Abstract
Rapid-onset dystonia with parkinsonism (RDP) or DYT12 dystonia is a rare form of primary, generalized dystonia. Patients do not present with any symptoms until triggered by a physiological stressor. Within days, patients will show both dystonia and Parkinson’s disease. Missense mutations resulting in a loss of function in the ATP1A3 gene have been identified as the cause of RDP. ATP1A3 encodes the α3 subunit of the Na+/K+-ATPase in neurons and cardiac cells. We have previously created a line of mice harboring a point mutation of the Atp1a3 gene (mouse homolog of the human ATP1A3 gene) that results in a loss of function of the α3 subunit. The Atp1a3 mutant mice showed hyperactivity, spatial learning and memory deficits, and increased locomotion induced by methamphetamine. However, the full spectrum of the motor phenotype has not been characterized in the Atp1a3 mutant mice and it is not known whether triggers such as restraint stress affect the motor phenotype. Here, we characterized the motor phenotype in normal heterozygous Atp1a3 mutant mice and heterozygous Atp1a3 mutant mice that have been exposed to a restraint stress. We found that this type of trigger induced significant deficits in motor coordination and balance in the mutant mice, characteristic of other genotypic dystonia mouse models. Furthermore, stressed mutant mice also had a decreased thermal sensitivity and alterations in monoamine metabolism. These results suggest that the Atp1a3 mutant mouse models several characteristics of RDP and further analysis of this mouse model will provide great insight into pathogenesis of RDP.
Keywords: DYT12 dystonia, rapid-onset dystonia with parkinsonism, ATP1A3, Na+/K+-ATPase, dopamine, sensory deficit
1. Introduction
Dystonia is a neurological movement disorder that is characterized by sustained contractions that cause twisting and repetitive movements or abnormal postures (Fahn, 1988). Dystonia can be classified according to the part of the body it affects. Generalized dystonia affects most or all of the body, while focal dystonia affects only a certain muscle group. Another classification of dystonia is by the etiology. Primary dystonia arises spontaneously and often has a genetic component, while secondary dystonia is a result of another neurological or metabolic disease. To date, there are at least 20 forms of inherited, monogenic dystonias, but only half have identified genes (Breakefield et al., 2008; Camargos et al., 2008; Fuchs et al., 2009; Muller, 2009).
Parkinson’s disease is another neurological movement disorder that is characterized by four hallmark features: resting tremors, akinesia, slowness of movement, and postural instability (Jankovic, 2008). The majority of patients are over the age of 50 and have Parkinson’s disease without a specific known cause (Jankovic, 2008). However, in 5–10% of patients there is a known genetic component, which usually results in Parkinson’s disease earlier in life. Generally, both forms of Parkinson’s disease results in neurodegeneration of dopaminergic neurons in the substantia nigra, which causes the motor symptoms.
These two neurological disorders can occur together. For example, rapid-onset dystonia with parkinsonism (RDP) or DYT12 dystonia is a rare form of generalized, primary dystonia with Parkinson’s disease features. DYT12 dystonia patients do not present with any symptoms until triggered by a physiological stressor (e.g. fever), typically in late adolescence or early adulthood (Brashear et al., 1997; Dobyns et al., 1993). After this trigger, symptoms appear minutes to days and are permanent thereafter (Brashear et al., 2007; Dobyns et al., 1993; Kamm et al., 2004; Linazasoro et al., 2002; Pittock et al., 2000; Zaremba et al., 2004). Symptoms first affect the face, then the arms, and finally the legs, commonly referred to as a rostrocaudal gradient (Brashear et al., 2007). Although a dopaminergic system alteration is proposed to be part of the pathophysiological basis of DYT12 dystonia (Brashear et al., 1998a; Brashear et al., 1998b), there is no neurodegeneration of dopaminergic neurons as in Parkinson’s disease (Muller et al., 2002). Additionally, patients have little relief from dopaminergic treatments, such as L-DOPA (Brashear et al., 2007).
The inheritance of DYT12 dystonia has been determined to be autosomal dominant with reduced penetrance (de Carvalho Aguiar et al., 2004). The causative gene of DYT12 dystonia is the ATP1A3 gene (Brashear et al., 2007; de Carvalho Aguiar et al., 2004). ATP1A3 encodes the α3 subunit of the Na+/K+-ATPase. The Na+/K+-ATPase regulates the electrochemical gradients of sodium (Na+) and potassium (K+) ions through active transport. These ions are essential for numerous cellular functions including regulation of cellular osmolarity and action potentials of excitable membranes (Dobretsov and Stimers, 2005). The α3 subunit is one of four α isoforms that serve as the catalytic component of the Na+/K+-ATPase by hydrolyzing ATP (Lingrel and Kuntzweiler, 1994). The α3 subunit is exclusively expressed in neurons and cardiac muscle cells (Lingrel et al., 2007). Seven missense mutations in the ATP1A3 gene have been identified in DYT12 dystonia patients (Brashear et al., 2007; Zanotti-Fregonara et al., 2008). Recently, six of these mutations have been shown to impair the activity or stability of the α3 subunit in cultured cells (de Carvalho Aguiar et al., 2004). This suggests that these missense mutations lead to a reduction or loss-of-function resulting in haploinsufficiency of the Na+/K+-ATPase in neurons and cardiac cells (de Carvalho Aguiar et al., 2004). It has been proposed that haploinsufficiency exacerbates the cellular response to a physiological stressor in DYT12 dystonia patients, similar to stress induced channelopathies such as myasthenia gravis or chronic fatigue syndrome (Breakefield et al., 2008; Cannon, 2006).
Currently no animal model has been characterized for DYT12 dystonia. However, we previously created a point mutation in the Atp1a3 gene at the exon/intron boundary before the fourth intron, which resulted in an aberrant splicing and lethality of homozygous mutant mice (Moseley et al., 2007). Heterozygous mice carrying this Atp1a3 mutation can survive to adulthood and show an approximate 50% reduction in α3 subunit expression (Moseley et al., 2007). Heterozygous Atp1a3 mutant (Het) mice exhibit alterations in spatial learning and hyperactivity, but no alteration in anxiety-like behaviors. Additionally, Het mice show alterations in the dopaminergic system (Moseley et al., 2007). Het mice have not been subjected to an comprehensive test battery of motor behaviors as the other genotypic mouse models of dystonia (Dang et al., 2005; Dang et al., 2006a; Grundmann et al., 2007; Page et al., 2010; Sharma et al., 2005; Yokoi et al., 2006; Yokoi et al., 2008; Yokoi et al., 2010). Additionally, as symptoms in patients with DYT12 dystonia are triggered by a physiological stressor, the effects of stress on these mice were left unanswered (Breakefield et al., 2008; Muller, 2009).
