Abstract
Our previous proteomic study demonstrated that oxidative stress and antioxidant delphinidin regulated the cellular level of p27kip1 (referred to as p27) as well as some heat shock proteins in human colon cancer HT 29 cells. Current study was conducted to validate and confirm the regulation of these proteins using both in vitro and in vivo systems. The level of p27 was decreased by hydrogen peroxide in a dose-dependent manner in human colon carcinoma HCT 116 (p53-positive) cells while it was increased upon exposure to hydrogen peroxide in HT 29 (p53-negative) cells. However, high concentration of hydrogen peroxide (100 µM) downregulated p27 in both cell lines, but delphindin, one of antioxidative anthocyanins, enhanced the level of p27 suppressed by 100 µM hydrogen peroxide. ICR mice were injected with varying concentrations of hydrogen peroxide, delphinidin and both. Western blot analysis for the mouse large intestinal tissue showed that the expression of p27 was upregulated by 25 mg/kg BW hydrogen peroxide. To investigate the association of p27 regulation with hypoxia-inducible factor 1-beta (HIF-1β), the level of p27 was analyzed in wild-type mouse hepatoma hepa1c1c7 and Aryl Hydrocarbon Nuclear Translocator (arnt, HIF-1β)-defective mutant BPRc1 cells in the absence and presence of hydrogen peroxide and delphinidin. While the level of p27 was responsive to hydrogen peroxide and delphinidin, it remained unchanged in BPRc1, suggesting that the regulation of p27 requires functional HIF-1β. We also found that hydrogen peroxide and delphinidin affected PI3K/Akt/mTOR signaling pathway which is one of upstream regulators of HIFs. In conclusion, hydrogen peroxide and antioxidant delphinidin seem to regulate intracellular level of p27 through regulating HIF-1 level which is, in turn, governed by its upstream regulators comprising of PI3K/Akt/mTOR signaling pathway. The results should also encourage further study for the potential of p27 as a biomarker for intracellular oxidative or antioxidant status.
Keywords: p27kip1, biomarker, antioxidant, oxidative status, delphinidin
Introduction
Oxidative stress is caused by an imbalance between the production of reactive oxygen species and biological systems ability to readily detoxify the reactive intermediates [1,2]. The imbalance may be a consequence of reduced antioxidant capacity caused by disturbances in dietary intake, production and distribution of antioxidants, or an overabundance of reactive oxygen species (ROS) from an environmental or behavioral stressor, including tissue pathology. Oxidative stress has been implicated in a growing list of human diseases, including colon pathologies such as cancer.
Numerous physiological functions are controlled by redox-responsive signaling pathways. These cases of redox regulation typically involve the regulated production of nitric oxide (NO) or ROS by nitric oxide synthase (NOS) or NAD(P)H oxidase, respectively, and the effects of these compounds on specific signaling cascades. The redox-sensitive target molecules of these signaling cascades and their chemical modification by oxidative agents are, in most cases, not yet well understood [3].
Our previous study using proteomic approach demonstrated that p27kip1 (hereafter referred to as p27) and some heat shock proteins such as glucose regulated protein 78 (GRP78) and HSP105 were regulated by oxidative stress and antioxidant in human colon cancer cells [4]. p27 has been reported to prevent Cdk4 from adding phosphate residues to its principal substrate, the retinoblastoma (pRb) protein. The increased level of p27 protein typically causes cells to arrest at the G1 phase of the cell cycle. Likewise, p27 is able to bind other Cdk proteins when complexed to cyclin subunits such as cyclin E/cdk2 and cyclin A/cdk2 [5,6]. The expression of p27 has been reported to be regulated by hypoxia-inducible factor 1α (HIF-1α), which, in turn, is governed by PI3K/Akt/mTOR signaling pathway [6,7]. Accordingly, we attempted to confirm the hypoxia-inducible factor (HIF)-mediated regulation of p27 level by oxidative stress or antioxidant. We were also interested in studying the possibility of p27 as a biomarker for oxidative status in the living things. Our study suggests that p27 might be a useful biomarker for intracellular oxidative status and its expression is differentially controlled in human colon carcinoma HT 29 (p53-negative) and HCT 116 (p53-positive). Furthermore, the regulation of its expression not only requires functional HIF-1β but also is governed by PI3K/Akt/mTOR signaling pathway.
