Abstract
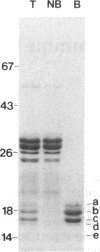
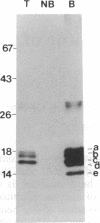
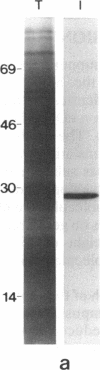
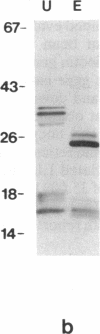
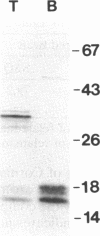
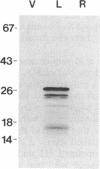
An alpha-amylase inhibitor that inhibits insect and mammalian alpha-amylases but not plant alpha-amylases, is present in seeds of the common bean (Phaseolus vulgaris). We have purified the alpha-amylase inhibitor by using a selective heat treatment in acidic medium and affinity chromatography with porcine pancreas alpha-amylase coupled to agarose. Under sodium dodecyl sulfate gel electrophoresis, the purified inhibitor gave rise to five bands with mobilities corresponding to molecular masses ranging from 14 to 19 kDa. N-terminal sequencing (up to 15 amino acids) of the polypeptides obtained from these bands resulted in only two different sequences matching two stretches of the amino acid sequence deduced from an already described lectin gene [Hoffman, L. M. (1984) J. Mol. Appl. Gen. 2,447-453]. This gene is different from but closely related to the genes that code for phytohemagglutinin, the major lectin of bean. Further evidence based on amino acid composition, identification of a precursor, and recognition of the product of the gene (expressed in Escherichia coli) by an anti-alpha-amylase inhibitor serum confirms that the inhibitor is encoded by this or a closely related lectin gene. This finding assigns a biological function, which has been described at the molecular level, to a plant lectin gene product and supports the defense role postulated for seed lectins. The lack of homology with other families of enzyme inhibitors suggests that this may be the first member of a new family of plant enzyme inhibitors.
Full text
PDF

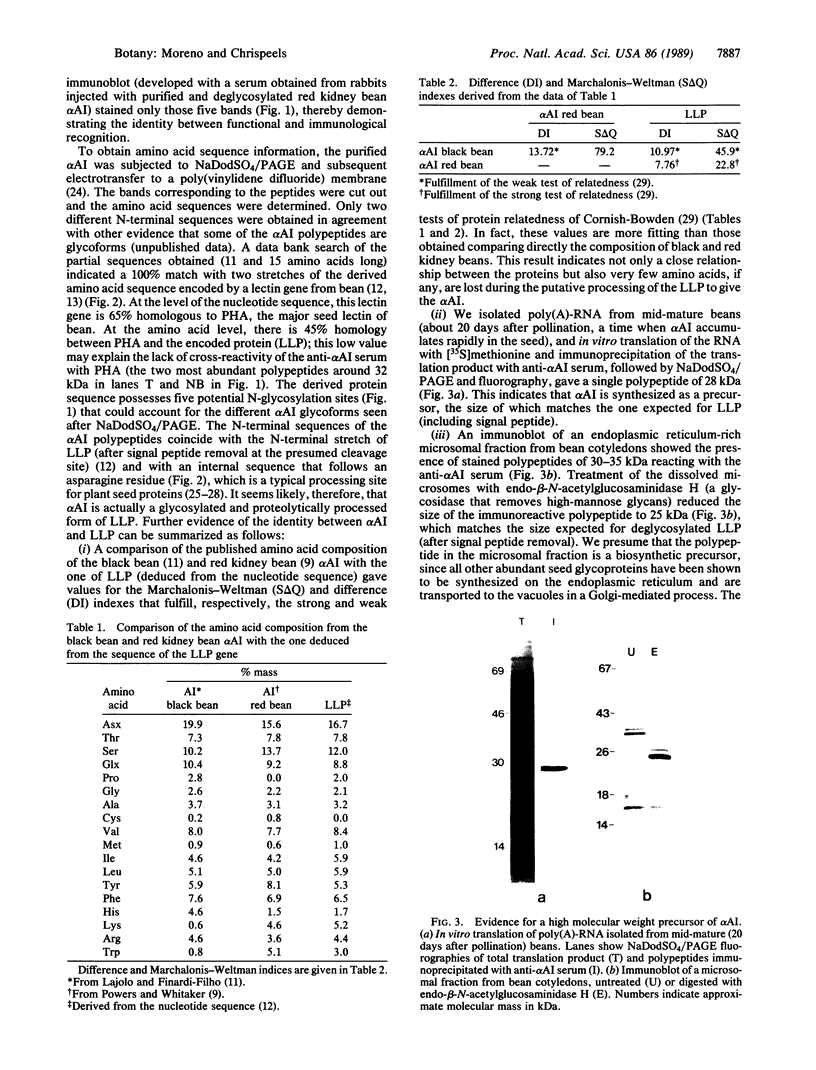
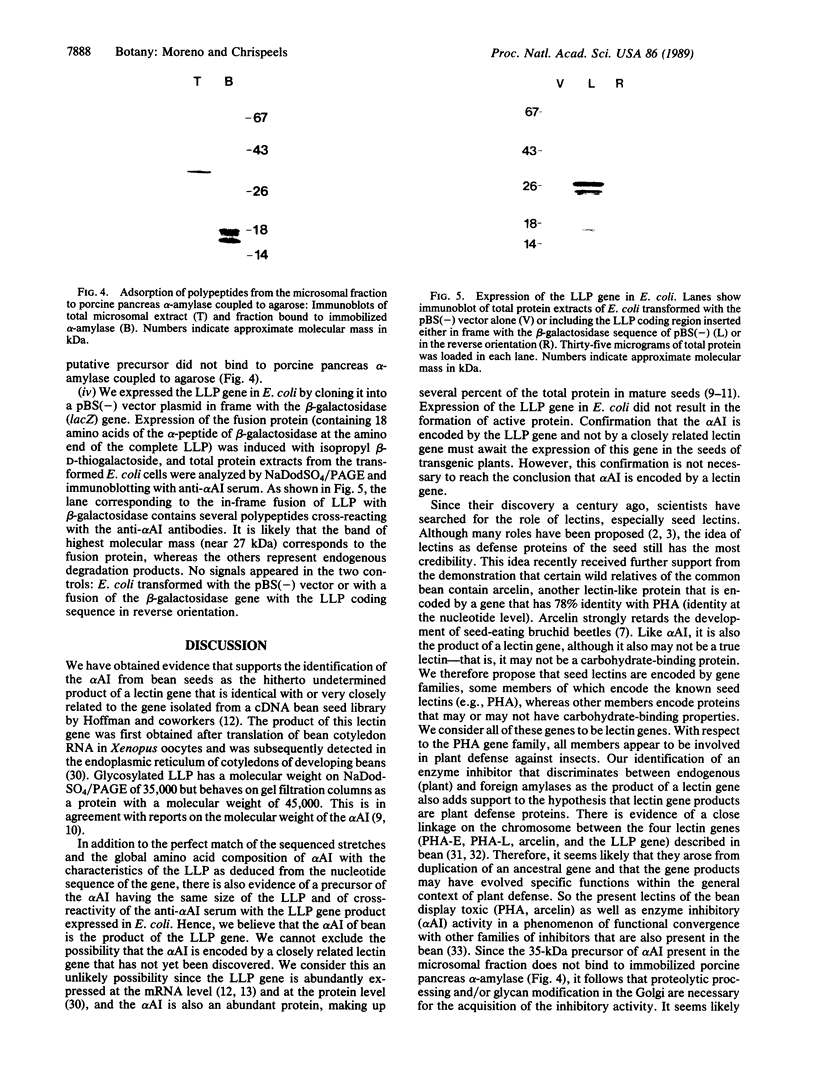