We attempted to address these questions by characterizing the motor behaviors in normal Het mice and Het mice that have been exposed to a restraint stress. Additionally, we have investigated the sensory system of these stressed Het mice as several studies have suggested a hypofunctioning sensory system in dystonia patients (Aglioti et al., 2003; Bara-Jimenez et al., 2000; Fiorio et al., 2007; Fiorio et al., 2008; Molloy et al., 2003; Peller et al., 2006; Sanger et al., 2001; Tamura et al., 2008; Tinazzi et al., 1999; Tinazzi et al., 2000; Tinazzi et al., 2002a; Tinazzi et al., 2002b; Tinazzi et al., 2004). Furthermore, we analyzed the voluntary activity levels of stressed Het mice as we hypothesized that the discomfort of running due to the dystonic-like symptoms would decrease voluntary activity levels. We found that non-stressed Het mice exhibit no deficits on the beam-walking test. After exposure to a restraint stress, however, female Het mice exhibited a dramatic deficit in the beam-walking and rotarod tests. Additionally, we have found alterations in the sensory system and voluntary activity levels in the stressed Het mice. Our data suggest that the stressed female Het mice model several characteristics of DYT12 dystonia. With the Het mice, the pathophysiology of the disease can be better elucidated and new treatments can be developed for DYT12 dystonia.
2. Materials and Methods
All experiments were carried out in compliance with the USPHS Guide for Care and Use of Laboratory Animals and approved by the Institutional Animal Use and Care Committee at the University of Alabama at Birmingham (UAB).
2.1 Mice
Heterozygous Atp1a3 mutant (Het) mice in C57BL/6 background were bred with wild-type C57BL/6 (WT) mice to produce experimental animals. The animals were genotyped by PCR as previously described (Moseley et al., 2007). These mice were housed under a 12 hour light, 12 hour dark cycle with ad libitum access to food and water. No spontaneous seizures were observed as reported in mice carrying another Atp1a3 mutation (Clapcote et al., 2009). Two cohorts of animals were used in the following experiments. The first cohort consisted of 15 WT (9 males and 6 females) and 19 Het (9 males and 10 females) of approximately 6 months old. The mice were first tested on the beam-walking test. Following the beam-walking test the mice were stressed using a restraint stress protocol. After the restraint stress exposure mice were tested in the following order: beam-walking test, grip strength test, wheel running, and tail flick sensory test (Figure 1). Two male animals died during the wheel running test. The second cohort consisted of 22 WT (10 males and 12 females) and 15 Het (7 males and 8 females) of approximately 6 months old. For this cohort, all mice were stressed using the same restraint stress protocol as the first cohort. Following the restraint stress exposure the mice were used in the following order: open field locomotion test, accelerated rotarod test, and high performance liquid chromatography (HPLC) (Figure 1). Mice were rested approximately one week in between experiments. All experiments were conducted by investigators blind to the genotype of the mice.
Figure 1. Experimental design.
Two cohorts of mice were used for all experiments. The first cohort of mice was first analyzed using the beam-walking test followed by a restraint stress and then another beam-walking test. After the second beam-walking test the following experiments were conducted: grip strength, wheel running, and tail flick. The second cohort of mice was first exposed to a restraint stress and then tested on the following experiments: rotarod, open field, and HPLC.
2.2 Restraint stress protocol
To expose mice to a physiological stressor, mouse movement was constrained using a Mouse Decapicone (Braintree Scientific) disposable restrainer. Each mouse was placed in the restrainer for two consecutive 60 minutes sessions a day for five continuous days. After five days, mice were allowed to rest for two weeks before experimentation. Herein, we define mice that have not undergone a restraint stress protocol as non-stressed mice and mice that have undergone a restraint stress protocol as stressed mice.
2.3 Motor behavior
2.3.1 Beam-walking test
The beam-walking test assesses motor coordination and motor learning as mice transverse a narrow beam of various diameters to a dark box at the other end. The test was performed as previously described (Carter, 2001). In brief, animals were first trained to transverse a medium square beam (approximately 14 mm wide) for two consecutive days with three trials each day. After training, the animals were tested on the following two consecutive days. On the first testing day animals transversed the medium square beam and a medium round beam (approximately 17 mm diameter). On the second testing day animals transversed a small round beam (approximately 10 mm diameter) and a small square beam (approximately 7 mm wide). Each beam was repeated twice consecutively and the time to transverse the beam and number of hind limb slips were recorded. The beam-walking test was performed twice on the same mice, once before the mice underwent a restraint stress protocol and again after the restraint stress protocol.
2.3.2 Accelerated Rotarod
The rotarod test assesses the ability of mice to maintain balance and coordination on an accelerating, rotating rod, as previously described (Dang et al., 2006b). The Economex accelerating rotarod (Columbus Instruments) started at an initial 4 rpm and accelerated at a rate of 0.2 rpm/s to a final rate of 28 rpm over two minutes. Mice were tested for two consecutive days with three trials each day. The trials each day were performed approximately one hour apart from each other. The latency for the mouse to fall was measured for each trial with a cutoff of two minutes.
2.4 Grip strength
To assess forelimb and hind limb strength, a grip strength meter (San Diego Instruments) was used. The meter records the force of a metal grate being pulled in newtons. To measure forelimb strength, the mice were held by their tail at an angle that prevented the hind limbs from grasping the metal grate and were quickly pulled back. To measure hind limb strength, the mice were held by the skin behind their neck and held by their tail at an angle that prevented the forelimbs from grasping the metal grate and were quickly pulled back. Each measurement was conducted three times and the maximum force of the three trials was used for statistical analysis.
2.5 Activity levels
2.5.1 Open field
An open field apparatus was used to measure spontaneous activity and stereotypic behaviors. The open field apparatus (Lafayette Instruments) is equipped with infrared sensors that detect breaks in the beams. This information is analyzed by the Digiscan System (Accuscan Instruments) as described previously (Cao and Li, 2002; Dang et al., 2005; Yokoi et al., 2009). The center of the chamber was exposed to bright illumination (produced by a 60W bulb). Each mouse was placed in the center of the open field box and activity was recorded for 15 minutes.
