Abstract
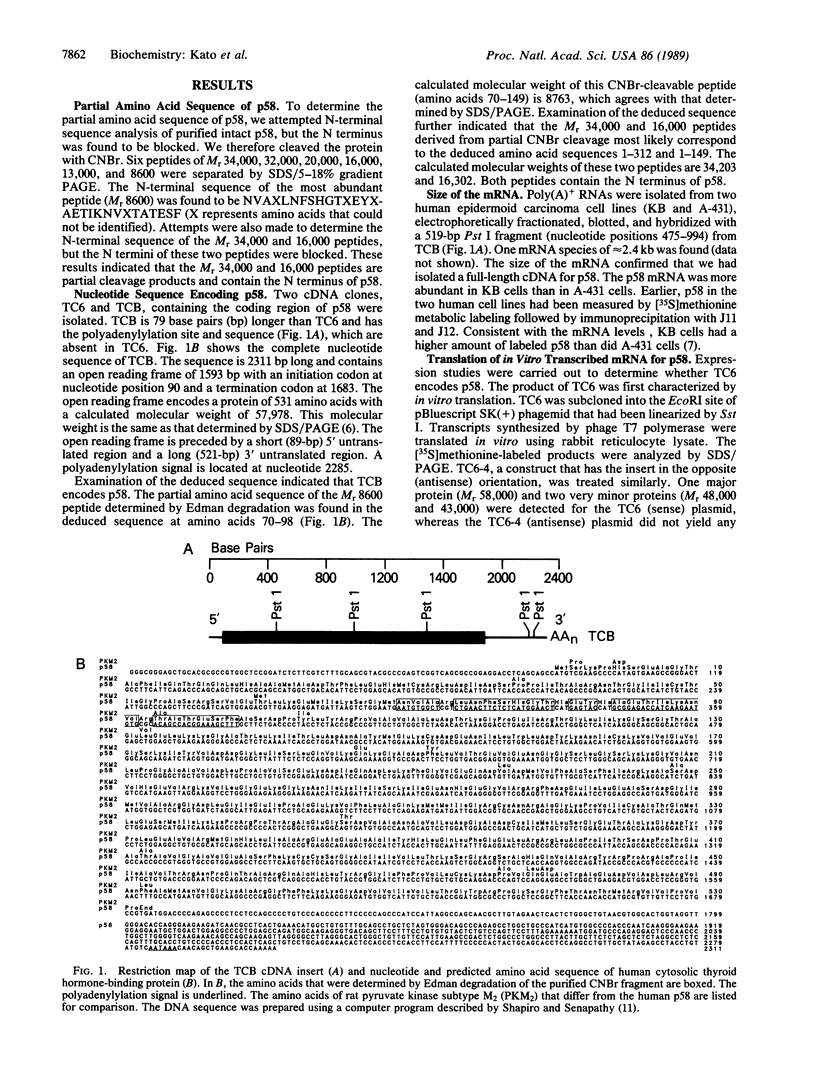
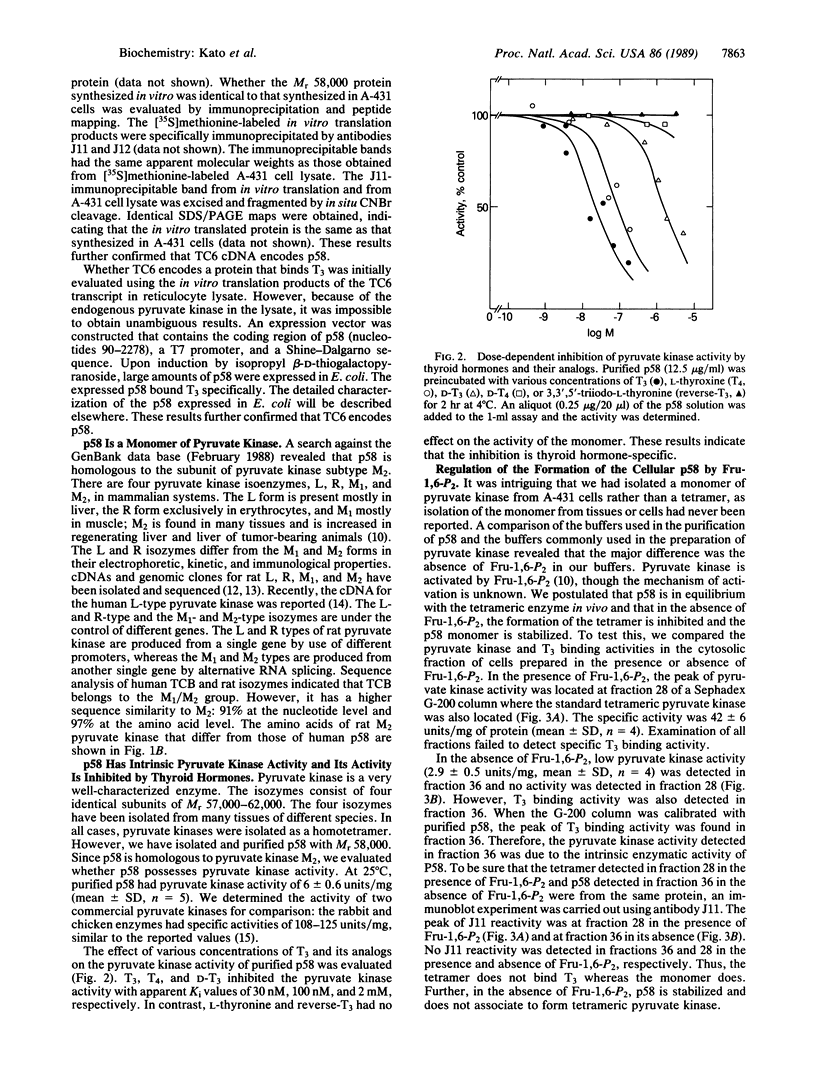
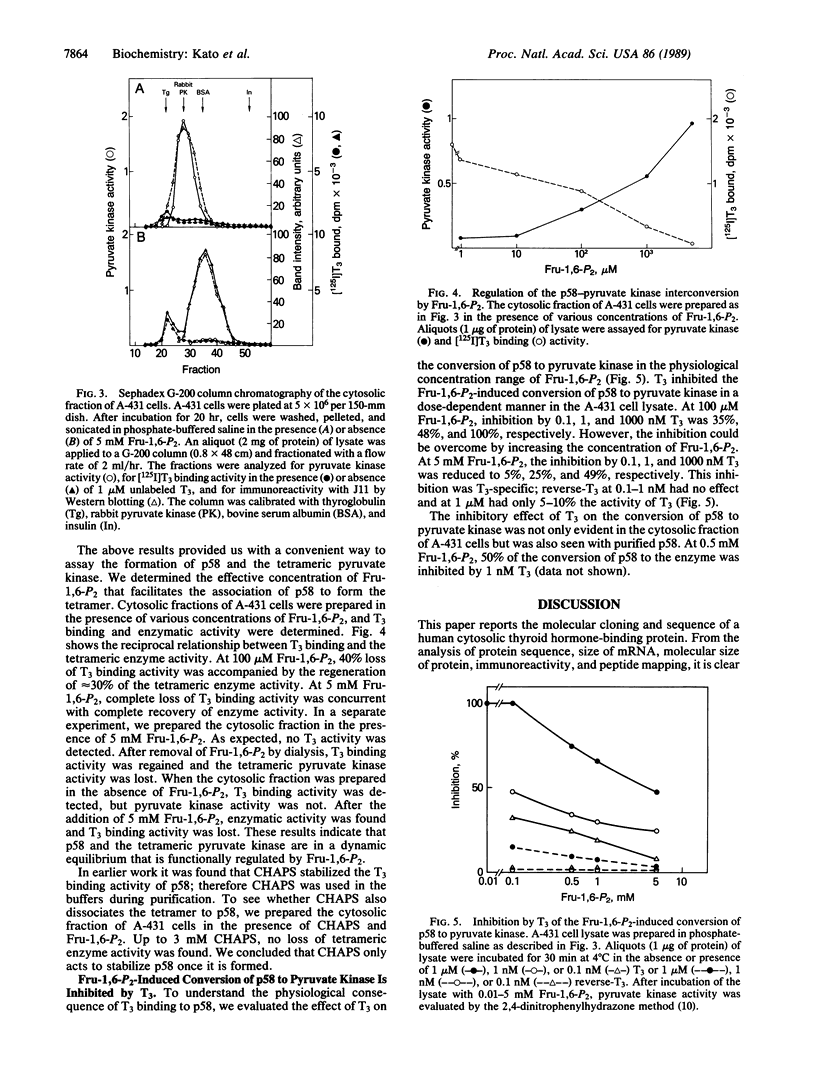
A cDNA clone encoding a human cytosolic thyroid hormone-binding protein (p58) has been isolated. The human sequence was found to be homologous to that of rat pyruvate kinase (EC 2.7.1.40) subtype M2. p58 is a monomer that has approximately 5% the enzymatic activity of the tetrameric pyruvate kinase M2. The tetrameric M2 does not bind 3,3',5-triiodo-L-thyronine (T3). Binding of p58 to T3 and its analogs resulted in the inhibition of its pyruvate kinase activity. The apparent Ki values of T3, L-thyroxine, and D-T3 are 30 nM, 100 nM, and 2 mM, respectively. L-Thyronine and 3,3',5'-triiodo-L-thyronine had no effect. This order of activity correlates with the thermogenic effects reported for T3 and its analogs. Conversion of p58 to the tetramer is reversible and is under the control of fructose 1,6-bisphosphate. The conversion is inhibited by T3 in a dose-dependent manner. Since pyruvate kinase is a key enzyme in regulating cellular ADP, ATP, and pyruvate, our findings suggest that p58 may be involved in mediating some of the cellular metabolic effects induced by thyroid hormones.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boado R. J., Campbell D. A., Chopra I. J. Nucleotide sequence of rat liver iodothyronine 5'-monodeiodinase (5' MD): its identity with the protein disulfide isomerase. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1297–1304. doi: 10.1016/s0006-291x(88)81282-8. [DOI] [PubMed] [Google Scholar]
