Abstract
Purpose
The aim of this in vitro study was to evaluate potential determinants of drug loss in different ECMO circuits.
Methods
Midazolam, morphine, fentanyl, paracetamol, cefazolin, meropenem and vancomycin were injected into three neonatal roller pump, two paediatric roller pump and two clinically used neonatal roller pump circuits, all with a silicone membrane, and two neonatal centrifugal pump circuits with polypropylene hollow-fibre membranes. Serial blood samples were taken from a post-oxygenator site. Drug recovery was calculated as the ratio between the determined and the theoretical maximum concentration. The latter was obtained by dividing dose by theoretical circuit volume.
Results
Average drug recoveries at 180 min in three neonatal silicone membrane roller pump circuits were midazolam 0.62%, morphine 23.9%, fentanyl 0.35%, paracetamol 34.0%, cefazolin 84.3%, meropenem 82.9% and vancomycin 67.8%. There was a significant correlation between the lipophilicity of the drug expressed as log P and the extent of drug absorption, p < 0.001. The recovery of midazolam and fentanyl in centrifugal pump circuits with hollow-fibre membrane oxygenator was significantly higher compared to neonatal roller pump circuits with silicone membranes: midazolam 63.4 versus 0.62%, fentanyl 33.8 versus 0.35%, p < 0.001. Oxygenator size and used circuits do not significantly affect drug losses.
Conclusions
Significant absorption of drugs occurs in the ECMO circuit, correlating with increased lipophilicity of the drug. Centrifugal pump circuits with hollow-fibre membrane oxygenators show less absorption for all drugs, most pronounced for lipophilic drugs. These results suggest that pharmacokinetics and hence optimal doses of these drugs may be altered during ECMO.
Keywords: Pharmacokinetics, ECMO, Sedatives, Analgesics, Antibiotics, Drug sequestration
Introduction
Extracorporeal membrane oxygenation (ECMO) is a form of prolonged temporary cardiopulmonary bypass for cardiac and/or respiratory failure unresponsive to other treatment. In general, patients on ECMO receive more than 10 drugs during ECMO for sedation, analgesia and treatment of underlying or concomitant conditions [1]. Altered pharmacokinetics of several drugs such as morphine, midazolam, vancomycin and gentamicin have been observed during ECMO; the volume of distribution is generally increased, whereas clearance is decreased [2–5]. Evidence-based dosing regimes on ECMO are sparse, with only a few clinical–pharmacological trials [6]. Absorption of medication in ECMO circuits appears to be one of the reasons for the increased volume of distribution. The studies on this subject have left important questions unanswered [7–11]. Levels of drug absorption by polymers, silicone rubber and other materials have been linked to the drugs’ lipophilicity. Assuming this holds for ECMO membranes and tubing as well, there might be a correlation between drug loss and lipophilicity, expressed as log P (or the partition coefficient between 1-octanol and water). Other system-related factors affecting drug loss include the type of pump and circuit, reduced absorption by hollow-fibre membranes, shorter tubing and circuits with a centrifugal pump or roller pump systems. As the total absorptive capacity is linked to the total surface area, paediatric systems, with their larger membrane oxygenators and longer tubing, are expected to show larger absorption, although this has yet to be established in trial settings. Finally, if absorption is maximized by saturation of the surface, one would expect clinically used ECMO circuits to show less absorption than freshly blood-primed ECMO circuits, but reports are contradictory [9, 11].
Aiming at evaluating these aspects, we set out to test drug loss in different ECMO circuits. The drugs studied are some of the most frequently used sedatives (midazolam), analgesics (fentanyl, morphine, paracetamol) and antibiotics (vancomycin, meropenem, cefazolin) in ECMO patients treated in the two ICUs participating in this study. To assess the effect of lipophilicity on absorption these drugs were also chosen to reflect a wide range of log P values.
Materials and methods
The study was conducted at the ICU of the Sophia Children’s Hospital, Erasmus MC, Rotterdam, the Netherlands, and the neonatal intensive care unit, University Hospitals Leuven, Belgium. The same investigators conducted the experiments at both sites. Three different ECMO circuits were compared (Table 1). Contrary to the Leuven ECMO circuits, the standard setup of the ECMO circuit in the Sophia Children’s Hospital, Erasmus MC, Rotterdam, includes a hemofilter.
Table 1.
