Abstract
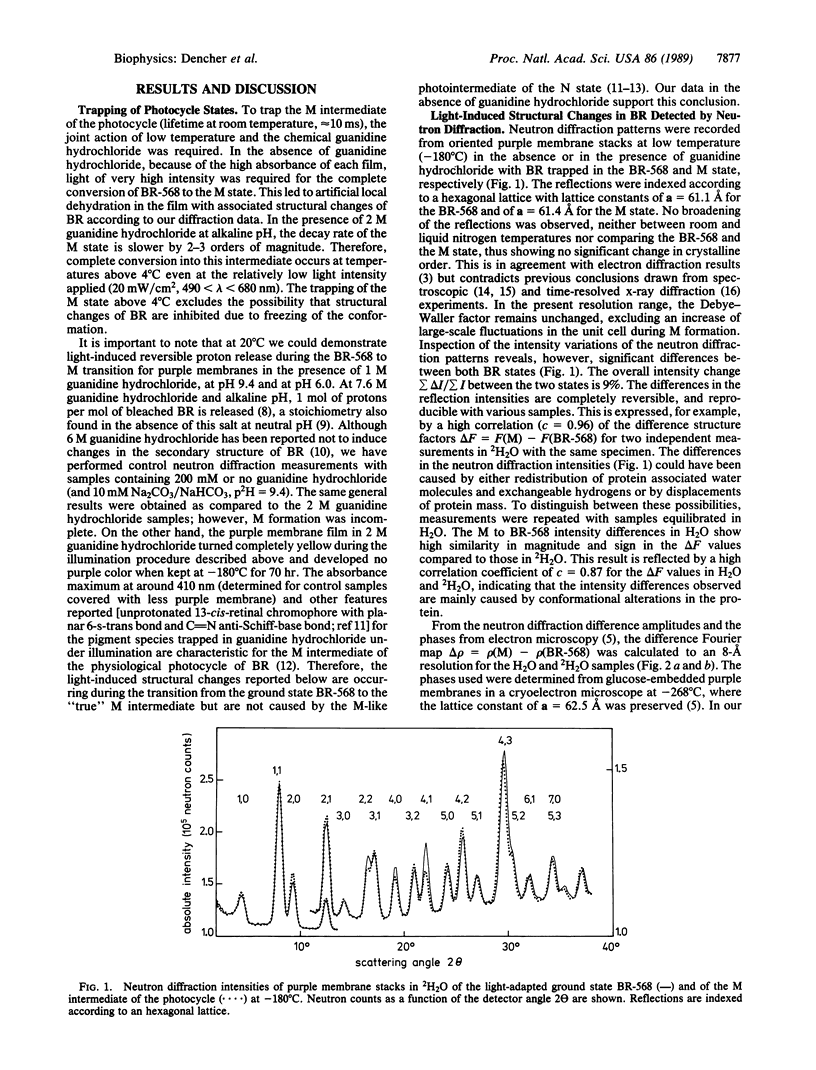
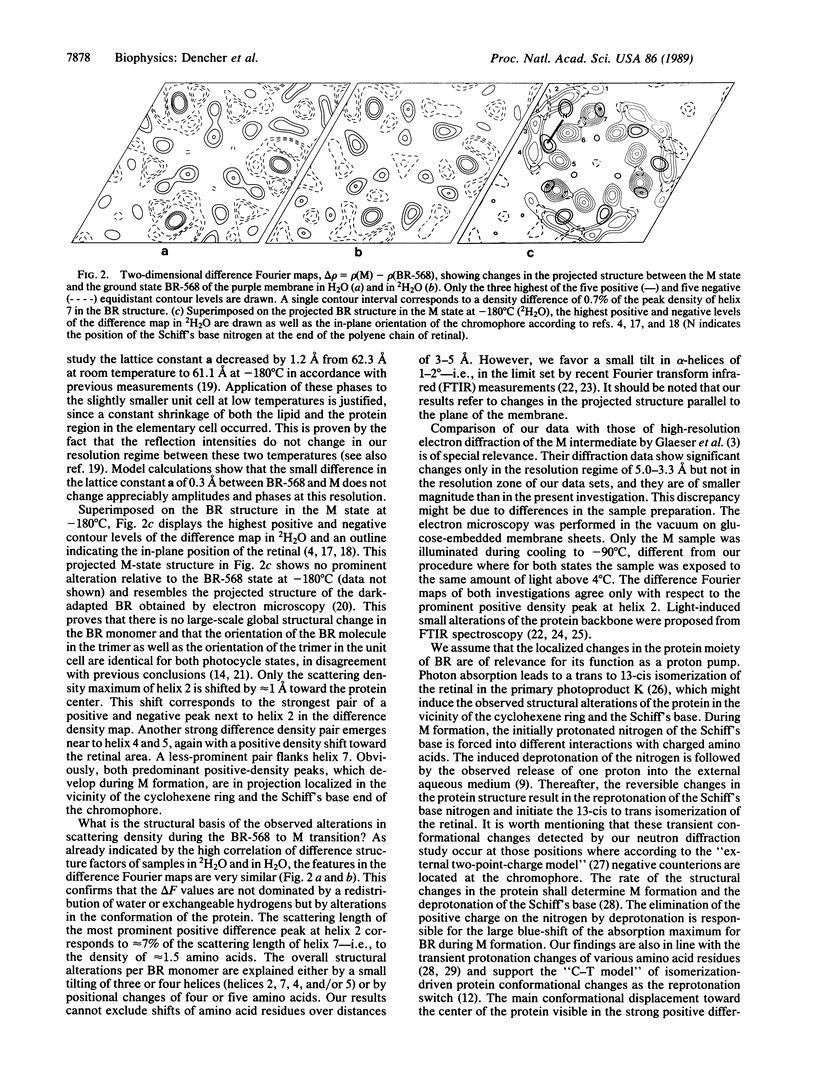
A neutron diffraction study of spectroscopic states for the light-energized proton pump bacteriorhodopsin (BR) is presented. The photocycle states BR-568 and M were generated at temperatures above 4 degrees C and were measured after trapping at--180 degrees C. In the BR-568 to M-state transition, which is known to be a key step in transmembrane proton pumping, reversible structural changes of the protein were detected. These structural alterations occur in the neighborhood of the cyclohexene ring and at the Schiff's base end of the chromophore retinal. They are interpreted as a 1-2 degree tilt of three or four of the transmembrane alpha-helices or as positional changes of four or five amino acids. The structural changes observed are inherent in the transport mechanism of bacteriorhodopsin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Cone R. A. Light activates rotations of bacteriorhodopsin in the purple membrane. Biophys J. 1984 Jun;45(6):1039–1049. doi: 10.1016/S0006-3495(84)84251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley K., Dollinger G., Eisenstein L., Singh A. K., Zimányi L. Fourier transform infrared difference spectroscopy of bacteriorhodopsin and its photoproducts. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4972–4976. doi: 10.1073/pnas.79.16.4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braiman M. S., Ahl P. L., Rothschild K. J. Millisecond Fourier-transform infrared difference spectra of bacteriorhodopsin's M412 photoproduct. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5221–5225. doi: 10.1073/pnas.84.15.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Time-resolved x-ray diffraction study of photostimulated purple membrane. Biophys J. 1985 Mar;47(3):387–393. doi: 10.1016/S0006-3495(85)83930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamoto J. H., Dupuis P., El-Sayed M. A. On the protein (tyrosine)-chromophore (protonated Schiff base) coupling in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7083–7087. doi: 10.1073/pnas.81.22.