Abstract
In wound healing transforming growth factor β1 (TGFβ1), utilizing the Smad signaling pathway, advances connective tissue deposition, the transformation of fibroblasts into myofibroblasts and wound contraction. The compound SB-505124 disrupts the Smad signaling pathway by blocking activin receptor-like kinase phosphorylation of select Smad signaling proteins. Four full thickness excisional square 2×2 cm wounds were made on the rat dorsum. On day 2, the pair of wounds on the left received 1 uM SB-505124 in gel, and the pair on the right, controlz, received gel alone. Wounds were covered with nonocclusive dressings and treated redressed daily for 4 days. No differences in day 14 wound sizes between treatment groups were found. H&E stained sections revealed increased cell density in SB-505124 treated wounds. Polarized light microscopy showed collagen fiber bundles birefringence intensity and organization were equivalent between treatment groups. Myofibroblast populations, identified by α-smooth muscle actin staining, were the norm in controls but absent in SB-505124 treated wounds, which was confirmed by Western blot analysis. Blocking the Smad signaling pathway diminished connective tissue deposition and generated a deficiency in myofibroblast numbers, but wound contraction was unimpaired. The absence of myofibroblasts may be related to the blocking of the Smad signaling pathway or it may be related to the generation of less tension in treated wounds, related to reduced deposited connective tissue. These findings support the notion that wound contraction does not require the generation of myofibroblast contractile forces, but rather the organization of newly deposited collagen fiber bundles by forces related to fibroblast locomotion.
Keywords: wound contraction, myofibroblasts, Smad signaling
INTRODUCTION
Open wound healing follows a time sequence of events, after controlling bleeding. The repair process starts with the lag or inflammatory phase, followed by the proliferative phase, and finally the remodeling phase. The infiltration of inflammatory cells, whose functions include eliminating the establishment of microbial colonization as well as the release of soluble factors that initiate the proliferative phase characterize the lag phase. Repair’s proliferative phase consist of the infiltration of fibroblasts, their proliferation, synthesis and the deposition of a new connective tissue matrix, granulation tissue, which replaces the fibrin matrix and participates in the wound contraction closure of full thickness open wounds. The transformation of fibroblasts into myofibroblasts and the development of a new vasculature are morphological hallmarks of granulation tissue. Repair’s remodeling phase of repair follows the proliferative phase of repair. Its features include, reducing the density of myofibroblasts through apoptosis, a loss vascular density and a connective tissue matrix with few cells. Transforming growth factor beta 1 (TGFβ1) is an important growth factor for promoting wound repair process and fibrosis (Massague, 2000).
Wound contraction play an important role in the closure of full thickness wounds, where surrounding skin is pulled into the defect by forces that develop within the granulation tissue. The myofibroblast, a specialized fibroblast characterized by cytoplasmic stress fibers enriched in α smooth muscle actin (SMA), is the accepted cell phenotype responsible for wound contraction (Gabbiani, 2003). As the newly synthesized connective tissue matrix becomes organized into thicker collagen fiber bundles, tension develops, which plays a physical role in the transformation of the fibroblast into the myofibroblast (Tomasek et al., 1992). Biochemically, TGFβ1 promotes the transformation of fibroblasts into myofibroblasts in monolayer culture (Desmouliere et al., 1993). Promoting the myofibroblast phenotype would be expected to optimize wound contraction; likewise preventing the appearance of myofibroblasts would be expected to retard or inhibit wound contraction. TGFβ1 has numerous activities that include the promotion of fibroblast proliferation, increased deposition of connective tissue and the transformation of fibroblasts into myofibroblasts. These TGFβ1 activities utilize the Smad signaling pathway (Itoh et al., 2000). The initiation of the Smad signaling pathway requires the binding of TGFβ1 to a cell’s plasma membrane receptor, which combines with a second membrane receptor, TGF-β type I and type II receptors. The complex initiates intracellular kinase and phosphatase enzymatic activities that include the phosphorylation of members of the Smad signaling pathway family of proteins (Massague, 2000). The activin receptor-like kinase (ALK) 5 is the kinase involved in the phosphorylation of Smad2 and 3, which are critical for the promotion of granulation tissue (Itoh et al., 2000). SB-505124 [2-(5-benzo[1,3]dioxol-5-yl-2-tert-butyl-3Himidazol-4-yl)-6-methylpyridine hydrochloride], a small synthetic kinase inhibitor that freely diffuses into cells, is a competitive inhibitor of ALK 5, which has the capacity to interrupt the Smad signaling pathway by inhibiting the phosphorylation of Smad 3 (DaCosta Byfield et al., 2004). A histological and biochemical investigation of SB-505124 on the closure of rat open wounds examines the mechanism for wound contraction either through fibroblast organization of collagen fiber bundles or myofibroblasts generation of cell contractile forces.