2.5.2 Wheel running
Mice were tested for voluntary activity levels using a standard wheel running chamber (Lafayette Instruments). The chamber is equipped with an optical sensor to detect the speed and number of revolutions of the wheel. This information was collected by a computer at five minute intervals across six consecutive days. The first three days of recordings were excluded from analysis due to acclimation and novelty of the wheel. Mice were housed under a 12 hour light, 12 hour dark cycle with food and water accessible ad libitum.
2.6 Tail flick
Mice were tested for perception of pain using the Tail Flick Analgesia Meter (San Diego Instruments). The mouse was placed in an acrylic restrainer with the distal end of its tail protruding under a heat lamp. The heat lamp was then manually switched on. In the first five seconds the lamp reached a temperature of approximately 40°C and the temperature rose from there to 50°C at 10 seconds and 57°C at 15 seconds. The timer started once the heat lamp was turned on and automatically stopped when the mouse produced a strong reaction to the heating and moved the tail. This latency to respond was limited to 15 seconds to prevent injury to the mouse.
2.7 HPLC
To determine various neurochemical levels in striatum, high performance liquid chromatography (HPLC) was conducted using striatal extracts from mice as previously described (Dang et al., 2005; Yokoi et al., 2006) except using 50 mM potassium phosphate buffer with 0.5 mM octyl sulfate (Sigma-Aldrich) and 8% acetonitrile as running buffer. Dopamine (DA), serotonin (5-HT), 3,4-dihyroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), and 3-methoxytyramine (3-MT) were measured by the Neurochemistry Core Lab, Vanderbilt University Medical Center, Nashville, TN (Lindsey et al., 1998).
2.8 Statistics
Statistics was performed using the SAS/STAT 9.1 (SAS Institute). Multifactorial ANOVA was used to analyze data with genotype, sex, age, and body weight as variables in the models for all experiments except the beam-walking test. Means and standard errors of the mean (SEM) were derived by the least squares (LS) means method. The beam-walking test data were analyzed by logistic regression (GENMOD) with negative binominal distribution using GEE model using genotype, sex, age and body weight as variables. The data were analyzed after log transformation to obtain a normal distribution. WT mice were normalized to zero. Significance was assigned by a p value of less than 0.05.
3. Results
3.1 Motor deficits in female heterozygous Atp1a3 mutant mice
3.1.1 Beam-walking test
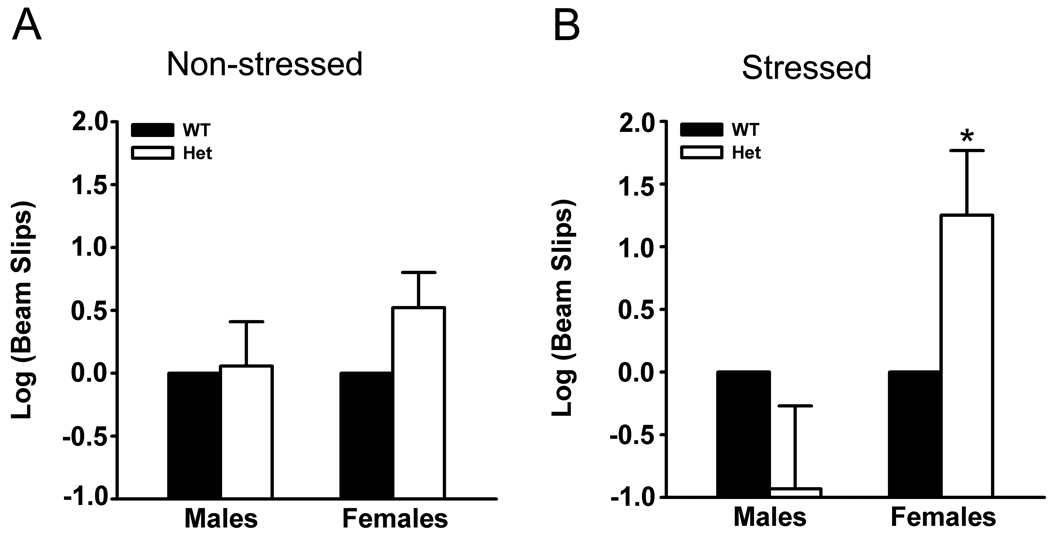
A beam-walking test was used to assess motor coordination and motor learning deficits in heterozygous Atp1a3 mutant (Het) mice (Carter et al., 1999). No statistical difference was observed in both non-stressed male and female Het mice when compared to non-stressed wild- type (WT) mice (p>0.05, Figure 2A). As the symptoms of DYT12 dystonia are absent until triggered by a physiological stressor (Brashear et al., 2007; Dobyns et al., 1993; Kamm et al., 2004; Linazasoro et al., 2002; Pittock et al., 2000; Zaremba et al., 2004), the effects of stress on motor behavior was examined. Mice were exposed to a restraint stress for five days followed by a two-week rest period. In contrast to non-stressed female Het mice, stressed female Het mice exhibited a 250% increase in hind limb slips in the beam-walking test compared to stressed female WT mice (p<0.05, Figure 2B). Similar to non-stressed male Het mice, there was no significant increase in the number of hind limb slips in stressed male Het mice compared to stressed male WT mice (p>0.05, Figure 2B). It has been demonstrated that sensitivity to restraint stress is sex-dependent (Koehl et al., 2006) and thus it is possible that under the current conditions we failed to induce the dystonic-like phenotype in stressed male Het mice. As stress induced motor deficits in the beam-walking test, the remaining experiments were conducted on stressed animals only.
Figure 2. Hind limb slips on the beam-walking test.
Non-stressed male and female mice showed no significant difference in hind limb slips (A). However, stressed female Het mice showed an increase in slips compared to stressed female WT mice, while stressed male Het mice showed no significant difference compared to stressed male WT mice (B). Bars represent means with standard errors of the mean. * p < 0.05.