Materials and Methods
Cell culture and conditions
Mouse hepatoma hepa1c1c7 cell and its mutant BPRc1 (lacking HIF-1β) cells [8] were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA), and the human colon cancer cell lines (HCT 116 and HT 29) were purchased from the Korean Cell Line Bank (Seoul, Korea). Mouse hepatoma cells and human colon cancer cells were cultured in α-MEM and RPMI containing 10% FBS, respectively, and grown at 37℃ under a 5% CO2-95% air atmosphere.
Preparation of cell extracts and western blot
After cells were exposed to samples for 12 and 24 h, culture medium was removed and washed with 5 ml PBS twice. Adherent cells were collected into an eppendorf tube (2 ml) using 1 ml of 0.25 M sucrose solution, sonicated and centrifuged at 10,500 ×g for 15 min. The supernatant was used for immunoblot assay [4].
Animal experiment
Healthy male ICR mice weighing 20-22 g were obtained from DaeHan Biolink Co., Ltd. (Seoul, Korea). The animal room was maintained under controlled conditions of 22-26℃ and light-dark periods of 12 h, with free access to commercial diet and water. Thirty five mice were randomly assigned to five treatment groups and were acclimatized for 1 week before treatment. Mice were administered with hydrogen peroxide at dosages of 25 and 50 mg/kg body weight (BW) and delphinidin at 20 mg/kg BW thrice a week. The control group was administered with the same volume of 0.1% saline. All thirty five animals were sacrificed after 7 days of exposure. Large intestinal tissues from the mice were immediately scraped to collect epithelia, frozen in liquid nitrogen and stored in a -70℃ deep freezer for immunoblot assay. Large intestinal epithelia were homogenized in 300 ml lysis buffer (0.5% NP40, 1% Triton X-100, 1% protease inhibitor, 150 mM NaCl and 10 mM Tris, pH 7.4) and centrifuged at 15,000 ×g for 30 min. Protein concentration was measured using Bradford method (Bio-Rad, Hercules, CA, USA) for equal loading in Western blot. The experimental protocol was approved by the committee on Animal Care and Research Ethics of Kyungpook National University.
Statistical analysis
Statistical significance of data was tested by analysis of variance, followed by Duncan's multiple range test, using SPSS software (SPSS Inc., Chicago, IL). P < 0.05 was considered to be statistically significant.
Results
Regulation of expression of p27 by hydrogen peroxide and delphinidin
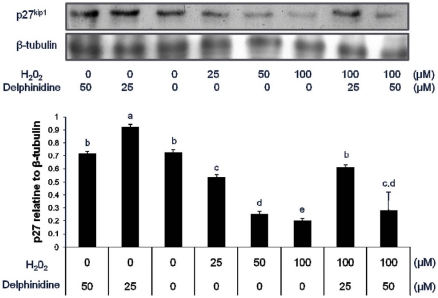
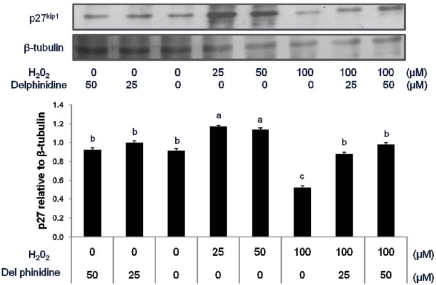
The level of p27 protein was decreased upon exposure to hydrogen peroxide in a dose-dependent manner in human colon carcinoma HCT 116 and HT 29 cell lines although the upregulation of p27 by hydrogen peroxide was more pronounced in HCT 116 cells carrying wild type p53. In particular, p27 expression suppressed by hydrogen peroxide was restored to untreated control level by cotreatment with delphinidin (Fig. 1 and 2). Delphinidin had a limited effect on p27 expression although it enhanced the level of p27 in the presence of 100 µM H2O2. More specifically, the level of p27 was increased upon exposure to 25 µM delphinidin in HCT 116 cells but remained unchanged in HT 29 cells treated with delphinidin.
Fig. 1.
Change in the expression of cell cycle inhibitor, p27kip1 in HCT 116 cells treated with hydrogen peroxide and delphinidin for 12 h. Human colon carcinoma HCT 116 cells (wild type p53) were exposed to various doses of H2O2 and delphinidin for 12 h, followed by determination of p27kip1 level by Western blot. Data are representative of three independent experiments as mean ± SD. Values on bars not sharing a common superscript significantly differ from each other (P < 0.05).
Fig. 2.