MATERIALS AND METHODS
Male Sprague-Dawley rats weighing 300 gm were placed under anesthesia with inhalational Isoflurane, and their backs shaved. On each rat’s back, 4 full-thickness square excisional wounds, 2×2 cm were made. The wounds tracing were made and the wounds photographed with a ruler in place. The rats were returned to his cage upon recovering from anesthesia. On day 2 the rats were again placed under anesthesia and one pair of wounds received 1uM SB-505124 (Sigma-Aldrich, St. Louis, MO) in a Pluronic F127 gel (Malmsten, 2002). The other pair of wounds was treated with Pluronic gel alone, and then all the wounds were covered with a nonconcussive, Tegaderm, dressings. After complete recovery from anesthesia, each rat was returned to his cage. Daily treatments and dressing changes were repeated daily over the next 4 days. On day 14, the rats were euthanized. The wounds were re-photographed and wound tracings made. To document wound areas, the paper tracings made on day 0 and day 14 were cut out, the paper weighed to the nearest mg and area reported in relative area units (RAU). The healed wounds with the rim of surrounding skin were excised. Granulation tissue from half the healing wounds was acquired for biochemical analysis. The other half of the excised tissue was taken for histological evaluation. Portions of tissues were fixed in 10% formaldehyde, embedded, sectioned, and stained with either hematoxylin-eosin (H&E) or Sirius red (Ehrlich et al., 1999). Other tissue samples were placed in cassettes, submerged in Tissue-Tek O.C.T. compound (Sakura Finetek, Torance, CA), frozen in liquid nitrogen and stored at −80°. Frozen cryosections 4 to 6 µm thick were cut, fixed in buffered 4% paraformaldehyde, permeablized with 0.1% Triton X-100 detergent, and immuno-stained with monoclonal antibody directed to α SMA (Sigma-Aldrich). The α SMA antibody complex was recognized by a rhodamine-conjugated sheep-anti-mouse antibody (Jackson Immuno Research Labs, West Grove, PA). Filamentous actin was demonstrated by rhodamine-phalloidin staining (Invitrogen Corp. Carlsbad, CA). Tissues to undergo biochemical analysis were homogenized in lysis buffer (0.1% (SDS) in 10 mM Tris HCl pH 6.8), boiled for 5 minutes, cleared by centrifugation, and stored frozen (−80o). The protein concentrations were determined and 20 µg of protein was subjected to SDS polyacrylamide gel electrophoresis (PAGE). By electrophoresis proteins were transferred from the PAGE gel to a PVDF membrane (Millipore Corp, Bedford, MA). The membrane was incubated in 5% dried milk solution to block nonspecific protein-binding sites before incubating with the primary antibodies (α SMA or β actin). After treating with primary antibody, membranes were exhaustively rinsed and then incubated with an anti mouse IgG peroxidase-conjugated secondary antibody, followed with exhaustive washes (8). The membrane was subjected to Super Signal West Dura chemiluminescence detection system, following the manufacturer’s instructions (Pierce Biotechnology, Rockford, IL).
RESULTS
The closure of full thickness open wounds proceeded mostly by wound contraction, which was confirmed by histology (Fukuchi et al., 2001). The gross appearance of typical wounds presented in Fig. 1 showed wounds on the days 0 and 14. The determined wound areas showed the initial wound size averaged for all wounds was 32.4 ± 3.89 RAU (mean ± standard error). At 14 days, the control gel alone treated wounds contracted to an average of 2.97 ± 1.62 RAU (n = 12) whereas the wound average size of the SB-505124 treated group was 2.34 ± 0.79 RAU (n = 12). The enhanced wound contraction in the SB-505124 treated wounds was insignificant, where the mean difference 0.03 ± 0.26; had a p value of 0.7. The bar graph presented in Fig. 2 shows the mean RAU of wound closure by contraction for the SB-505124 treated wounds compared to control wounds. Topically administrated SB-505124 did not affect wound contraction. By H&E staining reepithelialization played a minor role in the closure of these wounds, as seen by the same small tongue of epidermal cells pushing into the granulation tissue compartment from the wound edges. The epidermis made a minor advancement into the wound site, illustrating epithelialization playing a minor role in full excision rat open wound closure.
