Abstract
Calcium plays a crucial role in many cellular processes. Its functions are directly dependent on the high specificity for Ca2+ exhibited by the proteins and ion carriers that bind divalent ions. To elucidate the basis for this specificity we have calculated the relative energies of solvation of calcium and magnesium ions in complexes with cyclo(-L-Pro-Gly-)3, a small synthetic peptide that binds Ca2+ with an affinity comparable to those of the naturally occurring proteins. The results show that the ion selectivity of the peptide resides in the difference in the solvation energies of the competing ions in water. Although the peptide is able to complex Mg2+ better than Ca2+ in the stoichiometries in which cyclo(-L-Pro-Gly-)3 binds divalent ions, it is not always able to provide as much stabilization for Mg2+ as water does. These results also explain why cyclo(-L-Pro-Gly-)3 binds Ca2+ and Mg2+ with different stoichiometries and indicate the source for expected differences in the structures of complexes of the two ions.
Full text
PDF

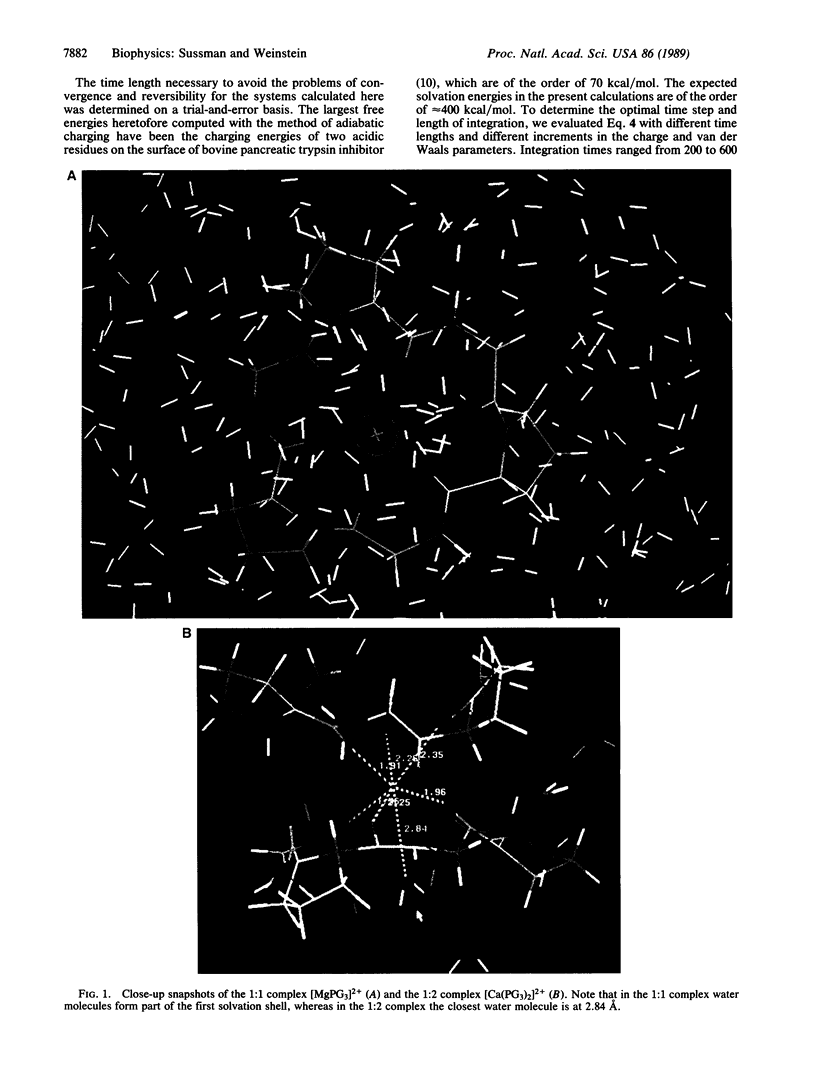
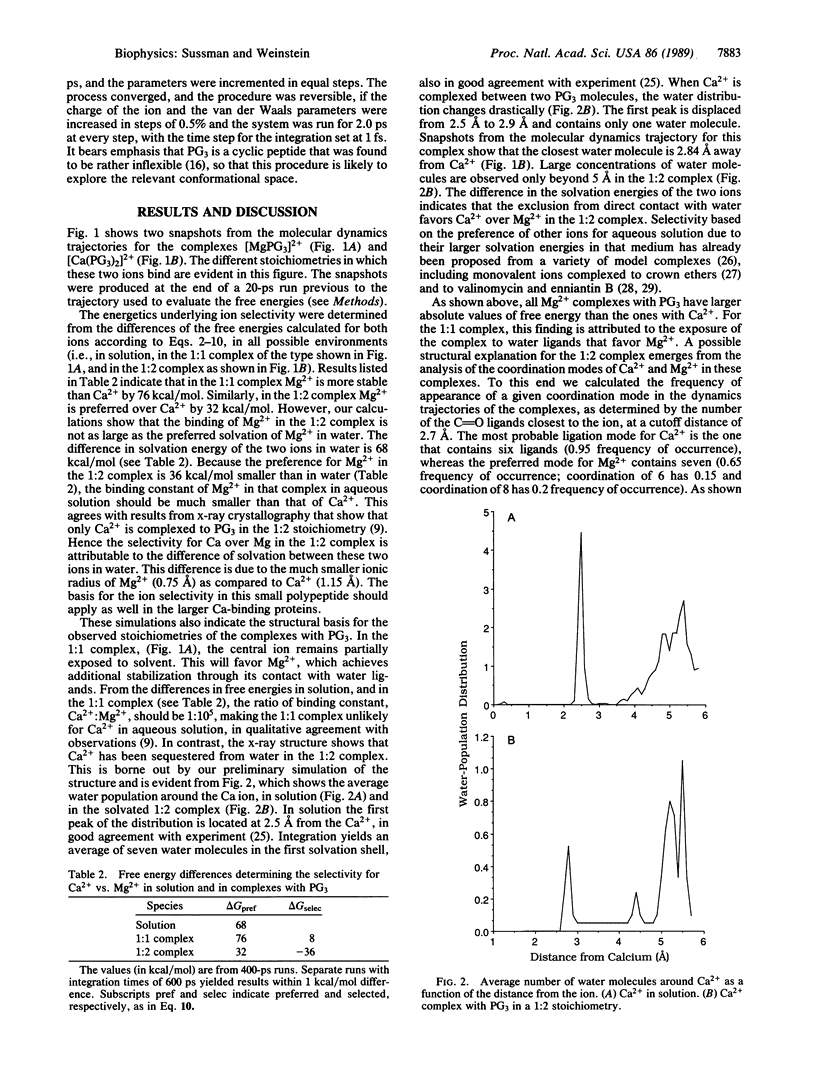

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bash P. A., Singh U. C., Brown F. K., Langridge R., Kollman P. A. Calculation of the relative change in binding free energy of a protein-inhibitor complex. Science. 1987 Jan 30;235(4788):574–576. doi: 10.1126/science.3810157. [DOI] [PubMed] [Google Scholar]
- Bash P. A., Singh U. C., Langridge R., Kollman P. A. Free energy calculations by computer simulation. Science. 1987 May 1;236(4801):564–568. doi: 10.1126/science.3576184. [DOI] [PubMed] [Google Scholar]
- Drakenberg T., Forsén S., Thulin E., Vogel H. J. The binding of Ca2+, Mg2+ and Cd2+ to tryptic fragments of skeletal muscle troponin C. Cadmium-113 and proton NMR studies. J Biol Chem. 1987 Jan 15;262(2):672–678. [PubMed] [Google Scholar]
- Herzberg O., James M. N. Refined crystal structure of troponin C from turkey skeletal muscle at 2.0 A resolution. J Mol Biol. 1988 Oct 5;203(3):761–779. doi: 10.1016/0022-2836(88)90208-2. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Hori K., Kushick J. N., Weinstein H. Structural and energetic parameters of Ca2+ binding to peptides and proteins. Biopolymers. 1988 Dec;27(12):1865–1886. doi: 10.1002/bip.360271202. [DOI] [PubMed] [Google Scholar]
