Abstract
Leukocyte-endothelial interactions play central roles in many pathological conditions. However, the mechanisms responsible for non-uniform spatial distribution of adhering leukocytes to endothelial cells in microvascular networks in vivo are not clear. We used a combination of in vitro and in vivo methodologies for understanding of this complex phenomenon. A mouse cremaster muscle model was used to study the spatial distribution of leukocyte-endothelial cell interaction in vivo. A PDMS based synthetic microvascular network (SMN) device was used to study interactions of functionalized microspheres using a receptor-ligand system in a (endothelial) cell-free environment for the in vitro studies. Our in vivo and in vitro findings indicate that both leukocytes in vivo and microspheres in vitro preferentially adhere near bifurcation within 1-2 vessel diameters from the bifurcation. This adhesion pattern was found to be independent of the diameter of the vessels. These findings support our hypothesis that the fluidic patterns near bifurcations/junctions, and not the presence or absence of endothelial cells and/or receptors on their surfaces, may be the driving force behind the preferential adhesion patterns of leukocytes near bifurcations.
Introduction
The inflammatory cascade is upregulated during injury (e.g. wound healing), the development of infections (e.g. bacterial infiltration) and a number of other pathologies (e.g. myocardial infarction). A key component of this process is the adhesion of leukocytes to the microvascular endothelium. This adhesion cascade is mediated, in part, by adhesive bonds which form between glycoproteins (ligands) present on the leukocytes and cognate glycoproteins (receptors) present on the endothelium. The evolving signals then recruit leukocytes to the site of inflammation through a multistep process involving dilation of capillaries to increase blood flow near the site of inflammation, changes in molecular profile of endothelial cells, and leukocyte transmigration through the endothelium and accumulation at the affected site(Springer1994). The level of leukocyte-endothelial cell adhesion process is controlled by a combination of shear force changes generated by blood flow movements in microcirculation (Lawrence, McIntire, and Eskin1987;Luscinskas et al.1994)and upregulation of adhesion molecules (e.g. selectins and integrins) expressed on the endothelial cells(Luscinskas et al.1994). Leukocytes initially roll on the endothelium following interaction between selectins such as E-selection, P-selectin and their corresponding ligands on the leukocytes such as sialyl Lewisx ( SLex), P-selectin– glycoprotein ligand-1 (PSGL-1) and L-selectin(Lawrence, McIntire, and Eskin1987;Carlos and Harlan1994;Luscinskas et al.1994). Firm adhesion of the leukocytes on the endothelium is subsequently mediated by integrins such as ICAM-1, VCAM-1 and their corresponding ligand such as the β2 –integrins and α4β1 respectively expressed on leukocytes(Carlos and Harlan1994;Luscinskas et al.1994).
A key feature of all microvascular networks is the presence of junctions and bifurcations which has been extensively studied in larger vessels in different pathological conditions (Cunningham and Gotlieb2005) involving leukocyte recruitments such as atherosclerosis(Richter et al.2004;Cicha et al.2009;Cunningham and Gotlieb2005;Jerome and Lewis1984). These regions of the vasculature also exhibit complex fluidic patterns and deviate from the normal laminar flow observed in the microvasculature leading to other inflammatory responses(Jerome and Lewis1984). Several reports have indicated that leukocytes preferentially adhere near bifurcations in microvascular networks (Sumagin, Lamkin-Kennard, and Sarelius2009) but this phenomenon and its underlying mechanisms have not been systematically studied. Of special interests is whether shear force changes or possibly a special subclass of endothelial cells are responsible for this increased adhesion near bifurcations. While techniques such as intra vital microscopy have been used in the past to study leukocyte-endothelial interactions in vivo(Zarbock and Ley2009;Kiani et al.2002), realistic in vitro models of microvascular networks for these types of studies have been rare. Parallel flow chamber which have been used routinely to study leukocyte-endothelium interactions in vitro(Lawrence, McIntire, and Eskin1987;Lawrence et al.1990) do not accurately replicate the complex morphology observed in the microvascular networks such as bifurcations, cross-sectional area changes, and tortuous path lengths, all of which are important features found in the in vivo microcirculation(Gaehtgens1992;Prabhakarpandian et al.2008).