Change in the expression of cell cycle inhibitor, p27kip1 in HT 29 cells treated with hydrogen peroxide and delphinidin for 12 h. Human colon carcinoma HT 29 cells (nonfunctional p53) were exposed to various doses of H2O2 and delphinidin for 12 h, followed by determination of p27kip1 level by Western blot. Data are representative of three independent experiments as mean ± SD. Values on bars not sharing a common superscript significantly differ from each other (P < 0.05).
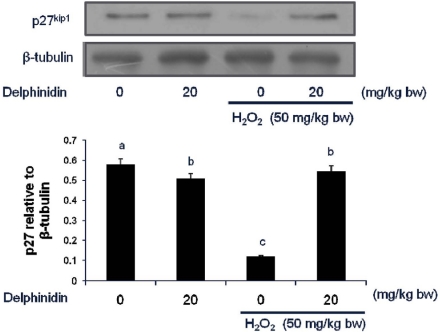
The similar regulation pattern has been observed in mouse large intestine tissues. That is, p27 expression was significantly reduced by hydrogen peroxide (50 mg/kg BW) and restored to control level by injection with 20 mg/kg delphinidin (Fig. 3). Similar to those observed in cultured cells, delphinidin did not change the level of p27 in large intestinal tissue in the absence of hydrogen peroxide.
Fig. 3.
Modulation of p27kip1 protein expression in large intestinal tissue of mice treated with delphinidin and hydrogen peroxide. ICR mice were injected with varying doses of H2O2 and delphinidin three times for a week, and epithelia of large intestine were collected and analyzed for p27kip1 level. Data are representative of three independent experiments as mean ± SD. Values on bars not sharing a common superscript significantly differ from each other (P < 0.05).
Role of hypoxia-inducible factor in p27 regulation
In order to investigate whether the regulation of p27 level by oxidative stress and antioxidant is mediated by hypoxia-inducible factor(s), the levels of p27 were analyzed in the presence of varying concentrations of H2O2 and delphinidin in wild type mouse hepatoma hepa1c1c7 cells and its mutant BPRc1 cells with defective HIF-1β which is essential for HIF-mediated transcriptional regulation. Interestingly enough, while wild type hepa1c1c7 cells containing functional HIF-1β were responsive to treatment with hydrogen peroxide or delphinidin, BPRc1 defective in HIF-1β was not responsive to either hydrogen peroxide or delphinidin (Fig. 4). The amplitude of fluctuation in p27 level in hepa1c1c7 cells was much larger than in BPRc1 cells (Fig. 4), suggesting involvement of HIF-1β in p27 regulation.
Fig. 4.
Effect of hydrogen peroxide and delphinidin on the level of p27kip1 in wild type hepa1c1c7 (A) and HIF-1β-deficient BPRc1 (B) cells. Mouse hepatoma hepa1c1c7 (HIF-1β-positive) and its mutant BPRc1 (HIF-1β-negative) were treated with various doses of delphinidin in the presence of 100 µM H2O2, followed by the determination of p27kip1 level. Data are representative of three independent experiments as mean ± SD. Values on bars not sharing a common superscript significantly differ from each other (P < 0.05).
Delphinidin and hydrogen peroxide affect PI3K/Akt/mTOR signaling pathway
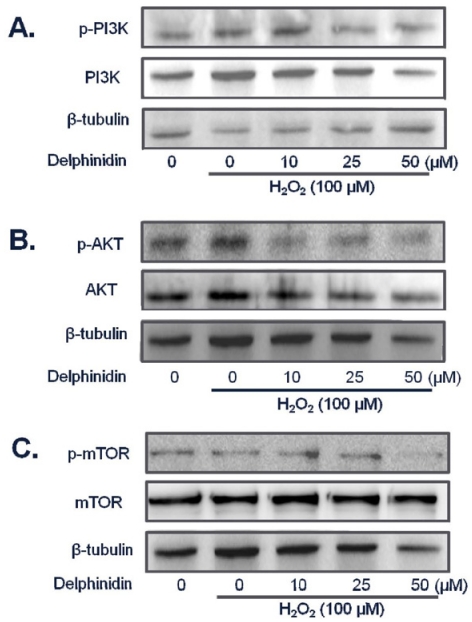
Gathering all the results from our experiment and other studies, we hypothesized that one of the possible route that HIF-1 might be regulated into is the PI3K/AkT/mTOR signaling pathway. We, therefore, investigated the expression levels and phosphorylation of major proteins in the pathway using HCT 116 human colon cancer cell line. In the cells treated with different concentrations of delphinidin and hydrogen peroxide, the levels of p-mTOR, p-AKT and p-PI3K were reduced by delphinidin while they were not affected by hydrogen peroxide (Fig. 5).