Fig. 1. Gross appearance of 14 day healing open rat wounds.
Full excision wounds undergoing contraction are presented. The SB-505124 treated wounds are on the left and control wounds are on the right.
Fig. 2. Bar graft comparing treated and untreated open wound areas at day 14.
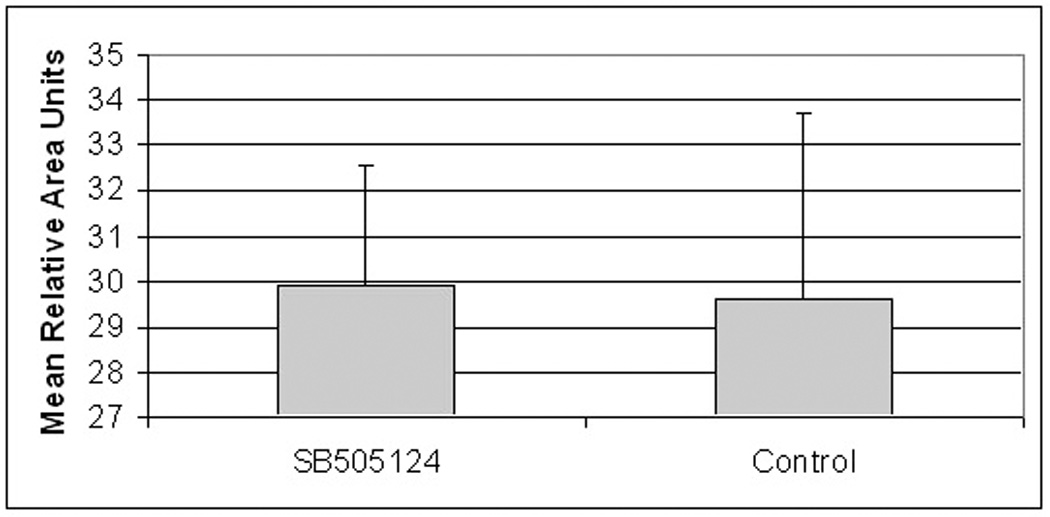
Bar graph shows the mean Relative Area Units (RAU) changes from day 0 to day 14 in the SB- 505124 treated and untreated, control wounds. Differences in wound areas are the same with a calculated p value of 0.70.
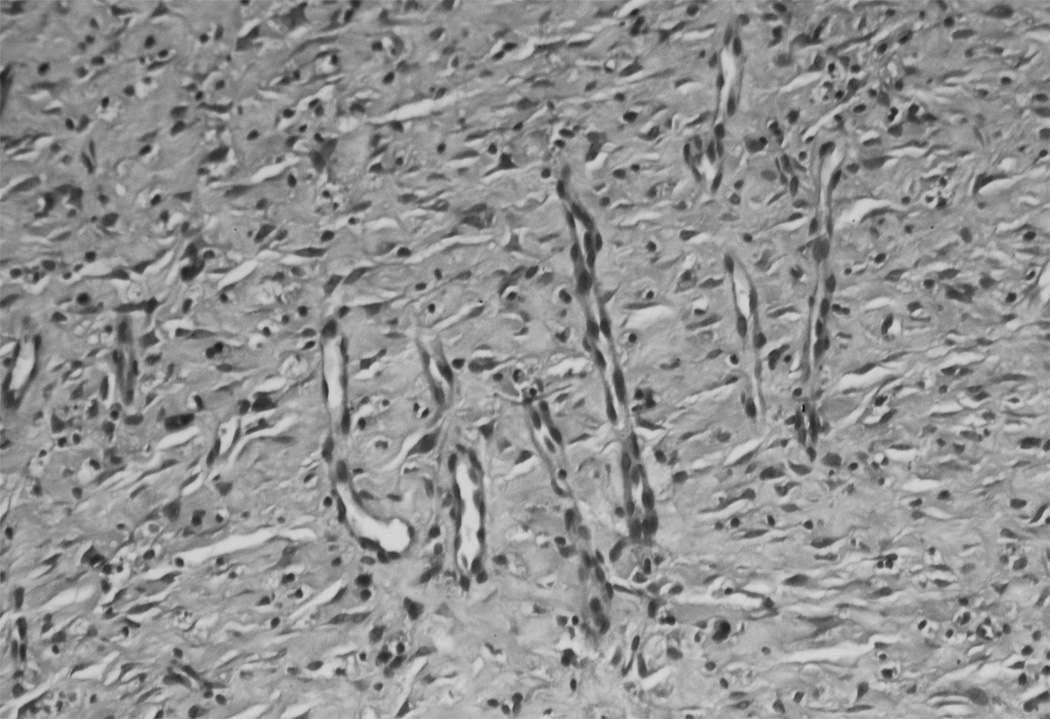
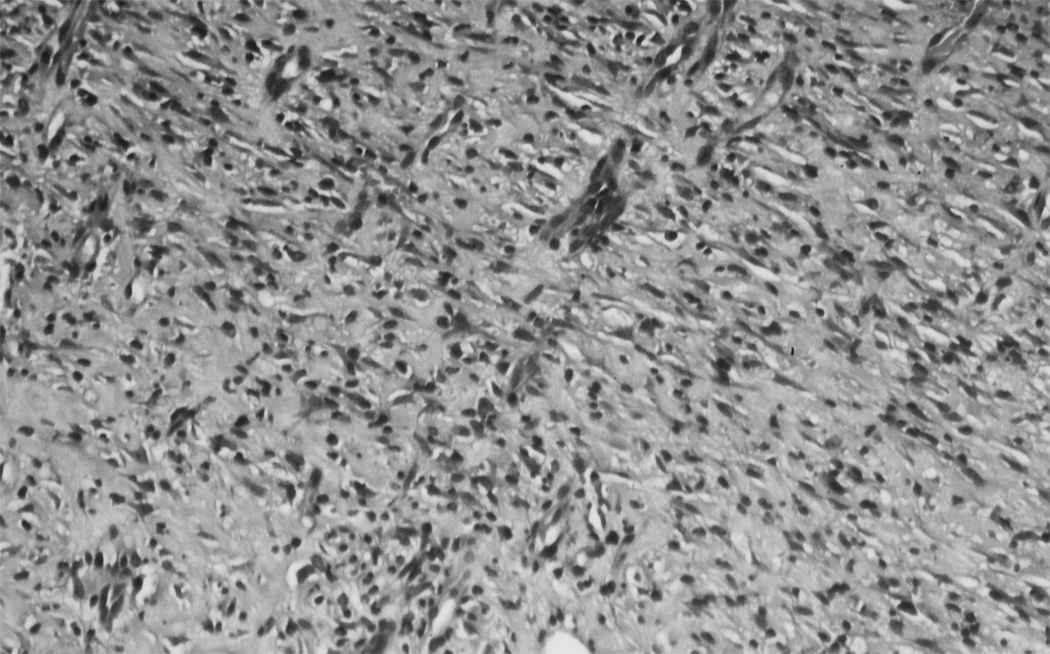
Granulation tissue from SB-505124 treated wounds showed histological differences in their cellular density and composition. H&E stained sections of treated wounds revealed an increase in cell density as compared to controls. The increase in cell density was related to a reduction in connective tissue deposited between cells, which was expected with the inhibition of the Smad signaling pathway (Fig. 3). SB-505124 treated and control wounds stained with Sirius red, viewed with a polarized light, showed a similar birefringence pattern and intensity with controls and SB-505124 treated wounds (Fig. 4). Though the connective tissue content in treated wounds was reduced content, the organization of the granulation tissue collagen fiber bundles was equivalent compared to the connective tissue organization in control granulation tissue. The inhibition of Smad signaling by SB-505124 reduced the quantity of deposited collagen, but there no alteration in the organization of the collagen fiber bundles deposited in treated or control wounds was evident.
Fig. 3. H&E histology of treated and untreated granulation tissue from 14 day rat wounds.
H&E stained cross section of healing wounds granulation tissue shows blood vessels and fibroblasts. In panel A a typical control wound with a great deal of connective tissue separating cell populations. Panel B form a typical SB-505124 treated wound show less connective tissue deposited between cell populations.
Fig. 4. Birefringence of granulation tissue from SB-505124 treated and untreated wounds.
Cross sections of 14 day healed wounds stained with Sirius Red were viewed under polarized light, which reveals the pattern of organized collagen fiber bundles. The patterns are similar in control wounds (panel A) and SB-505124 treated wounds (panel B).