Recently we developed a novel microfluidic device; synthetic microvascular network (SMN), that mimics the in vivo microvasculature(Prabhakarpandian et al.2008;Rosano et al.2009). In the current study, we compared the spatial leukocyte adhesion patterns in the microvasculature of mouse cremaster muscle to the adhesion patterns of functionalized microspheres using a ligand-receptor combination in a cell free SMN to determine the critical mechanisms responsible for the preferential adhesion of leukocytes near bifurcations. We hypothesized that the presence of endothelial cells is not the determining factor in the preferential leukocyte adhesion near bifurcations.
Materials and Methods
In vivo model
Male C57BL mice (5–7 weeks of age) were purchased from Harlan Laboratories (Frederick, MD). The open cremaster muscle preparation surgical procedure used in this study have been described before (Roth and Kiani1999). Briefly, mouse cremaster muscle was cut, flattened and pinned on a surgery board. Animals used to explore leukocyte-endothelial cell interaction were treated with TNF-α (500ng)/animal(Sumagin and Sarelius2006), by testicular injection, four hours prior to surgery. In order to visualize leukocyte-endothelial cell interactions, a fluorescent dye, Rhodamine, was used to label the leukocytes. Rhodamine 6G (0.4 mg/ kg body weight; Sigma, St. Louis, MO) with excitation and emission of 525nm and 555nm, respectively, was introduced into the mice by penile injection 30 seconds prior to imaging(Yuan et al.2005).
The microvascular networks were mapped using a modified Geographic Information System (GIS)(Roth and Kiani1999).A Nikon Eclipse FN1 fluorescent microscope was used to obtain the images of labeled leukocytes. The images were taken under 10× magnification with a Nikon digital camera (CoolSnap-EZ Monochrome, Tuscan, AZ) and controlled by the NIS-Elements AR 3.0 imaging software (Melville, NY). Adobe Photoshop was then used to create a collage of images which represents the entire microvascular network. The collage image was inserted into Autodesk MAP. Leukocytes that were immobilized for a minimum of 30 seconds were identified as firmly adhered leukocytes on the vessel wall and were marked on the collage image. The distance to the nearest bifurcation for each adhering leukocytes was then measured in each network. The normalized frequency of adhered leukocytes was calculated using the following formula:
Skewness, which is a measure of how symmetrical data points are distributed about the mean and Kurtosis, a measure which describes the shape of the distribution peak, were calculated by SigmaStat 3.1 (Systat software, Inc. Chicago, IL,). Data are presented as mean ± standard error of the mean (SEM).
In vitro model
The microvascular network of a cremaster muscle was mapped using a modified Geographic Information Systems (GIS)(Roth and Kiani1999). SMNs were created using soft lithography techniques described previously(Rosano et al.2009).
The inlet of the SMN chip was connected to a 1mL syringe using microbore tubing. A 8-port perfusion manifold (Harvard Apparatus) was used to connect the outlets of the microvascular chip. The inlet of the perfusion manifold was connected to a 1mL syringe (without a plunger) held in the vertical position above the chip. Avidin (Invitrogen, Carlsbad, CA) at a concentration of 20μg/ml was pipette into the syringe. The syringe at the inlet was set to refill at 1ul/min until the entire channels were filled. The flow was stopped, the tubing's clamped and the SMN chip was then stored at 4C overnight.