Description of tested circuits
| Description | Neonatal roller pump | Neonatal centrifugal pump | Paediatric roller pump | Neonatal (used) roller pump |
|---|---|---|---|---|
| Priming volume (ml) | 350 | 200 | 900 | 350 |
| Tubing | Medtronic® Sh. 70 USP class VI 1/4 × 1/16 superTygon® | Intercept® CLASS VI 1/4 × 1/16 | Medtronic Sh. 70 USP class VI 3/8 × 3/32 superTygon® | Medtronic Sh. 70 USP class VI 1/4 × 1/16 superTygon® |
| Oxygenator | Medtronic® 1.5 m2 silicone membrane, Paediatric Extended Capacity Membrane Oxygenator | MEDOS HILITE® 800LT RHEOPARIN® coated polypropylene microporous hollow-fibre | Medtronic® I-2500-2A 2.5 m2 silicone Surgical Membrane Oxygenator | Medtronic® 1.5 m2 silicone membrane, Paediatric Extended Capacity Membrane Oxygenator |
| Heat exchanger | Medtronic® Heat Exchanger Monitoring adapter and Luer-lock | NA | Medtronic® Heat Exchanger Monitoring adapter and Luer-lock | Medtronic® Heat Exchanger Monitoring adapter and Luer-lock |
| Hemofilter | Hospal Multiflow 100 | NA | Hospal Multiflow 100 | Hospal Multiflow 100 |
| Remarks | Freshly primed, reference group | Freshly primed | Freshly primed | Clinically used for at least 48 h before experiment |
Manufacturers: Medtronic, Minneapolis, USA; Medos Medizintechnik AG, Stolberg, Germany; Hospal, Lyon, France
NA not applicable
ECMO circuits
New ECMO circuits were primed according to hospital-based protocols. The only exception was the age of erythrocytes used for priming: Leftover erythrocytes over 1 week old were used. ECMO circuits were primed with carbon dioxide, Ringer’s lactate solution, albumin, tris(hydroxymethyl)aminomethane, sodium bicarbonate and erythrocytes. Drug losses in three freshly primed neonatal roller pump circuits were used as reference values for comparison with two freshly primed neonatal centrifugal circuits, two freshly primed paediatric circuits, and two used neonatal circuits. Used circuits were tested within 6 h after decannulation, without replacement of their contents. Medication prior to decannulation consisted of continuous midazolam (150–200 µg/kg/h) and morphine (15–25 µg/kg/h) infusions for more than 12 h, cefotaxime (50 mg/kg twice daily) and amoxicillin (50 mg/kg three times daily), magnesium sulphate (50 mg/kg four times daily), hydrocortisone (1 mg/kg three times daily) and vancomycin (15 mg/kg). Temperature, hematocrit and pH values were maintained within normal ranges.
ECMO circuits were made continuous via an incorporated bridge connection. As the centrifugal circuits lacked a bridge connection, these circuits were made continuous with the use of a reservoir bag containing 50 mL of priming fluid. The ECMO circuit was filled to maximal capacity with pre-oxygenator pressures of 250 mmHg. Before injection of the drugs an equal volume of fluid was subtracted from the ECMO circuit. The volume of the neonatal roller pump circuit was estimated at 350 mL, that of the paediatric circuit volume at 900 mL and that of the centrifugal circuit volume (including reservoir) at 200 mL. Flow rates were set at 350 mL/min for neonatal circuits and 1,000 mL/min for paediatric circuits.
Drug administration
Drugs were injected at 5-s intervals into a pre-bladder injection site simulating actual drug administration in patients. The line was flushed with at least 3 mL of physiological saline solution (0.9%) in between injection of the different drugs to avoid crystallization or depot effects. Drugs were dosed according to a standardized weight for a newborn (3 kg) and for an older child (15 kg). The order of drug injection was the following (neonatal/paediatric): fentanyl 15 μg/75 μg, morphine 0.6 mg/3 mg, midazolam 0.6 mg/3 mg, paracetamol 45 mg/250 mg, cefazolin 150 mg/750 mg, meropenem 60 mg/300 mg, vancomycin 30 mg/250 mg.