Fig. 5.
The levels of PI3K (A), Akt (B), mTOR (C) and their phosphorylated forms in HCT 116 cells treated with delphinidin and hydrogen peroxide. Human colon carcinoma HCT 116 cells were exposed to various doses of delphinidin in the presence of 100 µM H2O2, followed by the determination of PI3K, Akt, mTOR and their phosphorylation.
Discussion
Oxidative stress has been implicated in an enormous variety of physiological and pathological processes, including aging, cancer, diabetes, atherosclerosis, neurological disorders, autoimmune diseases such as arthritis [9,10]. ROS have been found to induce many types of DNA damage. In the face of the deleterious effects of oxidative stress, aerobic organisms have developed a wide array of different mechanisms to maintain genomic stability. These mechanisms include constitutive and inducible antioxidants, oxidant defense enzymes such as catalase and superoxide dismutase (SOD), DNA repair enzymes, and mechanisms of genomic surveillance, such as cell cycle checkpoint systems. Our preliminary study demonstrated that oxidative stress such as exposure to H2O2 treatment modulated the level of p27, which is Cdk inhibitor (CDKI) protein.
The CDKI p27 inhibits the kinase activity of cyclin-cdk complexes, particularly cyclin E-cdk2 and cyclin D-cdk4 activity, resulting in cell cycle arrest [11]. Regulation of p27 by mitogenic stimuli involves p27 transcription, translation, stability, and localization, and results in release of the cyclin-cdk complexes. Cyclin D-cdk4 then phosphorylates the retinoblastoma protein pRB, which attenuates repression of the mitogenic E2F transcription factor, resulting in G1-to-S progression [12]. It is a matter of debate whether the induction of p27 by hypoxia is HIF-1α-dependent [13-17].
Although p27 was shown to be induced by hypoxia in several studies, the present study demonstrated that the mode of p27 regulation by oxidative stress is complex. For instance, the level of p27 was decreased by hydrogen peroxide but increased by delphinidin in wild type human colon cancer cell (HCT 116). In HT 29 cells, p27 was slightly upregulated upon exposure to hydrogen peroxide at the concentrations of 25 and 50 µM like other reports [14,15,17]. The discrepancy in p27 regulation by hydrogen peroxide between two cell lines suggests that p27 regulation could be affected by the function of p53. Common downregulation of p27 in both cells exposed to the highest concentration of H2O2 (100 µM) and p53-independent upregulation by delphinidin suggest the existence of other mechanisms independent of p53 in regulating p27 level [13].
It has been reported that p27 level is associated with HIF-1, a transcription factor, which is critical to cell survival under hypoxic conditions [12-17]. HIF-1 is composed of the O2- and growth factor-regulated HIF-1α subunit, and the constitutively expressed HIF-1β subunit (aryl hydrocarbon receptor nuclear translocator, ARNT), both of which belong to the basic-helix-loop-helix (bHLH)-PAS (PER, ARNT, SIM) protein family [18-21]. The data also supported that p27 regulation is closely related to HIF-1 as mouse hepatoma cells (BPRc1) with defective HIF-1β was resistant to hydrogen peroxide and delphinidin while wild type hepa1c1c7 cells were responsive to them (Fig. 4).
The PI3K-Akt pathway is also intricately linked to HIF-1 regulation, not only by inducing HIF-1α translation in response to growth factors but also through the regulation of HIF-1α protein degradation [22]. In particular, oxidative stress was also known to stabilize HIF-1α and increase its intracellular level by similar mechanism to hypoxia [23-25].
Thus findings made so far support hypothesis that oxidative stress and antioxidant regulate p27 by affecting PI3K-Akt signaling pathway which, in turn, regulates HIF-1. In fact, we found that delphinidin at 25 µM or higher inhibited phosphorylation of PI3K and Akt in the presence of 100 µM H2O2 without affecting the levels of the kinases. Phosphorylation of mTOR was also inhibited by 50 µM delphinidin. Taken together, antioxidants like delphinidin might prevent from carcinogenesis by modulating HIF-1 and PI3K/Akt signaling pathway while ROS such as H2O2 act in the opposite way. Furthermore, p27 deserves further study as a biomarker for antioxidant potential.
Footnotes
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (314-2008-1-F00069 & 2009-0083853).