Prior to experiments, the SMN chip was taken out from 4°C and allowed to come to room temperature. A 3 way valve (Upchurch Scientific, Oak Harbor, WA) was used to connect a syringe with PBS. One port served as a waste port, and the second port of the valve was connected to one of the arms of the 1/16” Y-connector (Cole Parmer, Vernon Hills, Illinois). The second arm of the connector was connected to the syringe loaded with 2 μm biotinylated particles at a concentration of 5×105/ml. The outlet arm of the Y connector joined to 1/16” PEEK tubing served as the inlet port. The connected syringes were then placed on a programmable syringe pump (PHD 200, Harvard Apparatus, MA) at a flow rate of 10μl/min. The Y connector was initially primed with PBS followed by the biotinylated particles by switching the 3-way valve connected to PBS to go to the waste port. The flow was gradually reduced to a flow rate of 2.5μl/min (corresponding to shear rate of ~ 250 sec-1) and the PEEK tubing was inserted into the inlet of the SMN chip. At the first sign of entry of particles into the network, a timer was started and the particles flow was continued for 5 minutes. At the end of 5 minutes, PBS from the 3-way valve was turned on to flow via the Y connector into the inlet port and the flow from the particle syringe was stopped. PBS flush of particles was continued for about a minute to completely wash off particles from the inlet port. A scan of the entire SMN was performed using the automated stage at 4X objective on an epi-fluorescence microscope ( NIKON TE 2000, Melville, NY). A cooled CCD camera, Retiga Exi (Qimaging, Surrey, BC, Canada) was used for acquiring images. The acquired images were post processed using NIKON Elements software. The distance of each of the adhered particles to the center of the bifurcation was measured for each branch of SMN.
Results
In vivo model
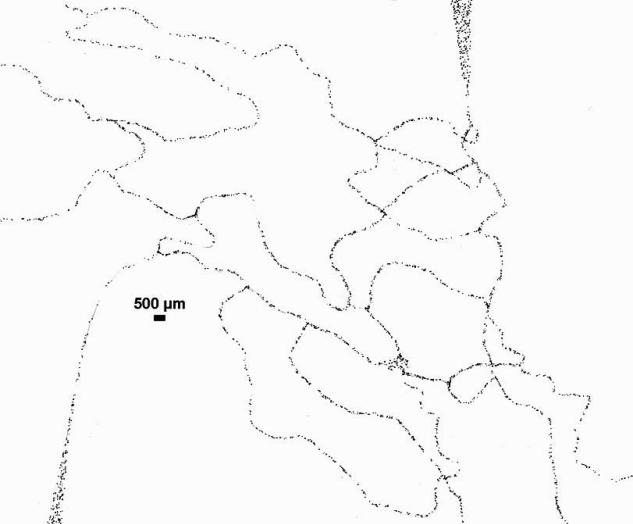
Adhesion pattern of Rhodamine labeled leukocytes in the vicinity of a bifurcation from one of the microvascular networks used in our study is shown in Figure 1. Vessel diameters (total of 90 vessels) and adhesion patterns of adhered leukocytes (total of 2,733 adhered leukocytes) in four microvascular networks were analyzed. The adhered leukocytes located exactly at the center of a bifurcation were assumed to have a minimum distance of zero from the bifurcation. The distance of each subsequent leukocyte was measured from this center of the bifurcation.
Figure 1.

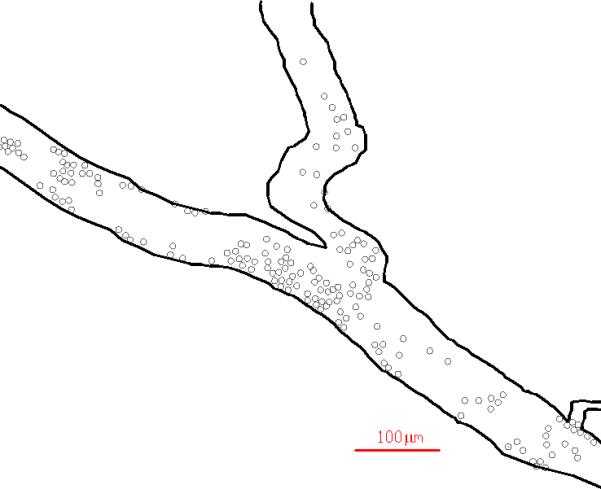
A typical example of spatial distribution of Rhodamine labeled leukocytes in a vessel of the mouse cremaster muscle showing their preferential adhesion near bifurcations. Both raw (top panel) and post processed (bottom panel) images are shown.