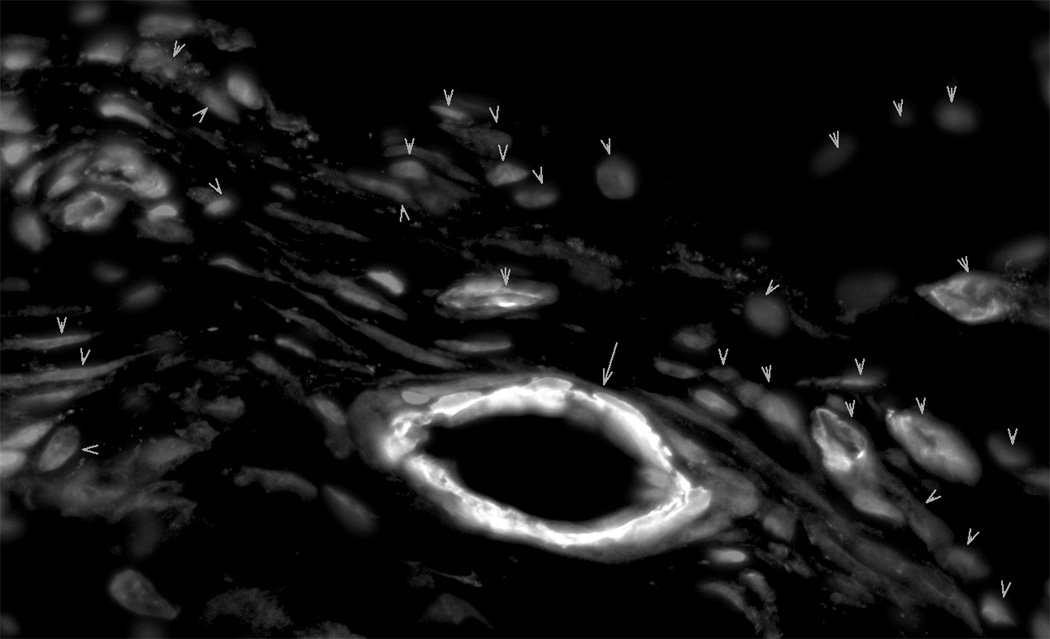
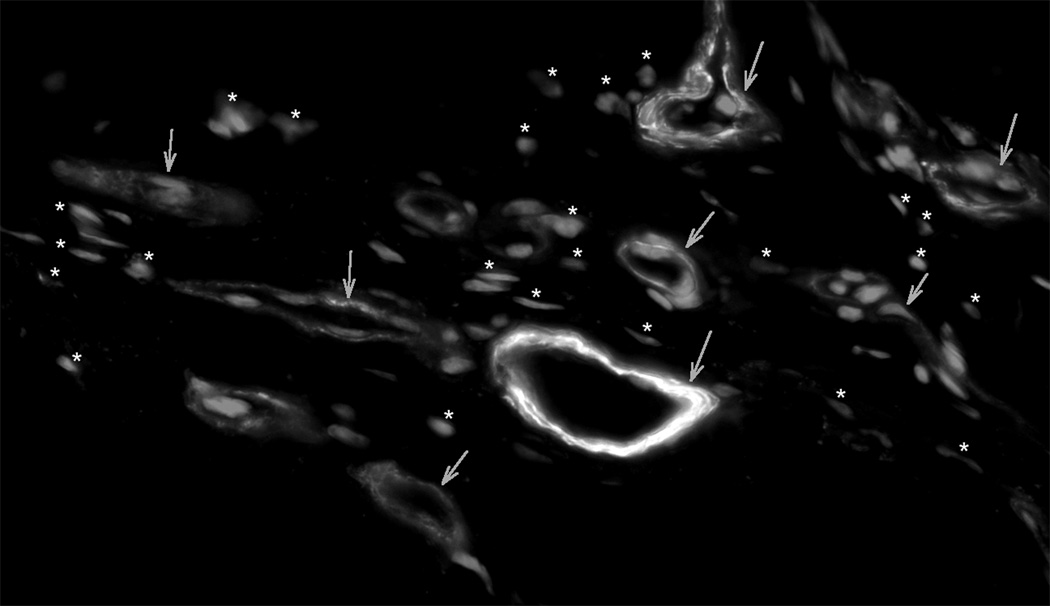
Myofibroblasts, the hallmark cell of granulation tissue, have been characterized by their prominent cytoplasmic stress fibers, which contain the α SM isoform of actin. Myofibroblasts with fluorescent stained α SMA stress fibers were prominent in granulation tissue from 14 day control healing wounds. In contrast, granulation tissue from SB-505124 treated wounds had reduced numbers of myofibroblasts as identified by fluorescent stained cytoplasmic α SMA stress fibers (Fig. 5). The reduction in fluorescent stained myofibroblasts was not attributed to a defect in immuno-staining. Blood vessels containing smooth muscle cells showed equivalent intense fluorescent staining in both control and treated wounds (Fig. 5).