Samples
Samples were taken from a post-oxygenator line before injection and 2, 4, 6, 8, 10, 30, 60 and 180 min after injection. Whole blood was collected in polypropylene tubes containing ethylenediaminetetraacetate (EDTA) and chilled to 4°C until further processing. The blood samples were centrifuged (6 min at 3,000×g) after which the plasma supernatant was transferred to polypropylene cryogenic vials with polyethylene screw caps (Nalgene Labware, Rochester, NY, USA). Samples were stored at −80°C until analysis. Absence of drug absorption by pipettes used to transfer samples (PVC, glass and polypropylene) was confirmed by testing the recovery of fentanyl morphine and midazolam from an aqueous standard after having been in a pipette tip for 3 min.
Quantification of analytes in plasma
Drugs were quantified via ultra-performance liquid chromatography with tandem mass spectrometry detection (UPLC-MS/MS) (Waters Corp., Milford, MA, USA).
Antibiotics and analgesics/sedatives were quantified via two similar methods.
See the method published by Ahsman et al. [12]. The limits of quantification (LOQ) were 0.2 ng/mL (CFZ, MEM), 0.7 ng/mL (VAN), 0.5 ng/mL (fentanyl), 5 ng/mL (midazolam), 2.5 ng/mL (morphine) and 1 μg/mL (paracetamol).
Ratio of whole blood/plasma concentrations
Theoretical maximum concentration in blood was calculated by dividing the administered dose by the volume of the ECMO circuit. However, since drug assays were performed in plasma, the ratio between the plasma and the blood concentration had to be determined to calculate drug recovery. The blood plasma ratio was calculated as the average of the concentration in spiked whole blood samples divided by spiked plasma samples. This was done in triplicate; the mean value was used to calculate whole blood concentrations from measured plasma concentrations.
Data analysis
Data were plotted and analysed with Graphpad Prism v4.03 (Graphpad Software Inc., La Jolla, CA, USA). In the used circuits, pre-existing drug levels (as assessed from the samples taken at 0 min) were subtracted from subsequent measurements. Concentrations were converted from plasma to the corresponding whole blood concentrations using the blood plasma ratio. To calculate the percentage of drug recovered from the circuit, the drug concentration 180 min after infusion was divided by the theoretical concentration, as calculated from the administered amount of drug divided by the estimated circuit volume. The log P values for the individual drugs are derived from the University of Alberta Drugbank website [13]. A Student’s t test (p < 0.05) was used to assess statistical significance of a difference in recoveries in neonatal versus paediatric and used versus new circuits. A non-linear sigmoidal curve fit was applied to plot the recovery versus log P, based on theoretical binding kinetics. Correlation between log P values and recovery rates was calculated by using a two-sided Spearman test.
Results
Ratio of whole blood/plasma
The average ratios of whole blood/plasma for each drug were 1.41 (meropenem), 1.23 (vancomycin), 1.21 (cefazolin), 0.94 (morphine), 0.90 (paracetamol), 0.77 (fentanyl) and 0.75 (midazolam). There was no discernible trend in ratio versus incubation time. The relative standard deviation over all whole blood samples was 10% or less.
Drug loss
Table 2 lists the average recoveries after 180 min and range for each category.
Table 2.
Recovery in % (range) of drugs after 180 min of circulation
| System | Neonatal roller pump (n = 3) | Neonatal centrifugal pump (n = 2) | Paediatric roller pump (n = 2) | Neonatal (used) roller pump (n = 2) |
|---|---|---|---|---|
| MDZ | 0.62 (0.47 to 0.73) | 63.4 (61.6 to 65.2)* | 0.74 (0.66 to 0.81) | −0.06 (−0.93 to 0.81) |