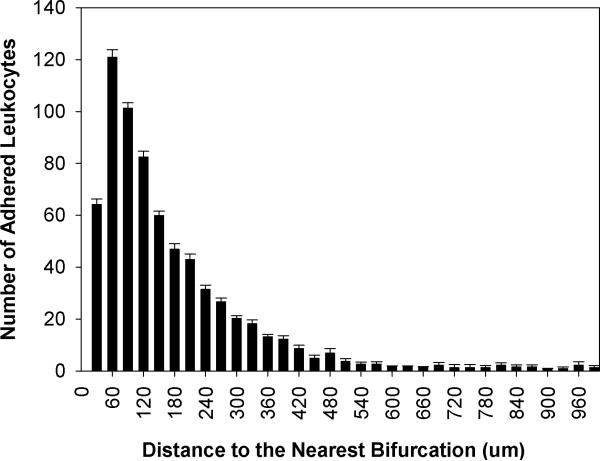
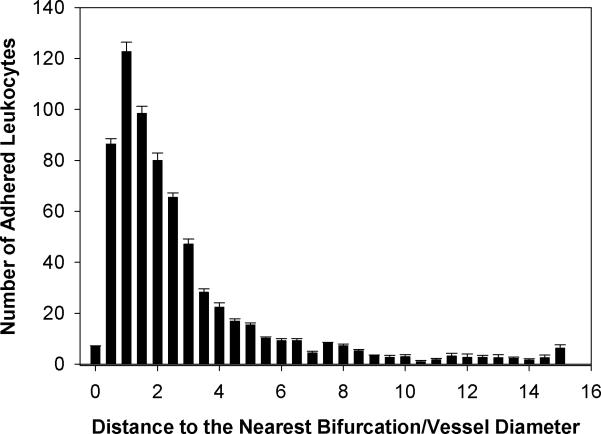
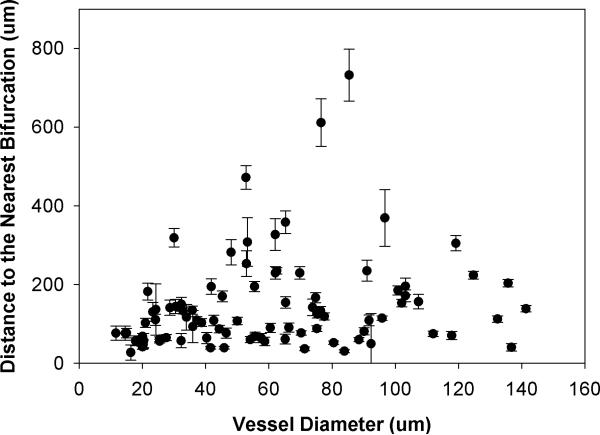
Spatial distribution of adhered leukocytes along the vessels throughout all four networks - had a peak at 60μm to the nearest bifurcation which corresponded with the average vessel diameter of 61.07μm in all the networks (Figure 2). Table 1 shows the summary of these adhesion data. A skewness of 2.9 and kurtosis of 9.6 for the adhesion patterns displays a large shift of the distance distribution to the left, indicating that leukocytes adhere preferentially near bifurcations. Furthermore, our findings indicate that adhered leukocytes mainly accumulate within the normalized distance of one vessel diameter from the nearest bifurcation and the number of adhered leukocytes decreases with an increasing distance from the nearest bifurcation (Figure 3). As shown in Figure 4, the average distance of adhered leukocytes to the nearest bifurcation in vivo did not correlate with vessel size.
Figure 2.
Distribution of the number (Mean ± SEM) of adhered leukocyte as a function of distance from the nearest bifurcation. This histogram is skewed to the left indicating that leukocytes preferentially adhere near bifurcations with the peak occurring at 60 μm from the nearest bifurcation. This peak is at the average diameter (61.07μm) of all vessels in the four networks used in this study.
Table 1.