Fig. 5. The Identification of myofibroblasts by α SM actin staining in granulation tissue.
Cryosections cut from granulation tissue of 14 day healing wounds had α SM actin stained stress fibers in both myofibroblasts and blood vessel smooth muscle cells. In addition, nuclei were fluorescently stained with DAPI. Panel A from control granulation tissue shows myofibroblasts, where α SM actin stained blood vessels are marked with arrows and myofibroblasts are marked with arrow heads. Panel B shows granulation tissue from a typical SB-505124 treated wound, where stained α SM actin fluorescent stress fibers are restricted to blood vessel smooth muscle cells (arrows). There α SM actin unstained wound fibroblasts are identified by their fluorescent stained nuclei (*). Granulation tissue from treated wounds did not contain myofibroblasts
Western blot analysis was employed to confirm the absence of α SMA immuno-staining of myofibroblasts in SB-505124 treated healing wounds. The great density of blood vessels with their elevated concentrations of smooth muscle cells in granulation tissue created a high background of α SMA protein in granulation tissue lysates. Reduced levels of α SMA were evident in the SB-505124 treated wound granulation tissue lysates as compared to granulation tissue isolated from controls, see Fig. 6. Western blot analysis showed a reduction in α SMA in SB-505124 treated wound granulation tissue compared to controls. The protein findings in Fig. 6 confirmed the immuno-histological findings that SB-505124 treated wounds have granulation tissue with a reduced density of myofibroblasts. Here in the absence of myofibroblasts wound contraction was not impaired in the SB-505124 topically treated wounds.
Fig. 6. Western blots from granulation tissue lysates from 14 day healing wounds.
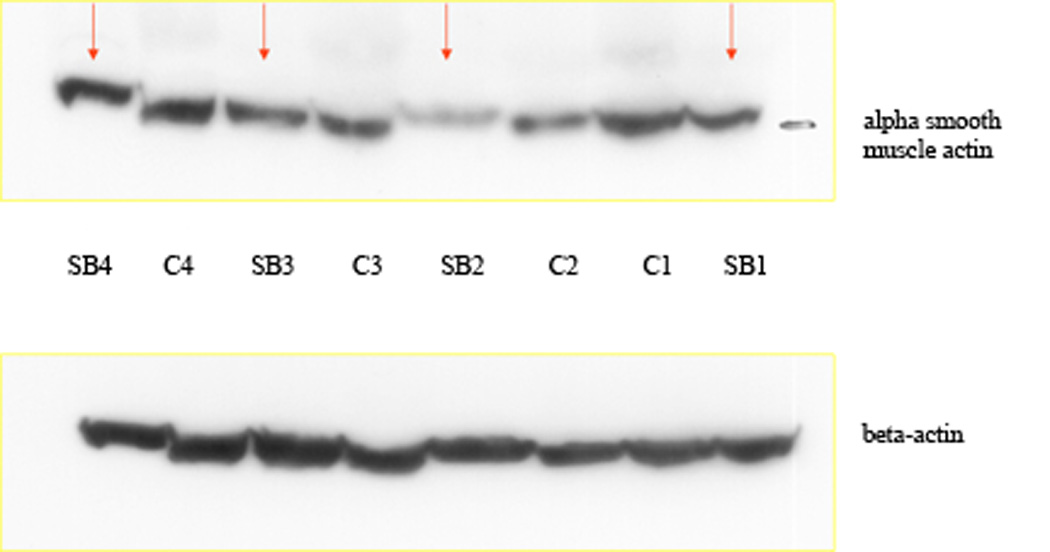
Western blots of granulation tissue lysates from pairs of treated and untreated wounds from the same rat are shown. The top panel presents α SM actin protein bands and the bottom panel presents β actin protein bands. The arrows point to the treated rat granulation tissue (SB-505124) and the number identifies the rat, from which the pair of healing wound granulation tissue was harvested.