3.1.2 Accelerated Rotarod
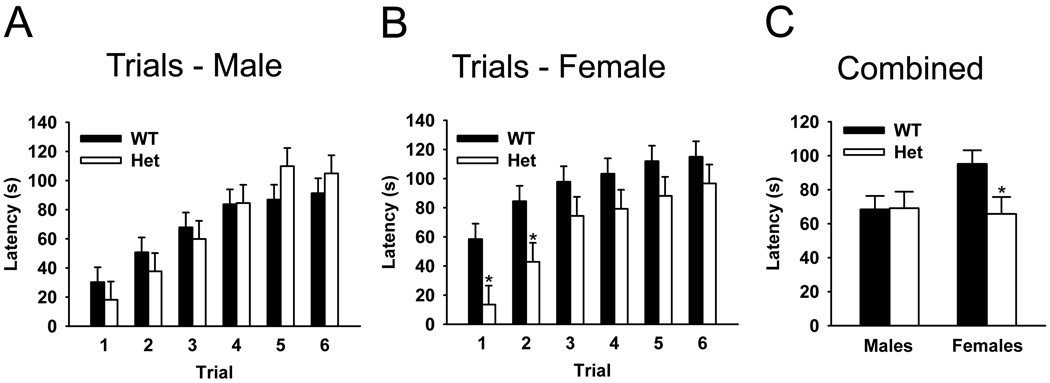
The rotarod test, another test for motor coordination and motor learning, was used on stressed mice. Stressed female Het mice exhibited a significantly decreased latency to fall from the rotarod compared to stressed female WT mice (F1,17=5.07, p<0.05, Figure 3C). In contrast, stressed male Het mice did not exhibit a deficit compared to stressed male WT mice (F1,14=0.00, p>0.05, Figure 3A,C). Similar to the beam-walking test, this suggests that the stressed female Het mice have motor deficits.
Figure 3. Latency to fall on the accelerated rotarod test.
Stressed male Het mice showed no significant alterations in performance on the rotarod test by trial (A) or with all trials combined compared to stressed male WT mice (C). However, stressed female Het mice showed a significant decrease in latency to fall compared to stressed female WT mice in both individual trials and with the trials combined (B, C). Bars represent means with standard errors of the mean. * p < 0.05.
3.2 Grip strength
To determine if the motor deficits exhibited in the beam-walking and rotarod tests arose from a muscular weakness and not a neurological deficit, the grip strength of the stressed mice were assessed for both the forelimbs and the hind limbs. There was no significant difference between stressed Het and stressed WT mice in forelimb strength in males (F1,16=2.06, p>0.05, Supplementary Figure 1A) or females (F1,13=1.19, p>0.05, Supplementary Figure 1A). Additionally, there was no significant difference in hind limb strength between stressed Het and WT mice in males (F1,16=2.57, p>0.05, Supplementary Figure 1B) or females (F1,13=0.93, p>0.05, Supplementary Figure 1B). This suggests that stressed Het mice do not have a significant change in grip strength or a muscular deficiency.
3.2 Activity levels of heterozygous Atp1a3 mutant mice
3.2.1 Spontaneous activity levels
Several mouse models of dystonia have shown alterations in activity levels and circling behavior (Dang et al., 2005; Dang et al., 2006a; Grundmann et al., 2007; Shashidharan et al., 2005; Yokoi et al., 2006; Yokoi et al., 2008). In addition, we previously demonstrated that non-stressed Het mice exhibit an increase in activity levels. Therefore, to monitor stressed Het mice for spontaneous activity, an open field experiment was used. However, in stressed Het mice there were no significant differences in horizontal activity (F1,33=1.20, p>0.05, Figure 4A) or vertical activity (F1,33=1.00, p>0.05, Figure 4A) compared to stressed WT mice, regardless of sex. However, stressed Het mice exhibited a significant decrease in clockwise circling (F1,33=6.33, p<0.05, Figure 4B) but no significant difference in counterclockwise circling compared to stressed WT mice (F1,33=0.29, p>0.05, Figure 4B). Alterations in activity and circling have been linked to alterations in the dopaminergic system (Kim et al., 2000; Viggiano et al., 2003), suggesting that the stressed Het mice might have alterations in this system.
Figure 4. Open field test to measure spontaneous activity levels.
Stressed Het mice, regardless of sex, showed no significant difference in horizontal activity (A) or vertical activity compared to stressed WT mice (A). Stressed Het mice, regardless of sex, showed a significant decrease in clockwise (CW) circling (B), but no significant difference in counterclockwise circling (CCW) (B). Bars represent means with standard errors of the mean. * p < 0.05.
3.2.2 Voluntary activity levels
To monitor for voluntary activity levels, a standard cage equipped with a wheel was used. No significant difference was observed in cumulative distance for four days for stressed male Het mice compared to stressed male WT mice (F1,14=0.24, p>0.05, Figure 5B). In contrast, the stressed female Het mice diverged into two separate groups from stressed female WT mice (Figure 5C). One group was significantly hyperactive when compared to stressed female WT mice (F2,13=45.2, n=7, p<0.05, Figure 5D) and another was significantly hypoactive when compared to stressed female WT mice (F2,13=45.2, n=3, p<0.0001, Figure 5D). This suggests that stressed female Het mice have alterations in voluntary activity levels.
Figure 5. Voluntary activity levels measured by wheel running.
Stressed male Het mice showed no difference in the distribution (A) or the mean (B) of cumulative distance run. However, stressed female Het mice showed a divergence in the distribution (C) with one group being increased in activity compared to stressed WT mice (Het – Hyperactive) and the other being decreased in activity compared to stressed WT mice (Het – Hypoactive) (D). Circles in (A) and (C) represent individual animal cumulative distances. Numbers in parenthesis in (C) indicates number of animals. Bars in (B) and (D) represent means with standard errors of the mean. * p < 0.05 compared to stressed WT mice. ** p < 0.01 compared to stressed WT mice.
3.3 Sensory deficits in heterozygous Atp1a3 mutant mice
A standard tail flick sensory experiment was conducted to test for sensory deficits in stressed Het mice. Stressed female Het mice showed a significant increase in latency to respond to the stimulus compared to stressed female WT mice (F1,13=5.36, p<0.05, Figure 6). In contrast, there was no significant difference between stressed male Het and WT mice (F1,13=0.07, p>0.05, Figure 6). This suggests that there is a sensory system alteration in stressed female Het mice.
Figure 6. Tail flick sensory test to determine perception of a warm stimulus.