- Bronk J. R. Thyroid hormone: effects on electron transport. Science. 1966 Aug 5;153(3736):638–639. doi: 10.1126/science.153.3736.638. [DOI] [PubMed] [Google Scholar]
- Cardenas J. M., Hubbard D. R., Anderson S. Subunit structure and hybrid formation of bovine pyruvate kinases. Biochemistry. 1977 Jan 25;16(2):191–197. doi: 10.1021/bi00621a005. [DOI] [PubMed] [Google Scholar]
- Cheng S. Y., Gong Q. H., Parkison C., Robinson E. A., Appella E., Merlino G. T., Pastan I. The nucleotide sequence of a human cellular thyroid hormone binding protein present in endoplasmic reticulum. J Biol Chem. 1987 Aug 15;262(23):11221–11227. [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Geetha-Habib M., Noiva R., Kaplan H. A., Lennarz W. J. Glycosylation site binding protein, a component of oligosaccharyl transferase, is highly similar to three other 57 kd luminal proteins of the ER. Cell. 1988 Sep 23;54(7):1053–1060. doi: 10.1016/0092-8674(88)90120-1. [DOI] [PubMed] [Google Scholar]
- Hagiwara M., Mamiya S., Hidaka H. Selective binding of L-thyroxine by myosin light chain kinase. J Biol Chem. 1989 Jan 5;264(1):40–44. [PubMed] [Google Scholar]
- Imamura K., Tanaka T. Pyruvate kinase isozymes from rat. Methods Enzymol. 1982;90(Pt E):150–165. doi: 10.1016/s0076-6879(82)90121-5. [DOI] [PubMed] [Google Scholar]
- Jedrzejak J., Heyduk T., Kochman M. An approach to the elucidation of the quaternary structure role in the activity of pyruvate kinase studies on the immobilized enzyme. Int J Biochem. 1983;15(5):695–702. doi: 10.1016/0020-711x(83)90194-5. [DOI] [PubMed] [Google Scholar]