DISCUSSION
SB-505124, a small molecular inhibitor, at 1 µM inhibits both Smad signaling and gene transcription induced by TGFβ1 (DaCosta Byfield et al., 2004). SB-505124, a member of a new class of small molecule inhibitors related to imidazole inhibitors of p38, inhibit the TGF-β type I receptor serine/threonine kinase known as activin receptor-like kinase (ALK) 5 (Harrison et al., 2005). SB-505124 is selective in its inhibitory activity by blocking ALK5 and the phosphorylation of select Smad signaling proteins. Phosphorylated Smad2 and Smad3 combine with Smad4, enter the cell’s nucleus, where the transcription of genes that advance wound repair and fibrosis are transcribed (Massague, 2000). An example of Smad signaling in wound repair is the conversion of fibroblasts to myofibroblasts (Desmouliere et al., 1993). Activins are members of the TGFβ super family that control activities such as cell proliferation, cell differentiation, immune responses as well as wound repair. In response to trauma, granulation tissue is deposited, which contains activins (Hubner et al., 1996). By histology a wounded transgenic mouse that over-expresses the activin bA chain deposits a marked increase in granulation tissue (Munz et al., 1999). Activin levels are directly proportional to the levels of deposited granulation tissue. Thus, over expression of activin may prove useful for promoting wound healing, whereas inhibiting activin expression may control connective tissue deposition, which may have value in situations of excessive fibrosis.
SB-505124 selectively inhibits ALK5 phosphorylation of Smad signaling proteins and is 3 to 5 times more potent than a related ALK5 inhibitor, SB-431542. In culture fibroblast treated with TGFβ1, show enhanced Smad phosphorylation and proliferation, while epithelial cells enter apoptosis (DaCosta Byfield et al., 2004). TGFβ1 treated epithelial cells receiving nanomolar concentrations of SB-505124 failed to phosphorylate Smad 2 and 3 and reduced numbers of these treated cells underwent apoptosis. Our in vivo studies show 1 µM SB-505124 is effective at inhibiting the conversions of fibroblasts to myofibroblasts and reducing the deposition of connective tissue in granulation tissue. The possibility exists that inhibiting endogenous TGFβ1 signaling will change the mechanism of wound closure from wound contraction to enhanced reepithelialization. TGFβ1 promotes keratinocyte apoptosis retarding keratinocyte migration. SB505124 inhibiting TGFβ1 signaling opens up the possibility of enhanced keratinocyte migration and wound closure by reepithelialization. SB505124 treated open wounds histologically did not show enhanced reepithelialization. However, in the findings presented here wound contraction is independent of the Smad signaling pathway.
SB505124 topically treated open rat wounds has reduced connective tissue deposition and a deficiency in myofibroblasts. TGFβ1 promotes the transformation of fibroblasts into myofibroblasts (Desmouliere et al., 1993), but tension also promotes the transformation of fibroblasts into myofibroblasts (Li et al., 2007). The birefringence patterns and intensity of connective tissues in treated and untreated wounds are equivalent, suggesting no alteration in the organization of collagen fiber bundles by SB 505124. In granulation tissue with reduced numbers of myofibroblasts, the expectation is impaired wound contraction. However, wound closure by wound contraction is unaffected in SB505124 treated wounds. A possible explanation of the consequence of less deposited connective tissue is reduced tension within granulation tissue. In vitro fibroblast collagen lattice contraction studies report reduced levels of collagen generate enhanced lattice contraction (Bell et al., 1979). It is reported with free floating fibroblast populated collagen lattices (FPCL), lattice contraction proceeds in the absence of myofibroblasts (Ehrlich, 1988; Ehrlich & Rajaratnam, 1990). The contraction of FPCL generates minimal tension. Lattice contraction proceeds by the collagen organization and not by cell contractile forces. Free floating FPCL share similarities with SB505124 treated open wounds, lacking myofibroblasts, more uniform organized collagen fiber bundles and less tension. Treating free floating FPCL with TGFβ1 enhances lattice contraction (Montesano and Orci, 1988). In contrast the attached FPCL is populated with myofibroblasts, which is the result of tension (Tomasek et al., 1992). Released myofibroblast populated collagen lattices show very rapid lattice contraction with the contraction of resident myofibroblasts. In this model myofibroblasts under tension cause lattice contraction by cell contraction generated forces.
Another example of wound contraction proceeding in the absence of myofibroblasts is in vanadate treated open rat wounds (Ehrlich et al., 1999). Wound contraction in presence of vanadate shows the more uniform packing of collagen fiber bundles deposited in granulation tissue. Berry and coworkers studies of contracting open pilonidal sinus excisional wounds in 15 patients found fibroblasts were the major cell population in these contracting human wounds (Berry et al., 1998). Myofibroblasts were a minor cell population, representing about 10% of the fibroblast population. The closure of pilonidal sinus excisional wounds by wound contraction represents a major volume change in that large wound cavity. Wound contraction of pilonidal sinus excisional wounds proceeds through the compaction and organization of collagen fibers; rather than by cell contraction forces.