| MOR | 23.9 (14.6 to 35.8) | 32.1 (31.9 to 32.3) | 30.5 (28.6 to 32.4) | 29.8 (17.1 to 42.6) |
| FEN | 0.35 (0.15 to 0.50) | 33.8 (32.4 to 35.3)* | 0.28 (0.18 to 0.37) | 0.54 (0.36 to 0.72) |
| PAR | 34.0 (29.4 to 41.8) | 44.2 (40.8 to 47.6) | 44.9 (44.3 to 45.4) | 47.3 (42.4 to 52.3) |
| CFZ | 84.3 (72.4 to 100.8) | 97.9 (92.5 to 103.3) | 49.4 (44.7 to 54.1) | 76.7 (65.7 to 87.6) |
| MEM | 82.9 (69.1 to 101.4) | 89.1 (76.4 to 101.7) | 58.1 (54.4 to 61.9) | 72.9 (60.1 to 85.7) |
| VAN | 67.8 (49.2 to 95.3) | 67.1 (61.6 to 72.6) | 54.4 (43.4 to 65.3) | 53.8 (47.4 to 60.3) |
* Statistically significant deviation (group averages, two-tailed, p < 0.001) from new neonatal system with roller pump
Neonatal roller pump circuits
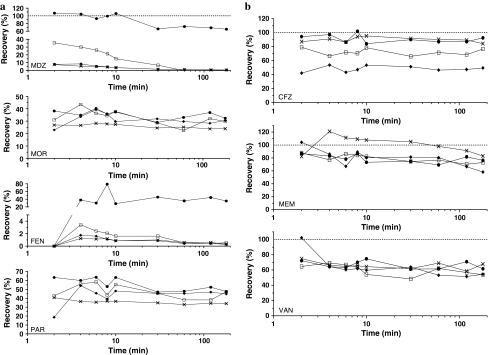
In the three neonatal roller pump circuits, fentanyl, midazolam, morphine and paracetamol showed significant reduced drug recovery within the first 2 min (Fig. 1a). While morphine and paracetamol reached an apparent steady state within 10 min, midazolam and fentanyl did not: a decrease in recovery appeared to continue for at least 120 min. Drug recoveries after 2 min for fentanyl, midazolam, morphine and paracetamol were 1.3, 7.5, 27 and 40.7%, respectively. After 180 min, drug recovery decreased to 0.4, 0.6, 24 and 34.1%, respectively.
Fig. 1.
Average recovery versus time for a analgesics and sedatives, b antibiotics. Neonatal roller pump circuits (×), neonatal centrifugal pump circuits (filled circles), paediatric roller pump circuits (filled diamonds) and neonatal used roller pump circuits (open squares)
Antibiotic recovery was much higher (Fig. 1b). After 2 min, 87% of cefazolin, 82% of meropenem and 75% of vancomycin were recovered in the plasma samples. After 180 min, an apparent steady state had been reached at 85, 83 and 68% of the expected concentration, respectively. For most drugs, especially the lipophilic drugs (midazolam, morphine), a large fraction of the administered dose appears to have been lost within 2 min of circulation. Despite having been injected first, fentanyl starts to appear only after 4 min, indicating strong pooling or absorption in the early phase of the experiment.
Correlation between recovery and log P
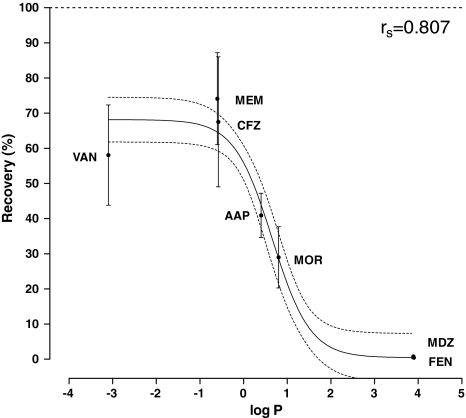
Since there were no statistically significant differences in recovery between all of the roller pump circuits, these systems were all combined (n = 8) to assess the correlation between recovery and lipophilicity. A sigmoidal curve best described the data, without apparent patterns in residuals versus log P values (Fig. 2). There was a significant correlation between log P and recovery (df = 53, r spearman = 0.807, p < 0.001).
Fig. 2.
Recovery of drugs in roller pump circuits (n = 8) versus their lipophilicity, expressed as log P values. Displayed are the means and 95% confidence intervals for each drug, with a non-linear sigmoidal curve fit (solid line) and its 95% confidence interval (dashed lines)
Centrifugal versus roller pump circuits
There was a significant difference in drug recovery between the roller pump and centrifugal pump circuits for midazolam (0.6 vs. 63%, p < 0.001) and fentanyl (0.4 vs. 34%, p < 0.001). Morphine recovery appeared higher in the centrifugal circuits, but without statistical significance (24.0 vs. 32.2%, p = 0.38). Drug recovery was comparable in both circuits for paracetamol, vancomycin, meropenem and cefazolin after 180 min.
Paediatric versus neonatal circuits
Sedatives and analgesics losses were similar in neonatal and paediatric circuits at 180 min. Meropenem and cefazolin losses in the paediatric circuits were higher than those in neonatal circuits, but without statistical significance (Table 2).