Stressed female Het mice exhibited a significant increase in latency to respond to the stimulus compared to stressed female WT mice while stressed male Het mice exhibited no difference compared to stressed male WT mice. Bars represent means with standard errors of the mean. * p < 0.05.
3.4 Altered dopamine and serotonin correlations to activity levels
High performance liquid chromatography (HPLC) was used to analyze the striatal levels of dopamine, serotonin, and their metabolites. No significant differences were found between stressed Het and WT mice in dopamine, serotonin, or their metabolites (Supplementary Table 1). As dopamine and serotonin are involved in movement, the levels of these monoamines and their metabolites were correlated with activity levels from the open field test for each individual mouse. Serotonin (5-HT) was directly correlated to vertical activity in the stressed WT mice, regardless of sex. However, this correlation was absent in the stressed Het mice (Table 1). Additionally, the vertical activity of stressed Het mice was inversely correlated to dopamine (DA), 3,4-dihyroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and 5-hydroxyindolacetic acid (5-HIAA), regardless of sex. However, in the stressed WT mice these correlations did not exist (Table 1). This suggests that though there are no alterations in the levels of the monoamines or their metabolites, there is an alteration in the other, yet identified aspects of the dopaminergic, serotonergic, or both pathways in stressed Het mice.
Table 1.
Correlations among DA, 5-HT, and their metabolites and vertical activity
| Compared subjects | WT PCC | WT p | Het PCC | Het p |
|---|---|---|---|---|
| DA:VACTV | −0.188 | 0.264 | −0.642 | 0.010* |
| DOPAC:VACTV | −0.087 | 0.607 | −0.553 | 0.042* |
| HVA:VACTV | −0.205 | 0.224 | −0.708 | 0.003** |
| 5-HT:VACTV | 0.458 | 0.030* | −0.074 | 0.792 |
| 5-HIAA:VACTV | −0.130 | 0.445 | −0.592 | 0.020* |
PCC, Pearson Correlation Coefficients; VACTV, Vertical Activity; WT, Wild-type mice; Het, Heterozygous Atp1a3 mutant mice;
p < 0.05,
p < 0.01.
4. Discussion
To determine if the heterozygous Atp1a3 mutant (Het) mice models DYT12 dystonia, we have conducted a comprehensive characterization of motor behaviors, similar to other mouse models of dystonia, of the Het mice. We have found that stress could induce deficits in motor coordination and balance in the female Het mice. Stressed female Het mice also exhibited altered circling patterns and monoamine systems. Furthermore, we have found that the stressed female Het mice have a decrease in sensation similar to human patients with dystonia (Aglioti et al., 2003; Bara-Jimenez et al., 2000; Fiorio et al., 2007; Fiorio et al., 2008; Molloy et al., 2003; Peller et al., 2006; Sanger et al., 2001; Tamura et al., 2008; Tinazzi et al., 1999; Tinazzi et al., 2000; Tinazzi et al., 2002a; Tinazzi et al., 2002b; Tinazzi et al., 2004). Lastly, we have found alterations in voluntary activity levels in stressed female Het mice.
No animal model of DYT12 dystonia has previously been characterized but several mouse models have been created of other dystonias, primarily DYT1 and DYT11 dystonias (Dang et al., 2005; Dang et al., 2006a; Grundmann et al., 2007; Sharma et al., 2005; Yokoi et al., 2006; Yokoi et al., 2008; Yokoi et al., 2010). These animal models were tested for several key characteristics to indirectly assess whether the animal models dystonia. These experiments include the beam-walking test, rotarod, open field monitoring of activity, and analysis of neurotransmitter levels. Our results showed that stressed female Het mice exhibited a greater tendency to slip during the transversal of the beam during the beam-walking test and fell from the rotarod significantly quicker than stressed female WT mice. Mouse models of DYT1 and DYT11 dystonias have shown similar decreases in motor performance (Dang et al., 2005; Dang et al., 2006a; Grundmann et al., 2007; Sharma et al., 2005; Yokoi et al., 2006; Yokoi et al., 2008; Yokoi et al., 2010). However, these mouse models have not shown a female predominance of deficits similar to our results. Furthermore, it has not been reported that DYT12 dystonia affects female patients preferentially over males. A possible explanation is the sex-dependent effect restraint stress has (Koehl et al., 2006) and thus the male mice failed to receive a strong enough stressor to induce motor deficits. To determine if these motor deficits were derived from a muscular deficit, the forelimb and hind limb grip strength was assessed. No deficits were seen in either one of these in the Het mice compared to WT mice, suggesting that there are no alterations at the muscular level (Supplemental Figure 1). Similarly, in dystonia patients, the motor deficits are thought to be of a neurological origin and not a muscular deficit (Muller et al., 2002). Next, our results show that the stressed Het mice had no alterations in spontaneous activity levels but had a decrease in circling behavior. Alterations in circling behavior have previously been correlated with changes in the dopaminergic system (Kim et al., 2000; Viggiano et al., 2003). Using high performance liquid chromatography (HPLC) we were able to analyze the levels of dopamine, serotonin, and their metabolites. We found no gross differences between stressed Het and WT mice. However, as dopamine and serotonin have been shown to play a role in motor performance, individual neurotransmitter levels were correlated to the activity levels from the open field experiment of each mouse. Stressed Het mice had alterations in these correlations compared to stressed WT mice, which suggests that there are changes in the dopaminergic and serotonergic systems. Similarly, DYT1 and DYT11 dystonia mouse models have alterations in either dopamine (Dang et al., 2005; Shashidharan P et al., 2002; Yokoi et al., 2006) or the correlations of dopamine and serotonin with activity levels (Yokoi et al., 2006). Additionally, it has been demonstrated that DYT12 dystonia patients have a decrease in homovanillic acid, a metabolite of dopamine, in the cerebrospinal fluid (CSF) (Brashear et al., 1998a; Brashear et al., 1998b) but post-mortem analysis of DYT12 dystonia striatal dopamine, serotonin, and their metabolites have not been conducted. Lastly, DYT12 dystonia patients do not exhibit any symptoms until triggered by a physiological stressor (Brashear et al., 1997; Dobyns et al., 1993). Similarly, our results show that there were no motor deficits in Het mice until the mouse undergoes a restraint stress protocol. Taken together, our results suggest that the stressed female Het mice model several characteristics of other dystonia mouse models and DYT12 dystonia patients.