Summary of the distance from adhering leukocyte to the nearest bifurcation in vivo.
| Distance to the Nearest Bifurcation | |||||
|---|---|---|---|---|---|
| Total Number of Adhered Leukocytes | Min (um) | Max (um) | Mean(um)±SEM | Skewness | Kurtosis |
| 2773 | 0 | 1449.4 | 194.6 ± 4.6 | 2.9 | 9.6 |
The total number of adhered leukocytes in all four networks was 2,773 with a minimum of distance of zero, exactly at the center of the bifurcations, and a maximum distance of 1449.4 μm which represents adhered leukocytes at the center of a vessel with approximately 2900.0 μm in length. A skewness of 2.9 and kurtosis of 9.6 indicate a large shift of the distance distribution to the right, indicating that the leukocytes adhere preferentially near the bifurcations.
Figure 3.
Distribution of the number (Mean ± SEM) of adhered leukocyte as a function of number of vessel diameters from the nearest bifurcation. Consistent with the results shown in Figure 2, adhered leukocytes mainly accumulate within the normalized distance of one vessel diameter from the nearest bifurcation and the number of adhered leukocytes decreases with distance from the nearest bifurcation.
Figure 4.
Distribution of distance to the nearest bifurcation (Mean ± SEM) of adhered leukocytes as a function of vessel diameter. The average distance of adhered leukocytes to the nearest bifurcation in vivo did not correlate with vessel diameter.
In vitro Study
Overall, particle adhesion patterns in the Synthetic Microvascular Networks (SMN) exhibited patterns that were similar to those observed in the vivo data (Figure 5). Figure 6 shows that in the SMN with a diameter of 100 um, there was preferential adhesion of particles near bifurcations and the level of adhesion had a negative correlation with distance to the bifurcation. In this case the peak of adhesion was at one vessel diameter from the bifurcation, i.e. 100 μm. Since in vivo we measured leukocytes in a range of vessel diameters, we then fabricated SMN's of 50 and 25 μm constant depth and repeated the experimental procedure to determine if particle adhesion patterns were diameter dependent. As shown in Figure 7 and Figure 8 and consistent with data in the 100 μm vessels and our in vivo observation, there was preferential adhesion of particles near bifurcations and the level of adhesion had a negative correlation with distance to the bifurcation. However, the peak adhesion of particles in these smaller vessels was observed at two vessel diameters from the bifurcation. Hence for the 50um SMN chip, the maximal adhesion was observed at a distance of 100 μm from the bifurcation, and for the 25μm SMN chip the maximal adhesion was observed at a distance of 50 μm from the bifurcation. These findings support the hypothesis that the maximum distance for particle adhesion in junctions may be dependent on the diameter of the channels but this distance is always at the maximum of twice the diameter of the channels. It should be noted that the key difference between these different SMN chips is that the fluidic condition experienced at the bifurcations may be different due to the different diameters but the surface receptor (avidin) and the ligand (biotin) concentrations are similar.
Figure 5.
A typical example of spatial distribution of biotin conjugated particles in SMN chip with a diameter of 100μm. Image has been digitally inverted to show details of the adhered particles.
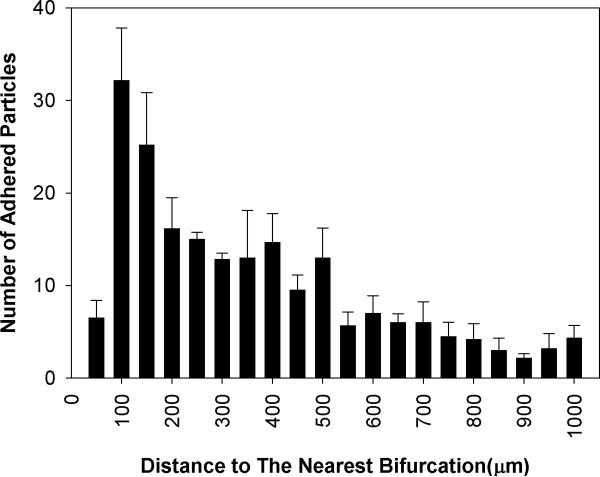
Figure 6.
Distribution of the number (Mean ± SEM) of adhered functionalized microspheres in SMN chip with a diameter of 100μm. Consistent with in vivo finding, biotin conjugated particles preferentially adhere near bifurcations with the peak occurring at a distance equal to the diameter of the vessel (100 μm).