Used versus new circuits
The average pre-existing concentrations in the used circuits were midazolam 344 ng/mL, morphine 16 ng/mL, fentanyl less than 0.5 ng/mL, paracetamol less than 1 μg/mL, cefazolin less than 0.2 ng/mL, meropenem less than 0.2 ng/mL, vancomycin 10.5 ng/mL.
Midazolam loss in the first 10 min in used circuits was lower than that in new ECMO circuits: 4.1 versus 26.1%, p = 0.0004. This difference had disappeared after 180 min (Table 1). There were no other significant differences in drug loss between used and freshly primed neonatal roller pump circuits.
Discussion
This comprehensive in vitro study enabled us to answer several questions regarding absorption of drugs in ECMO circuits. First of all, drug loss is correlated to the individual drugs’ log P values and absorption might therefore be predicted from a drug’s log P value. More lipophilic drugs such as fentanyl and midazolam disappeared almost completely, whereas the less lipophilic antibiotics showed much lower loss (10–35%).
Secondly, midazolam and fentanyl recoveries were significantly higher in the centrifugal ECMO circuits. For lipophilic drugs, circuit size and/or type of oxygenator seems to influence absorption. Others reported a marked difference in drug loss between polypropylene microporous or tubular membranes and silicone-based CPB membranes [14]. Our study was not designed to localize the site of drug loss and therefore only general conclusions may be drawn. Less PVC tubing, different oxygenators and inclusion of a hemofilter may all contribute to the differences found. Because of technical difficulties, temperatures of the centrifugal circuits were maintained at 29°C and not at 35–37°C. This discrepancy may have contributed to the differences found. On the other hand, Skacel et al. [15] showed no clear differences in drug losses between circuits maintained at low temperatures (24–25°C) and normal temperatures (37°C).
Thirdly, we found no significant difference between our paediatric and neonatal roller pump circuits; perhaps the effects of an increased dose and the increased polymer surface, combined with a relatively larger circulating volume, cancel each other out. If there is a saturation effect of binding sites, an increased initial dose or number of binding sites may increase or decrease the level of drug recovery. Although single dose studies show significant adsorption it is unclear what effect multiple doses will have on this process.
Finally, contrary to previous reports [9, 11], we found no significant difference in drug loss between freshly primed and used ECMO circuits after 180 min.
In the present study concentrations of most drugs declined within the first minutes of ECMO after which an apparent steady state was reached. This was not the case for midazolam and fentanyl; concentrations of these drugs declined continuously during the 3-h period. This suggests the presence of a greater amount of binding sites for midazolam and fentanyl than for the other compounds. As alternative explanations, the loading dose used is below the saturation threshold or steady state is reached after 3 h. This might explain the findings for the freshly primed circuits, but does not explain why similar absorption patterns were found in the used ECMO circuits. An alternative explanation—degradation by enzymes or other causes—is unlikely since both drugs are exclusively metabolized in the liver. In vitro experiments simulating continuous infusions or multiple dosing may help to clarify this issue.
Increased absorption could be a cause of the increased dose requirements seen for midazolam [16]. The increase, however, does not equal the greater than 90% loss of midazolam observed; apparently other factors affect drug absorption in the in vivo setting. Fentanyl shows the lowest recovery and takes a long time to appear at the other end of the circuit, which suggests pooling or a strong tendency to bind to any available binding site. This phenomenon has major clinical implications: as fentanyl and other lipophilic drugs will not be as effective when administered at a pre-oxygenator line they should be given directly to the patient instead to minimize drug loss. Morphine is absorbed to a lower extent, and is therefore the preferred opioid for ECMO patients.
Midazolam, fentanyl, morphine and vancomycin losses in the roller pump circuits were higher than those previously reported [7, 8, 11, 17]. Several factors may perhaps explain this discrepancy. Most studies are based on one to three ECMO circuits and we found substantial variability between individual circuits. There are several potential causes of these differences. A different t = 0 composition of the circulating contents might have affected adsorption, which could suggest varying degrees of protein binding or drug displacement by concomitantly present compounds. The degree of use (or ‘wear and tear’) of the circuits could affect drug adsorption. There is also some potential for inaccuracy in the injection of drugs into the circuit due to pooling of drugs in the pressure monitor bladder (especially for those drugs in a highly concentrated solution), which could cause variability in adsorption in new and used circuits alike. Finally, there could have been some sampling- or assay-related variability (although unlikely to be this large, and it is also unlikely that this effect would be consistently visible over all sampling times).