The sensory systems of previous animal models of dystonia have not been examined. However, the sensory system has been extensively studied in patients with dystonia. These studies have demonstrated a hypofunctioning sensory system in dystonia patients, with alterations in temporal and spatial discrimination and integration of sensory signals (Aglioti et al., 2003; Bara-Jimenez et al., 2000; Fiorio et al., 2007; Fiorio et al., 2008; Molloy et al., 2003; Peller et al., 2006; Sanger et al., 2001; Tamura et al., 2008; Tinazzi et al., 1999; Tinazzi et al., 2000; Tinazzi et al., 2002a; Tinazzi et al., 2002b; Tinazzi et al., 2004). Similarly, stressed female Het mice exhibited a hyposensitivity in the tail flick test. It has been suggested that disorders such as Parkinson’s disease and dystonia, have changes in their sensory systems due to alterations in the basal ganglia, which disrupts the integration of sensory and motor information (Kaji et al., 2005; Peller et al., 2006).
Lastly, we conducted a wheel running experiment to measure voluntary activity levels for four consecutive days. Previous animal models of dystonia have not undergone a similar test. We hypothesized that mice that exhibit dystonic-like symptoms would have decreased voluntary activity levels, due to the physical discomfort of running. Interestingly, stressed female Het mice diverged into a hyperactive and a hypoactive group compared to stressed female WT mice. These two groups likely represent a behaviorally penetrant group (hypoactive animals) and a behaviorally non-penetrant group (hyperactive group). Further experiments will be needed to explore this phenomenon further. This wheel running experiment has the potential to be used in other motor disorders, as voluntary activity may be altered similarly.
The ATP1A3 gene has been implicated in DYT12 dystonia (Brashear et al., 1997; Zanotti-Fregonara et al., 2008) and haploinsufficiency of the α3 subunit of the Na+/K+-ATPase during a physiological stressor is believed to be the cause (de Carvalho Aguiar et al., 2004). We have analyzed the Het mice to test whether it models key characteristics of the DYT12 dystonia phenotype. We have observed no deficits in motor behavior in non-stressed Het mice. However, after exposure to a restraint stress, female Het mice exhibit motor deficits, abnormal circling pattern, and alterations in monoamine systems. Additionally, we have found that the stressed female Het mice had sensory deficits similar to patients with dystonia. We have also found alterations in voluntary activity levels in stressed female Het mice. Taken together, this suggests that the stressed female Het mice model several characteristics of DYT12 dystonia. Furthermore, because the stressor precipitates motor behavior deficits that were not present prior to stress, this animal model can be used to examine the physiological response to stress. Finally, as the stressed Het mice had a dystonic-like behavior phenotype that have been shown in mouse models of DYT1 and DYT11 dystonias, this mouse model will allow further dissection of the pathophysiology of DYT12 dystonia and facilitate designing and testing of novel therapeutics.
Supplementary Material
Supplementary Figure 1. Forelimb and hind limb grip strength. Stressed male and female Het mice showed no difference in grip strength for forelimb (A) or hind limb (B) compared to stressed male and female WT mice (A). Bars represent means with standard errors
Acknowledgments
We thank Andrea McCullough and Kevin Feng, and their staff for animal care, Miki Jinno and Alena Samal for their technical assistance, and Chad C. Cheetham and Atbin Doroodchi for their stimulating discussions and their assistance in editing this manuscript. This work was supported by National Institutes of Health (NIH) grants (NS37409, NS47466, NS47692, NS54246, NS57098, NS65273, and NS72872) and the start up fund from the Department of Neurology (UAB) to YL, and by NIH grant 5RO1 HL28573 to JBL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aglioti SM, Fiorio M, Forster B, Tinazzi M. Temporal discrimination of cross-modal and unimodal stimuli in generalized dystonia. Neurology. 2003;60:782–785. doi: 10.1212/01.wnl.0000046528.24693.5b. [DOI] [PubMed] [Google Scholar]
- Bara-Jimenez W, Shelton P, Sanger TD, Hallett M. Sensory discrimination capabilities in patients with focal hand dystonia. Ann Neurol. 2000;47:377–380. [PubMed] [Google Scholar]
- Brashear A, DeLeon D, Bressman SB, Thyagarajan D, Farlow MR, Dobyns WB. Rapid-onset dystonia-parkinsonism in a second family. Neurology. 1997;48:1066–1069. doi: 10.1212/wnl.48.4.1066. [DOI] [PubMed] [Google Scholar]
- Brashear A, Butler IJ, Hyland K, Farlow MR, Dobyns WB. Cerebrospinal fluid homovanillic acid levels in rapid-onset dystonia-parkinsonism. Ann Neurol. 1998a;43:521–526. doi: 10.1002/ana.410430417. [DOI] [PubMed] [Google Scholar]
- Brashear A, Butler IJ, Ozelius LJ, Kramer PI, Farlow MR, Breakefield XO, Dobyns WB. Rapid-onset dystonia-parkinsonism: a report of clinical, biochemical, and genetic studies in two families. Adv Neurol. 1998b;78:335–339. [PubMed] [Google Scholar]
- Brashear A, Dobyns WB, de Carvalho Aguiar P, Borg M, Frijns CJ, Gollamudi S, Green A, Guimaraes J, Haake BC, Klein C, Linazasoro G, Munchau A, Raymond D, Riley D, Saunders-Pullman R, Tijssen MA, Webb D, Zaremba J, Bressman SB, Ozelius LJ. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- Breakefield XO, Blood AJ, Li Y, Hallett M, Hanson PI, Standaert DG. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- Camargos S, Scholz S, Simon-Sanchez J, Paisan-Ruiz C, Lewis P, Hernandez D, Ding J, Gibbs JR, Cookson MR, Bras J, Guerreiro R, Oliveira CR, Lees A, Hardy J, Cardoso F, Singleton AB. DYT16, a novel young-onset dystonia-parkinsonism disorder: identification of a segregating mutation in the stress-response protein PRKRA. Lancet Neurol. 2008;7:207–215. doi: 10.1016/S1474-4422(08)70022-X. [DOI] [PubMed] [Google Scholar]