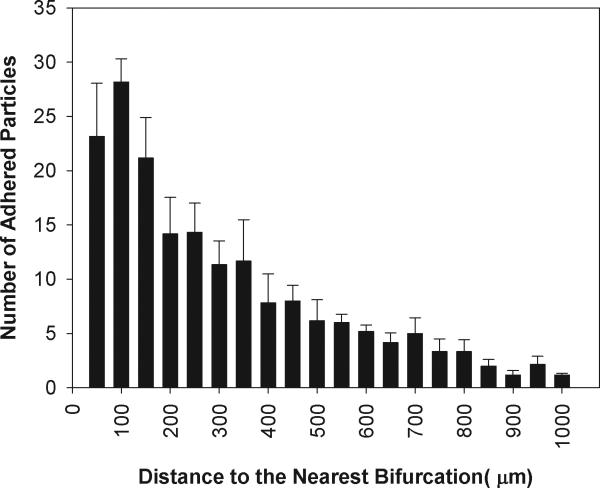
Figure 7.
Distribution of the number (Mean ± SEM) of adhered functionalized microspheres in SMN chip with a diameter of 50μm. Consistent with in vivo finding and those of 100 μm SMN, biotin conjugated particles preferentially adhere near bifurcations but in this case the peak occurs at a distance equal to the two vessel diameters (100 μm) from the bifurcation.
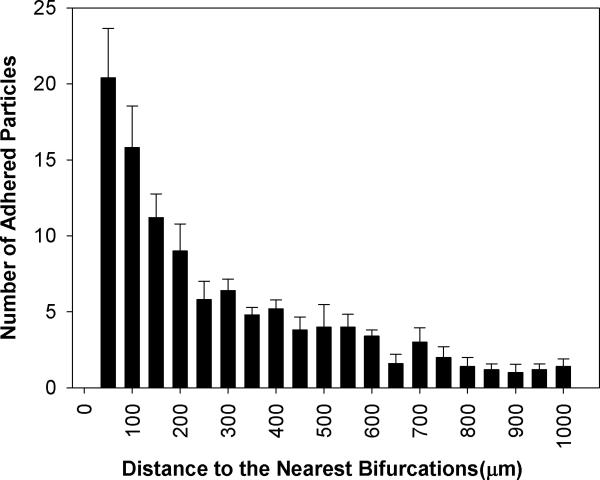
Figure 8.
Distribution of the number (Mean ± SEM) of adhered functionalized microspheres in SMN of diameter of 25μm. Consistent with in vivo finding and those of 100 μm and 50 μm SMNs, biotin conjugated particles preferentially adhere near bifurcations. Similar to the findings from 50 μm SMNs, the peak of adhesion occurs at a distance equal to the two vessel diameters (50 μm) from the bifurcation.
Discussion
Interplay of hemodynamic forces, shear rate, adhesion mechanics, and ligand-receptor interactions govern leukocyte adhesion to endothelial cells in various parts of microvascular networks in vivo(Tees and Goetz2003;Bienvenu and Granger1993). We have used the mouse cremaster muscle to study the spatial distribution of leukocyte-endothelial cell interaction in vivo and a PDMS synthetic microvascular network (SMN) device to study the spatial distribution of biotin conjugated microspheres to avidin coated surfaces in a (endothelial) cell-free environment in vitro. Overall, our in vivo and in vitro findings indicate that both leukocytes in vivo and ligand coated microspheres in vitro preferentially adhere near bifurcations supporting the hypothesis that the fluidic patterns, and not the presence or absence of endothelial cells and/or receptors on their surfaces, may be the driving force behind the preferential adhesion of leukocyte near bifurcations. This is also in agreement with recently observed data (Sumagin, Lamkin-Kennard, and Sarelius2009) that in bifurcating regions, fluid shear exhibits an effect on leukocyte endothelial cell interactions.