The variability makes it difficult to compare the results from different studies. Mehta et al. reported stable fentanyl concentrations in blood-primed circuits for up to 3 h, although in their wet primed circuit fentanyl loss at 3 h was 78% [8]. After 24 h fentanyl was no longer detectable indicating ongoing drug loss in the ECMO circuit, similar to our observations for midazolam and fentanyl. Both studies tested roller pump circuits with a Medtronic® silicone membrane, but experimental methods were distinctly different. Mehta et al. used a reservoir bag pre-primed with medication before connecting this to the ECMO circuit; altered distribution within the ECMO circuit or reservoir bag could result in different time-dependent elimination curves. Another potential cause of variation is the presence of a hemofilter in the ECMO circuit, which we now use routinely to manage the fluid balance and improve caloric intake [18–20]. There was no dialysate flow during the in vitro trial but drug loss by the hemofilter membrane might have occurred. We tested several drugs simultaneously to simulate actual medication administration in ECMO patients. Although we cannot completely rule out drug–drug interactions, we consider this experimental approach to accurately reflect daily clinical practice.
The goal of this in vitro study was to evaluate potential determinants of drug loss in different ECMO circuits. In this study we tried to mimic the clinical situation in which solutions of routinely used drugs are injected into blood-primed circuits at short intervals. Previous studies were done in aqueous media, [7, 17] or a spiked bag of blood to represent a patient [8]. The use of a whole blood system with assays in plasma required us to determine a ratio of whole blood concentration and plasma concentration and the estimation of the total volume, but the resulting experimental setup is close to the clinical situation. Without the use of a reservoir, baseline concentrations could not be measured. Instead, theoretical concentrations were estimated from dose and estimated volume of the ECMO circuits. This may have led to over- or underestimation of percentage drug losses. Priming volumes of all three circuits are known, however, and any error in estimated volume should not exceed 5%, and equally affects all drugs. The general trend therefore is clear: sedatives and analgesics are lost due to absorption by membranes or tubing, whereas antibiotics are affected to a much lesser extent. This confirms observations done in studies of cardiopulmonary bypass circuits [10, 21, 22].
Conclusions
Significant uptake of drugs occurs in the ECMO circuit, which could lead to unexpectedly low initial plasma concentrations and higher loading dose requirements for lipophilic drugs in particular. The log P value may be used to predict drug loss for roller pump circuits. Application of centrifugal pump circuits with hollow-fibre membrane oxygenators limits absorption for all drugs, notably lipophilic drugs. Oxygenator size and previous use of a circuit do not significantly affect drug losses. In combination with the interpatient variability that is inherent to critically ill children, these drug losses likely contribute to the altered pharmacokinetics observed in patients on ECMO.
Acknowledgments
The authors would like to thank the ECMO specialists Addie Koole and Jose Groenewold of the Sophia Children’s Hospital, Erasmus MC, Rotterdam, and Leen Vercaemst, perfusionist in the University Hospitals, Leuven, Belgium, for their support and cooperation.