- Kitagawa S., Obata T., Hasumura S., Pastan I., Cheng S. Y. A cellular 3,3',5-triiodo-L-thyronine binding protein from a human carcinoma cell line. Purification and characterization. J Biol Chem. 1987 Mar 15;262(8):3903–3908. [PubMed] [Google Scholar]
- MacDonald R. G., Pfeffer S. R., Coussens L., Tepper M. A., Brocklebank C. M., Mole J. E., Anderson J. K., Chen E., Czech M. P., Ullrich A. A single receptor binds both insulin-like growth factor II and mannose-6-phosphate. Science. 1988 Mar 4;239(4844):1134–1137. doi: 10.1126/science.2964083. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Noguchi T., Inoue H., Tanaka T. The M1- and M2-type isozymes of rat pyruvate kinase are produced from the same gene by alternative RNA splicing. J Biol Chem. 1986 Oct 15;261(29):13807–13812. [PubMed] [Google Scholar]
- Noguchi T., Yamada K., Inoue H., Matsuda T., Tanaka T. The L- and R-type isozymes of rat pyruvate kinase are produced from a single gene by use of different promoters. J Biol Chem. 1987 Oct 15;262(29):14366–14371. [PubMed] [Google Scholar]
- Obata T., Fukuda T., Willingham M. C., Liang C. M., Cheng S. Y. A cytoplasmic thyroid hormone binding protein: characterization using monoclonal antibodies. Biochemistry. 1989 Jan 24;28(2):617–623. doi: 10.1021/bi00428a030. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L., Mariash C. N., Kinlaw W. B., Wong N. C., Freake H. C. Advances in our understanding of thyroid hormone action at the cellular level. Endocr Rev. 1987 Aug;8(3):288–308. doi: 10.1210/edrv-8-3-288. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H., Schwartz H. L. Stereospecific transport of triiodothyronine from plasma to cytosol and from cytosol to nucleus in rat liver, kidney, brain, and heart. J Clin Invest. 