In the microvascular networks of the cremaster muscle, the highest level of leukocyte adhesion was at an average distance of one vessel diameter away from the center of the bifurcation and this peak did not change with vessel diameter. Similarly, in the synthetic micovascular networks (SMN) in vitro, the highest level of adhesion of biotin conjugated particles to avidin coated surfaces was also at an average distance of one vessel diameter for larger vessels (100μm). However, for smaller diameter vessels (50 μm or 25 μm), the highest adhesion level of particles occurred at a distance two vessel diameters from the bifurcation. Further studies are required to determine the mechanisms that may be responsible for this discrepancy between our in vivo and in vitro findings. The use of rigid particles conjugated with biotin molecule in the in vitro model versus flexible leukocytes bearing multiple ligands in the in vivo model may in part explain this discrepancy. Other factors such as the possible differential expression of receptors and ligands may also influence these adhesion patterns but the fact that such dissimilar in vivo and in vitro systems result in similar adhesion spatial patterns is a strong indication that hymodynamic forces, and not cellular characteristics, may be responsible for this phenomenon.
Leukocyte adhesion at or near bifurcations in vivo is commonly observed during pathological conditions such as atherosclerosis where turbulent blood flow and changes in shear stress in conjunction with recirculation zones are commonly reported(Richter et al.2004;Sharma, Mares, and Kini2009;Ridger et al.2008). This is consistent with our observations in the current study of preferential adhesion of leukocytes near bifurcations and our previous study showing that flow fluctuations at the junctions are correlated with increased cell/particle adhesion(Prabhakarpandian et al.2008). The findings from this study could provide a better understanding of the mechanisms behind these pathologies and, for example, providing appropriate targets for treatment.
In conclusion, the results of this study indicate that particles/cells adhere preferentially near bifurcations with a peak within 1-2 vessel diameters from the bifurcation. More importantly, our results support the hypothesis that hemodynamic forces and not cellular differences may be responsible for this non-uniform spatial distribution of leukocytes in microvascular networks in vivo.
Acknowledgement
This work was supported by NIH (2R44HL076034-02), the American Heart Association, and the Pennsylvania Department of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Bienvenu K, Granger DN. Molecular Determinants of Shear Rate-Dependent Leukocyte Adhesion in Postcapillary Venules. Am.J.Physiol. 1993;264(5 Pt 2):H1504–H1508. doi: 10.1152/ajpheart.1993.264.5.H1504. [DOI] [PubMed] [Google Scholar]
- 2.Carlos TM, Harlan JM. Leukocyte-Endothelial Adhesion Molecules. Blood. 1994 Oct 1;84(7):2068–101. [PubMed] [Google Scholar]
- 3.Cicha I, Beronov K, Ramirez EL, Osterode K, Goppelt-Struebe M, Raaz D, Yilmaz A, Daniel WG, Garlichs CD. Shear Stress Preconditioning Modulates Endothelial Susceptibility to Circulating TNF-Alpha and Monocytic Cell Recruitment in a Simplified Model of Arterial Bifurcations. Atherosclerosis. 2009 May 3; doi: 10.1016/j.atherosclerosis.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham KS, Gotlieb AI. The Role of Shear Stress in the Pathogenesis of Atherosclerosis. Lab Invest. 2005;85(1):9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 5.Gaehtgens P. Why Networks? Int.J.Microcirc.Clin.Exp. 1992;11(2):123–32. [PubMed] [Google Scholar]
- 6.Jerome WG, Lewis JC. Early Atherogenesis in White Carneau Pigeons. I. Leukocyte Margination and Endothelial Alterations at the Celiac Bifurcation. Am J Pathol. 1984;116(1):56–68. [PMC free article] [PubMed] [Google Scholar]