CONCLUSIONS
Square full thickness, 14 day excisional wounds made on the backs of rats were treated with SB-505124, which disrupts the Smad signaling pathway or gel vehicle. There was no difference in wound contraction between SB-505124 treated and vehicle treated wounds. Blocking TGFβ1 signaling through the Smad signaling pathway diminished connective tissue deposition, generated a deficiency of myofibroblasts, but wound contraction was unimpaired. These findings support the notion that wound contraction proceeds through the organization of collagen fiber bundles within granulation tissue by fibroblasts, rather than through contractile forces generated by myofibroblasts.
Acknowledgments
FUNDING STATEMENT
The funding associated with this manuscript is from NIH grant GM 056851.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
Contributor Information
Katherine Au, Division of Plastic Surgery, Penn State University College of Medicine, MS Hershey Medical Center, Hershey, PA, kau@shrinenet.org.
H. Paul Ehrlich, Division of Plastic Surgery, Penn State University College of Medicine, MS Hershey Medical Center, Hershey, PA.
REFERENCES
- Bell E, Ivarsson B, Merrill C. Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc. Natl. Acad. Sci. U. S. A. 1979;76:1274–1278. doi: 10.1073/pnas.76.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DP, Harding KG, Stanton MR, Jasani B, Ehrlich HP. Human wound contraction: collagen organization, fibroblasts, and myofibroblasts. Plast. Reconstr. Surg. 1998;102:124–131. doi: 10.1097/00006534-199807000-00019. discussion 132–134. [DOI] [PubMed] [Google Scholar]
- DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2004;65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell. Biol. 1993;122:103–111. doi: 10.1083/jcb.122.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP. Wound closure: evidence of cooperation between fibroblasts and collagen matrix. Eye (Lond) 1988;2 ( Pt 2):149–157. doi: 10.1038/eye.1988.28. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Rajaratnam JB. Cell locomotion forces versus cell contraction forces for collagen lattice contraction: an in vitro model of wound contraction. Tissue. Cell. 1990;22:407–417. doi: 10.1016/0040-8166(90)90070-p. [DOI] [PubMed] [Google Scholar]
- Ehrlich HP, Keefer KA, Myers RL, Passaniti A. Vanadate and the absence of myofibroblasts in wound contraction. Arch. Surg. 1999;134:494–501. doi: 10.1001/archsurg.134.5.494. [DOI] [PubMed] [Google Scholar]
- Fukuchi M, Imamura T, Chiba T, Ebisawa T, Kawabata M, Tanaka K, Miyazono K. Ligand-dependent degradation of Smad3 by a ubiquitin ligase complex of ROC1 and associated proteins. Mol. Biol. Cell. 2001;12:1431–1443. doi: 10.1091/mbc.12.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gray PC, Vale WW, Robertson DM. Antagonists of activin signaling: mechanisms and potential biological applications. Trends. Endocrinol. Metab. 2005;16:73–78. doi: 10.1016/j.tem.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hubner G, Hu Q, Smola H, Werner S. Strong induction of activin expression after injury suggests an important role of activin in wound repair. Dev. Biol. 1996;173:490–498. doi: 10.1006/dbio.1996.0042. [DOI] [PubMed] [Google Scholar]
- Itoh S, Itoh F, Goumans MJ, Ten Dijke P. Signaling of transforming growth factor-beta family members through Smad proteins. Eur. J. Biochem. 2000;267:6954–6967. doi: 10.1046/j.1432-1327.2000.01828.x. [DOI] [PubMed] [Google Scholar]
- Li Z, Dranoff JA, Chan EP, Uemura M, Sevigny J, Wells RG. Transforming growth factor-beta and substrate stiffness regulate portal fibroblast activation in culture. Hepatology. 2007;46:1246–1256. doi: 10.1002/hep.21792. [DOI] [PubMed] [Google Scholar]
- Malmsten M. Surfactants and polymers in drug delivery. New York, NY: Marcel Dekker; 2002. [Google Scholar]
- Massague J. How cells read TGF-beta signals. Nat. Rev. Mol. Cell. Biol. 2000;1:169–178. doi: 10.1038/35043051. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc. Natl. Acad. Sci. U. S. A. 1988;85:4894–4897. doi: 10.1073/pnas.85.13.4894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz B, Smola H, Engelhardt F, Bleuel K, Brauchle M, Lein I, Evans LW, Huylebroeck D, Balling R, Werner S. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO. J. 1999;18:5205–5215. doi: 10.1093/emboj/18.19.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughan MB. Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat. Rec. 1992;232:359–368. doi: 10.1002/ar.1092320305. [DOI] [PubMed] [Google Scholar]