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn M. P., Westerhausen J., Wallat I., Seiff F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2146–2150. doi: 10.1073/pnas.85.7.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama T., Nasuda-Kouyama A., Ikegami A., Mathew M. K., Stoeckenius W. Bacteriorhodopsin photoreaction: identification of a long-lived intermediate N (P,R350) at high pH and its M-like photoproduct. Biochemistry. 1988 Aug 9;27(16):5855–5863. doi: 10.1021/bi00416a006. [DOI] [PubMed] [Google Scholar]
- Pettei M. J., Yudd A. P., Nakanishi K., Henselman R., Stoeckenius W. Identification of retinal isomers isolated from bacteriorhodopsin. Biochemistry. 1977 May 3;16(9):1955–1959. doi: 10.1021/bi00628a031. [DOI] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Courtin J., van den Berg E., Winkel C., Lugtenburg J., Herzfeld J., Griffin R. G. Solid-state 13C NMR of the retinal chromophore in photointermediates of bacteriorhodopsin: characterization of two forms of M. Biochemistry. 1989 Jan 10;28(1):237–243. doi: 10.1021/bi00427a033. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ohno K., Takeuchi Y. Altered activity of bacteriorhodopsin in high concentrations of guanidine hydrochloride. J Biochem. 1980 Feb;87(2):491–495. doi: 10.1093/oxfordjournals.jbchem.a132769. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Ohno K., Takeuchi Y., Kagawa Y. Prolonged lifetime of the 410-nm intermediate of bacteriorhodopsin in the presence of guanidine hydrochloride. Biochem Biophys Res Commun. 1977 Apr 25;75(4):1111–1116. doi: 10.1016/0006-291x(77)91497-8. [DOI] [PubMed] [Google Scholar]
- Zaccai G. Structure and hydration of purple membranes in different conditions. J Mol Biol. 1987 Apr 5;194(3):569–572. doi: 10.1016/0022-2836(87)90683-8. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Tokaji Z., Dollinger G. Circular dichroic spectrum of the L form and the blue light product of the m form of purple membrane. Biophys J. 1987 Jan;51(1):145–148. doi: 10.1016/S0006-3495(87)83319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