Conflict of interest
The authors declare that they have no competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- CPB
Cardiopulmonary bypass
- CFZ
Cefazolin
- ECMO
Extracorporeal membrane oxygenation
- FEN
Fentanyl
- HPLC
High-performance liquid chromatography
- ICU
Intensive care unit
- LQD
Limits of quantification
- MDZ
Midazolam
- MEM
Meropenem
- MOR
Morphine
- PAR
Paracetamol
- UPLC-MS/MS
Ultra-performance liquid chromatography with tandem mass spectrometry detection
- VAN
Vancomycin
References
- 1.Buck ML. Pharmacokinetic changes during extracorporeal membrane oxygenation: implications for drug therapy of neonates. Clin Pharmacokinet. 2003;42:403–417. doi: 10.2165/00003088-200342050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Mulla H, McCormack P, Lawson G, Firmin RK, Upton DR. Pharmacokinetics of midazolam in neonates undergoing extracorporeal membrane oxygenation. Anesthesiology. 2003;99:275–282. doi: 10.1097/00000542-200308000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Peters JW, Anderson BJ, Simons SH, Uges DR, Tibboel D. Morphine pharmacokinetics during venoarterial extracorporeal membrane oxygenation in neonates. Intensive Care Med. 2005;31:257–263. doi: 10.1007/s00134-004-2545-5. [DOI] [PubMed] [Google Scholar]
- 4.Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005;60:265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaker RD, DiPiro JT, Bhatia J. Pharmacokinetics of vancomycin in critically ill infants undergoing extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1996;40:1139–1142. doi: 10.1128/aac.40.5.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rayyan M, Allegaert K. Pharmacotherapy during neonatal extracorporeal membrane oxygenation: toward an evidence-based approach. Crit Care. 2007;11:107. doi: 10.1186/cc5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulla H, Lawson G, von Anrep C, Burke M, Upton D, Firmin R, Killer H. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 8.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 9.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Koren G, Crean P, Klein J, Goresky G, Villamater J, MacLeod SM. Sequestration of fentanyl by the cardiopulmonary bypass (CPBP) Eur J Clin Pharmacol. 1984;27:51–56. doi: 10.1007/BF00553154. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt-Meht V, Annich G. Sedative clearance during extracorporeal membrane oxygenation. Perfusion. 2005;20:309–315. doi: 10.1191/0267659105pf827oa. [DOI] [PubMed] [Google Scholar]
- 12.Ahsman MJ, Wildschut ED, Tibboel D, Mathot RA. Microanalysis of beta-lactam antibiotics and vancomycin in plasma for pharmacokinetic studies in neonates. Antimicrob Agents Chemother. 2009;53:75–80. doi: 10.1128/AAC.00636-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M, Res NA. DrugBank: a knowledgebase for drugs, drug actions and drug targets. Nucleic Acids Res. 2008;36(Database issue):D901–D906. doi: 10.1093/nar/gkm958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen DA, Rosen KR, Silvasi DL. In vitro variability in fentanyl absorption by different membrane oxygenators. J Cardiothorac Anesth. 1990;4:332–335. doi: 10.1016/0888-6296(90)90041-D. [DOI] [PubMed] [Google Scholar]
- 15.Skacel M, Knott C, Reynolds F, Aps C. Extracorporeal circuit sequestration of fentanyl and alfentanil. Br J Anaesth. 1986;58:947–949. doi: 10.1093/bja/58.9.947. [DOI] [PubMed] [Google Scholar]
- 16.Ahsman MJ, Hanekamp M, Wildschut ED, Tibboel D, Mathot RAA. Population pharmacokinetics of midazolam and metabolites during venoarterial extracorporeal membrane oxygenation in neonates. Clin Pharmacokinet. 2010;49(6):407–419. doi: 10.2165/11319970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Mulla HLG, Woodland ED, Peek GJ, Killer H, Firmin RK, Upton DR. Effects of neonatal extracorporeal membrane oxygenation circuits on drug disposition. Curr Ther Res. 2000;61:11. doi: 10.1016/S0011-393X(00)90010-9. [DOI] [Google Scholar]
- 18.Hoover NG, Heard M, Reid C, Wagoner S, Rogers K, Foland J, Paden ML, Fortenberry JD. Enhanced fluid management with continuous venovenous hemofiltration in pediatric respiratory failure patients receiving extracorporeal membrane oxygenation support. Intensive Care Med. 2008;34:2241–2247. doi: 10.1007/s00134-008-1200-y. [DOI] [PubMed] [Google Scholar]
- 19.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA. Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med. 2004;32:1771–1776. doi: 10.1097/01.CCM.0000132897.52737.49. [DOI] [PubMed] [Google Scholar]
- 20.Blijdorp KCK, Wildschut ED, Gischler SJ, Houmes RJ, Wolff ED, Tibboel D. Haemofiltration in newborns treated with extracorporeal membrane oxygenation: a case-comparison study. Crit Care. 2009;13:R48. doi: 10.1186/cc7771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen DA, Rosen KR. Elimination of drugs and toxins during cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1997;11:337–340. doi: 10.1016/S1053-0770(97)90104-X. [DOI] [PubMed] [Google Scholar]
- 22.Bjorksten AR, Crankshaw DP, Morgan DJ, Prideaux PR. The effects of cardiopulmonary bypass on plasma concentrations and protein binding of methohexital and thiopental. J Cardiothorac Anesth. 1988;2:281–289. doi: 10.1016/0888-6296(88)90306-7. [DOI] [PubMed] [Google Scholar]