N, Hoover DR, Taha TE, Quinn TC, Mtimavalye L, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180(1):93–8. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 25.Taha TE, Kumwenda NI, Gibbons A, Broadhead RL, Fiscus S, Lema V, et al. Short postexposure prophylaxis in newborn babies to reduce mother-to-child transmission of HIV-1: NVAZ randomised clinical trial. Lancet. 2003;362(9391):1171–7. doi: 10.1016/S0140-6736(03)14538-2. [DOI] [PubMed] [Google Scholar]
- 26.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292(2):202–9. doi: 10.1001/jama.292.2.202. [DOI] [PubMed] [Google Scholar]