References
- 1.Haddad JE, Olver RE, Land SC. Antioxidant/Pro-oxidant equilibrium regulates HIF-1 and NF-κB redox sensitivity. J Biol Chem. 2000;275:21130–21139. doi: 10.1074/jbc.M000737200. [DOI] [PubMed] [Google Scholar]
- 2.Paron I, D'Elia A, D'Ambrosio C, Scaloni A, D'Aurizio F, Prescott A, Damante G, Tell G. A proteomic approach to identify early molecular targets of oxidative stress in human epithelial lens cells. Biochem J. 2004;378:929–937. doi: 10.1042/BJ20031190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith J, Ladi E, Proschel MM, Noble M. Redox state is a central regulator of the balance between self-renewal and differentiation in a dividing glial precursor cell. Proc Natl Acad Sci U S A. 2000;97:10032–10037. doi: 10.1073/pnas.170209797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jang CH, Lee IA, Ha YR, Lim J, Sung MK, Lee SJ, Kim JS. PGK1 induction by hydrogen peroxide treatment is suppressed by antioxidants in human colon carcinoma cells. Biosci Biotechnol Biochem. 2008;72:1799–1808. doi: 10.1271/bbb.80079. [DOI] [PubMed] [Google Scholar]
- 5.Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. doi: 10.1017/S1462399408000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green SL, Freiberg RA, Giaccia AJ. p21(Cip1) and p27(Kip1) regulate cell cycle reentry after hypoxic stress but are not necessary for hypoxia-induced arrest. Mol Cell Biol. 2001;21:1196–1206. doi: 10.1128/MCB.21.4.1196-1206.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackenbeck T, Knaup KX, Schietke R, Schödel J, Willam C, Wu X, Warnecke C, Eckardt KU, Wiesener MS. HIF-1 or HIF-2 induction is sufficient to achieve cell cycle arrest in NIH3T3 mouse fibroblasts independent from hypoxia. Cell Cycle. 2009;8:1386–1395. doi: 10.4161/cc.8.9.8306. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 9.Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol. 2003;23:359–369. doi: 10.1128/MCB.23.1.359-369.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floyd RA. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990;4:2587–2597. [PubMed] [Google Scholar]
- 11.Shackelford RE, Kaufmann WK, Paules RS. Oxidative stress and cell cycle checkpoint function. Free Radic Biol Med. 2000;28:1387–1404. doi: 10.1016/s0891-5849(00)00224-0. [DOI] [PubMed] [Google Scholar]
- 12.Massagué J. G1 cell-cycle control and cancer. Nature. 2004;432:298–306. doi: 10.1038/nature03094. [DOI] [PubMed] [Google Scholar]
- 13.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumor angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 15.Gardner LB, Li Q, Park MS, Flanagan WM, Semenza GL, Dang CV. Hypoxia inhibits G1/S transition through regulation of p27 expression. J Biol Chem. 2001;276:7919–7926. doi: 10.1074/jbc.M010189200. [DOI] [PubMed] [Google Scholar]
- 16.Mack FA, Patel JH, Biju MP, Haase VH, Simon MC. Decreased growth of Vhl-/- fibrosarcomas is associated with elevated levels of cyclin kinase inhibitors p21 and p27. Mol Cell Biol. 2005;25:4565–4578. doi: 10.1128/MCB.25.11.4565-4578.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horrée N, Gort EH, van der Groep P, Heintz AP, Vooijs M, van Diest PJ. Hypoxia-inducible factor 1 alpha is essential for hypoxic p27 induction in endometrioid endometrial carcinoma. J Pathol. 2008;214:38–45. doi: 10.1002/path.2244. [DOI] [PubMed] [Google Scholar]
- 18.Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Wang GL, Jiang BH, Rue EA. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semenza GL. HIF-1 and mechanism of hypoxia sensing. Curr Opin Cell Biol. 2001;13:167–171. doi: 10.1016/s0955-0674(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 21.Ardyanto TD, Osaki M, Tokuyasu N, Nagahama Y, Ito H. CoCl2-induced HIF-1alpha expression correlates with proliferation and apoptosis in MKN-1 cells: a possible role for the PI3K/Akt pathway. Int J Oncol. 2006;29:549–555. [PubMed] [Google Scholar]
- 22.Yee Koh M, Spivak-Kroizman TR, Powis G. HIF-1 regulation: not so easy come, easy go. Trends Biochem Sci. 2008;33:526–534. doi: 10.1016/j.tibs.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 23.Qutub AA, Popel AS. Reactive oxygen species regulate hypoxia-inducible factor 1alpha differentially in cancer and ischemia. Mol Cell Biol. 2008;28:5106–5119. doi: 10.1128/MCB.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266:12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 25.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]