- 7.Kiani MF, Yuan H, Chen X, Smith L, Gaber MW, Goetz DJ. Targeting Microparticles to Select Tissue Via Radiation-Induced Upregulation of Endothelial Cell Adhesion Molecules. Pharm.Res. 2002;19(9):1317–22. doi: 10.1023/a:1020350708672. [DOI] [PubMed] [Google Scholar]
- 8.Lawrence MB, McIntire LV, Eskin SG. Effect of Flow on Polymorphonuclear Leukocyte/Endothelial Cell Adhesion. Blood. 1987;70(5):1284–90. [PubMed] [Google Scholar]
- 9.Lawrence MB, Smith CW, Eskin SG, McIntire LV. Effect of Venous Shear Stress on CD18-Mediated Neutrophil Adhesion to Cultured Endothelium. Blood. 1990 Jan 1;75(1):227–37. [PubMed] [Google Scholar]
- 10.Luscinskas FW, Kansas GS, Ding H, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA., Jr. Monocyte Rolling, Arrest and Spreading on IL-4-Activated Vascular Endothelium Under Flow Is Mediated Via Sequential Action of L-Selectin, Beta 1-Integrins, and Beta 2-Integrins. J.Cell Biol. 1994;125(6):1417–27. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prabhakarpandian B, Pant K, Scott RC, Patillo CB, Irimia D, Kiani MF, Sundaram S. Synthetic Microvascular Networks for Quantitative Analysis of Particle Adhesion. Biomed.Microdevices. 2008 Mar 8; doi: 10.1007/s10544-008-9170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter Y, Groothuis A, Seifert P, Edelman ER. Dynamic Flow Alterations Dictate Leukocyte Adhesion and Response to Endovascular Interventions. J.Clin.Invest. 2004;113(11):1607–14. doi: 10.1172/JCI21007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridger V, Krams R, Carpi A, Evans PC. Hemodynamic Parameters Regulating Vascular Inflammation and Atherosclerosis: a Brief Update. Biomed.Pharmacother. 2008;62(8):536–40. doi: 10.1016/j.biopha.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 14.Rosano JM, Tousi N, Scott RC, Krynska B, Rizzo V, Prabhakarpandian B, Pant K, Sundaram S, Kiani MF. A Physiologically Realistic in Vitro Model of Microvascular Networks. Biomed.Microdevices. 2009 May 19; doi: 10.1007/s10544-009-9322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth NM, Kiani MF. Geographic Information Systems” Based Technique for the Study of Microvascular Networks. Ann.Biomed.Eng. 1999;27(1):42–47. doi: 10.1114/1.204. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SK, Mares AM, Kini AS. Coronary Bifurcation Lesions. Minerva Cardioangiol. 2009;57(5):667–82. [PubMed] [Google Scholar]
- 17.Springer TA. Traffic Signals for Lymphocyte Recirculation and Leukocyte Emigration: the Multistep Paradigm. Cell. 1994 Jan 28;76(2):301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 18.Sumagin R, Lamkin-Kennard KA, Sarelius IH. A Separate Role for ICAM-1 and Fluid Shear in Regulating Leukocyte Interactions With Straight Regions of Venular Wall and Venular Convergences. Microcirculation. 2009;16(6):508–20. doi: 10.1080/10739680902942271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumagin R, Sarelius IH. TNF-Alpha Activation of Arterioles and Venules Alters Distribution and Levels of ICAM-1 and Affects Leukocyte-Endothelial Cell Interactions. Am.J.Physiol Heart Circ.Physiol. 2006;291(5):H2116–H2125. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tees DF, Goetz DJ. Leukocyte Adhesion: an Exquisite Balance of Hydrodynamic and Molecular Forces. News Physiol Sci. 2003:18186–90. doi: 10.1152/nips.01444.2003. [DOI] [PubMed] [Google Scholar]
- 21.Yuan H, Goetz DJ, Gaber MW, Issekutz AC, Merchant TE, Kiani MF. Radiation-Induced Up-Regulation of Adhesion Molecules in Brain Microvasculature and Their Modulation by Dexamethasone. Radiat.Res. 2005;163(5):544–51. doi: 10.1667/rr3361. [DOI] [PubMed] [Google Scholar]
- 22.Zarbock A, Ley K. New Insights into Leukocyte Recruitment by Intravital Microscopy. Curr.Top.Microbiol.Immunol. 2009:334129–52. doi: 10.1007/978-3-540-93864-4_6. [DOI] [PubMed] [Google Scholar]