- Cannon SC. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu Rev Neurosci. 2006;29:387–415. doi: 10.1146/annurev.neuro.29.051605.112815. [DOI] [PubMed] [Google Scholar]
- Cao BJ, Li Y. Reduced anxiety-- and depression-like behaviors in Emx1 homozygous mutant mice. Brain Res. 2002;937:32–40. doi: 10.1016/s0006-8993(02)02461-7. [DOI] [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RJ, Morton AJ, Dunnett SB. Locomotor Behavior. In: Crawley J, editor. Current Protocols in Neuroscience. Vol. Canada: Wiley; 2001. [Google Scholar]
- Clapcote SJ, Duffy S, Xie G, Kirshenbaum G, Bechard AR, Rodacker Schack V, Petersen J, Sinai L, Saab BJ, Lerch JP, Minassian BA, Ackerley CA, Sled JG, Cortez MA, Henderson JT, Vilsen B, Roder JC. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci U S A. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, McNaught KS, Jengelley TA, Jackson T, Li J, Li Y. Generation and characterization of Dyt1 DeltaGAG knock-in mouse as a model for early-onset dystonia. Exp Neurol. 2005;196:452–463. doi: 10.1016/j.expneurol.2005.08.025. [DOI] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Pence MA, Li Y. Motor deficits and hyperactivity in Dyt1 knockdown mice. Neurosci Res. 2006a;56:470–474. doi: 10.1016/j.neures.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang MT, Yokoi F, Yin HH, Lovinger DM, Wang Y, Li Y. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006b;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+ -ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci. 2005;10:2373–2396. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- Dobyns WB, Ozelius LJ, Kramer PL, Brashear A, Farlow MR, Perry TR, Walsh LE, Kasarskis EJ, Butler IJ, Breakefield XO. Rapid-onset dystonia-parkinsonism. Neurology. 1993;43:2596–2602. doi: 10.1212/wnl.43.12.2596. [DOI] [PubMed] [Google Scholar]
- Fahn S. Concept and classification of dystonia. Adv Neurol. 1988;50:1–8. [PubMed] [Google Scholar]
- Fiorio M, Gambarin M, Valente EM, Liberini P, Loi M, Cossu G, Moretto G, Bhatia KP, Defazio G, Aglioti SM, Fiaschi A, Tinazzi M. Defective temporal processing of sensory stimuli in DYT1 mutation carriers: a new endophenotype of dystonia? Brain. 2007;130:134–142. doi: 10.1093/brain/awl283. [DOI] [PubMed] [Google Scholar]
- Fiorio M, Tinazzi M, Scontrini A, Stanzani C, Gambarin M, Fiaschi A, Moretto G, Fabbrini G, Berardelli A. Tactile temporal discrimination in patients with blepharospasm. J Neurol Neurosurg Psychiatry. 2008;79:796–798. doi: 10.1136/jnnp.2007.131524. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Gavarini S, Saunders-Pullman R, Raymond D, Ehrlich ME, Bressman SB, Ozelius LJ. Mutations in the THAP1 gene are responsible for DYT6 primary torsion dystonia. Nat Genet. 2009;41:286–288. doi: 10.1038/ng.304. [DOI] [PubMed] [Google Scholar]
- Grundmann K, Reischmann B, Vanhoutte G, Hubener J, Teismann P, Hauser TK, Bonin M, Wilbertz J, Horn S, Nguyen HP, Kuhn M, Chanarat S, Wolburg H, Van der Linden A, Riess O. Overexpression of human wildtype torsinA and human DeltaGAG torsinA in a transgenic mouse model causes phenotypic abnormalities. Neurobiol Dis. 2007;27:190–206. doi: 10.1016/j.nbd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- Kaji R, Urushihara R, Murase N, Shimazu H, Goto S. Abnormal sensory gating in basal ganglia disorders. J Neurol. 2005;252(4):IV13–IV16. doi: 10.1007/s00415-005-4004-9. [DOI] [PubMed] [Google Scholar]
- Kamm C, Boston H, Hewett J, Wilbur J, Corey DP, Hanson PI, Ramesh V, Breakefield XO. The early onset dystonia protein torsinA interacts with kinesin light chain 1. J Biol Chem. 2004;279:19882–19892. doi: 10.1074/jbc.M401332200. [DOI] [PubMed] [Google Scholar]
- Kim DS, Szczypka MS, Palmiter RD. Dopamine-deficient mice are hypersensitive to dopamine receptor agonists. J Neurosci. 2000;20:4405–4413. doi: 10.1523/JNEUROSCI.20-12-04405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Battle S, Meerlo P. Sex differences in sleep: the response to sleep deprivation and restraint stress in mice. Sleep. 2006;29:1224–1231. doi: 10.1093/sleep/29.9.1224. [DOI] [PubMed] [Google Scholar]
- Linazasoro G, Indakoetxea B, Ruiz J, Van Blercom N, Lasa A. Possible sporadic rapid-onset dystonia-parkinsonism. Mov Disord. 2002;17:608–609. doi: 10.1002/mds.10103. [DOI] [PubMed] [Google Scholar]
- Lindsey JW, Jung AE, Narayanan TK, Ritchie GD. Acute effects of a bicyclophosphate neuroconvulsant on monoamine neurotransmitter and metabolite levels in the rat brain. J Toxicol Environ Health A. 1998;54:421–429. doi: 10.1080/009841098158827. [DOI] [PubMed] [Google Scholar]
- Lingrel JB, Kuntzweiler T. Na+,K(+)-ATPase. J Biol Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- Lingrel JB, Williams MT, Vorhees CV, Moseley AE. Na,K-ATPase and the role of alpha isoforms in behavior. J Bioenerg Biomembr. 2007;39:385–389. doi: 10.1007/s10863-007-9107-9. [DOI] [PubMed] [Google Scholar]
- Molloy FM, Carr TD, Zeuner KE, Dambrosia JM, Hallett M. Abnormalities of spatial discrimination in focal and generalized dystonia. Brain. 2003;126:2175–2182. doi: 10.1093/brain/awg219. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na,K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Hedrich K, Kock N, Dragasevic N, Svetel M, Garrels J, Landt O, Nitschke M, Pramstaller PP, Reik W, Schwinger E, Sperner J, Ozelius L, Kostic V, Klein C. Evidence that paternal expression of the epsilon-sarcoglycan gene accounts for reduced penetrance in myoclonus-dystonia. Am J Hum Genet. 2002;71:1303–1311. doi: 10.1086/344531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U. The monogenic primary dystonias. Brain. 2009;132:2005–2025. doi: 10.1093/brain/awp172. [DOI] [PubMed] [Google Scholar]