- Kartha G., Varughese K. I., Aimoto S. Conformation of cyclo(-L-Pro-Gly-)(3) and its Ca and Mg complexes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4519–4522. doi: 10.1073/pnas.79.14.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Lifson S., Felder C. E., Shanzer A. Enniatin B and valinomycin as ion carriers: an empirical force field analysis. J Biomol Struct Dyn. 1984 Dec;2(3):641–661. doi: 10.1080/07391102.1984.10507598. [DOI] [PubMed] [Google Scholar]
- Linse S., Brodin P., Drakenberg T., Thulin E., Sellers P., Elmdén K., Grundström T., Forsén S. Structure-function relationships in EF-hand Ca2+-binding proteins. Protein engineering and biophysical studies of calbindin D9k. Biochemistry. 1987 Oct 20;26(21):6723–6735. doi: 10.1021/bi00395a023. [DOI] [PubMed] [Google Scholar]
- Lybrand T. P., McCammon J. A., Wipff G. Theoretical calculation of relative binding affinity in host-guest systems. Proc Natl Acad Sci U S A. 1986 Feb;83(4):833–835. doi: 10.1073/pnas.83.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R. E., Gariépy J., Saund A. K., Hodges R. S. Calcium-induced protein folding. Structure-affinity relationships in synthetic analogs of the helix-loop-helix calcium binding unit. J Biol Chem. 1981 Mar 25;256(6):2742–2751. [PubMed] [Google Scholar]
- Szebenyi D. M., Moffat K. The refined structure of vitamin D-dependent calcium-binding protein from bovine intestine. Molecular details, ion binding, and implications for the structure of other calcium-binding proteins. J Biol Chem. 1986 Jul 5;261(19):8761–8777. [PubMed] [Google Scholar]
- Tsai M. D., Drakenberg T., Thulin E., Forsén S. Is the binding of magnesium (II) to calmodulin significant? An investigation by magnesium-25 nuclear magnetic resonance. Biochemistry. 1987 Jun 16;26(12):3635–3643. doi: 10.1021/bi00386a057. [DOI] [PubMed] [Google Scholar]
- Warshel A., Sussman F., King G. Free energy of charges in solvated proteins: microscopic calculations using a reversible charging process. Biochemistry. 1986 Dec 30;25(26):8368–8372. doi: 10.1021/bi00374a006. [DOI] [PubMed] [Google Scholar]
- Warshel A., Sussman F. Toward computer-aided site-directed mutagenesis of enzymes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3806–3810. doi: 10.1073/pnas.83.11.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]