1985 Jan;75(1):147–154. doi: 10.1172/JCI111667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlajaniemi T., Helaakoski T., Tasanen K., Myllylä R., Huhtala M. L., Koivu J., Kivirikko K. I. Molecular cloning of the beta-subunit of human prolyl 4-hydroxylase. This subunit and protein disulphide isomerase are products of the same gene. EMBO J. 1987 Mar;6(3):643–649. doi: 10.1002/j.1460-2075.1987.tb04803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. H., Cardenas J. M. Single subunits of Sepharose-bound pyruvate kinase are inactive. Biochemistry. 1981 Apr 28;20(9):2532–2537. doi: 10.1021/bi00512a026. [DOI] [PubMed] [Google Scholar]
- Samuels H. H., Forman B. M., Horowitz Z. D., Ye Z. S. Regulation of gene expression by thyroid hormone. J Clin Invest. 1988 Apr;81(4):957–967. doi: 10.1172/JCI113449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. Automated preparation of DNA sequences for publication. Nucleic Acids Res. 1986 Jan 10;14(1):65–73. doi: 10.1093/nar/14.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling K., Brenner M. A., Sakurada T. Rapid effect of triiodothyronine on the mitochondrial pathway in rat liver in vivo. Science. 1980 Oct 17;210(4467):340–342. doi: 10.1126/science.7423197. [DOI] [PubMed] [Google Scholar]
- Tani K., Fujii H., Nagata S., Miwa S. Human liver type pyruvate kinase: complete amino acid sequence and the expression in mammalian cells. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1792–1795. doi: 10.1073/pnas.85.6.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]