- Page ME, Bao L, Andre P, Pelta-Heller J, Sluzas E, Gonzalez-Alegre P, Bogush A, Khan LE, Iacovitti L, Rice ME, Ehrlich ME. Cell-autonomous alteration of dopaminergic transmission by wild type and mutant (DeltaE) TorsinA in transgenic mice. Neurobiol Dis. 2010;39:318–326. doi: 10.1016/j.nbd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peller M, Zeuner KE, Munchau A, Quartarone A, Weiss M, Knutzen A, Hallett M, Deuschl G, Siebner HR. The basal ganglia are hyperactive during the discrimination of tactile stimuli in writer’s cramp. Brain. 2006;129:2697–2708. doi: 10.1093/brain/awl181. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Joyce C, O’Keane V, Hugle B, Hardiman MO, Brett F, Green AJ, Barton DE, King MD, Webb DW. Rapid-onset dystonia-parkinsonism: a clinical and genetic analysis of a new kindred. Neurology. 2000;55:991–995. doi: 10.1212/wnl.55.7.991. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Tarsy D, Pascual-Leone A. Abnormalities of spatial and temporal sensory discrimination in writer’s cramp. Mov Disord. 2001;16:94–99. doi: 10.1002/1531-8257(200101)16:1<94::aid-mds1020>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baxter MG, Petravicz J, Bragg DC, Schienda A, Standaert DG, Breakefield XO. Impaired motor learning in mice expressing torsinA with the DYT1 dystonia mutation. J Neurosci. 2005;25:5351–5355. doi: 10.1523/JNEUROSCI.0855-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidharan P, Sandu D, Potla U, Armata IA, Walker RH, McNaught KS, Weisz D, Sreenath T, Brin MF, Olanow CW. Transgenic mouse model of early-onset DYT1 dystonia. Hum Mol Genet. 2005;14:125–133. doi: 10.1093/hmg/ddi012. [DOI] [PubMed] [Google Scholar]
- Shashidharan P, Brin MF, Gujjari D, Sandu D, Olanow C. A transgenic mouse model for DYT1 Dystonia; 4th International Dystonia Symposium; 2002. [Google Scholar]
- Tamura Y, Matsuhashi M, Lin P, Ou B, Vorbach S, Kakigi R, Hallett M. Impaired intracortical inhibition in the primary somatosensory cortex in focal hand dystonia. Mov Disord. 2008;23:558–565. doi: 10.1002/mds.21870. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Frasson E, Bertolasi L, Fiaschi A, Aglioti S. Temporal discrimination of somesthetic stimuli is impaired in dystonic patients. Neuroreport. 1999;10:1547–1550. doi: 10.1097/00001756-199905140-00028. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Priori A, Bertolasi L, Frasson E, Mauguiere F, Fiaschi A. Abnormal central integration of a dual somatosensory input in dystonia. Evidence for sensory overflow. Brain. 2000;123(1):42–50. doi: 10.1093/brain/123.1.42. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Fiaschi A, Frasson E, Fiorio M, Cortese F, Aglioti SM. Deficits of temporal discrimination in dystonia are independent from the spatial distance between the loci of tactile stimulation. Mov Disord. 2002a;17:333–338. doi: 10.1002/mds.10019. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Rosso T, Fiaschi A, Gambina G, Farina S, Fiorio SM, Aglioti SM. The role of somatosensory feedback in dystonia: a psychophysical [correction of psycophysical] evaluation. Neurol Sci. 2002b;23(2):S113–S114. doi: 10.1007/s100720200095. [DOI] [PubMed] [Google Scholar]
- Tinazzi M, Fiorio M, Bertolasi L, Aglioti SM. Timing of tactile and visuo-tactile events is impaired in patients with cervical dystonia. J Neurol. 2004;251:85–90. doi: 10.1007/s00415-004-0282-x. [DOI] [PubMed] [Google Scholar]
- Viggiano D, Ruocco LA, Sadile AG. Dopamine phenotype and behaviour in animal models: in relation to attention deficit hyperactivity disorder. Neurosci Biobehav Rev. 2003;27:623–637. doi: 10.1016/j.neubiorev.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Dang MT, Li J, Li Y. Myoclonus, motor deficits, alterations in emotional responses and monoamine metabolism in epsilon-sarcoglycan deficient mice. J Biochem (Tokyo) 2006;140:141–146. doi: 10.1093/jb/mvj138. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Dang MT, Mitsui S, Li J, Li Y. Motor Deficits and Hyperactivity in Cerebral Cortex-specific Dyt1 Conditional Knockout Mice. J Biochem. 2008;143:39–47. doi: 10.1093/jb/mvm191. [DOI] [PubMed] [Google Scholar]
- Yokoi F, Dang MT, Miller CA, Marshall AG, Campbell SL, Sweatt JD, Li Y. Increased c-fos expression in the central nucleus of the amygdala and enhancement of cued fear memory in Dyt1 DeltaGAG knock-in mice. Neurosci Res. 2009;65:228–235. doi: 10.1016/j.neures.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi F, Yang G, Li J, De Andrade MP, Zhou T, Li Y. Earlier onset of motor deficits in mice with double mutations in Dyt1 and Sgce. J Biochem. 2010 doi: 10.1093/jb/mvq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanotti-Fregonara P, Vidailhet M, Kas A, Ozelius LJ, Clot F, Hindie E, Ravasi L, Devaux JY, Roze E. [123I]-FP-CIT and [99mTc]-HMPAO single photon emission computed tomography in a new sporadic case of rapid-onset dystonia-parkinsonism. J Neurol Sci. 2008;273:148–151. doi: 10.1016/j.jns.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Zaremba J, Mierzewska H, Lysiak Z, Kramer P, Ozelius LJ, Brashear A. Rapid-onset dystonia-parkinsonism: a fourth family consistent with linkage to chromosome 19q13. Mov Disord. 2004;19:1506–1510. doi: 10.1002/mds.20258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Forelimb and hind limb grip strength. Stressed male and female Het mice showed no difference in grip strength for forelimb (A) or hind limb (B) compared to stressed male and female WT mice (A). Bars represent means with standard errors