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A. Critical values for testing the significance of amino acid composition indexes. Anal Biochem. 1980 Jul 1;105(2):233–238. doi: 10.1016/0003-2697(80)90450-9. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Halling K. C., Halling A. C., Murray E. E., Ladin B. F., Houston L. L., Weaver R. F. Genomic cloning and characterization of a ricin gene from Ricinus communis. Nucleic Acids Res. 1985 Nov 25;13(22):8019–8033. doi: 10.1093/nar/13.22.8019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. J., Chandler P. M., Zurawski G., Button S. C., Spencer D. The biosynthesis and primary structure of pea seed lectin. J Biol Chem. 1983 Aug 10;258(15):9544–9549. [PubMed] [Google Scholar]
- Hoffman L. M., Ma Y., Barker R. F. Molecular cloning of Phaseolus vulgaris lectin mRNA and use of cDNA as a probe to estimate lectin transcript levels in various tissues. Nucleic Acids Res. 1982 Dec 11;10(23):7819–7828. doi: 10.1093/nar/10.23.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- King E. B., Vanderlaan M., Jensen R. H., Kromhout L. K., Hoffman J. W. Morphologic changes in rat urothelial cells during carcinogenesis: I. Histologic and cytologic changes. Cytometry. 1984 Sep;5(5):447–453. doi: 10.1002/cyto.990050503. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lis H., Sharon N. Lectins as molecules and as tools. Annu Rev Biochem. 1986;55:35–67. doi: 10.1146/annurev.bi.55.070186.000343. [DOI] [PubMed] [Google Scholar]
- Marshall J. J., Lauda C. M. Purification and properties of phaseolamin, an inhibitor of alpha-amylase, from the kidney bean, Phaseolus vulgaris. J Biol Chem. 1975 Oct 25;250(20):8030–8037. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Osborni T. C., Alexander D. C., Sun S. S., Cardona C., Bliss F. A. Insecticidal activity and lectin homology of arcelin seed protein. Science. 1988 Apr 8;240(4849):207–210. doi: 10.1126/science.240.4849.207. [DOI] [PubMed] [Google Scholar]
- Pick K. H., Wöber G. Proteinaceous alpha-amylase inhibitor from beans (Phaseolus vulgaris). Purification and partial characterization. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1371–1377. doi: 10.1515/bchm2.1978.359.2.1371. [DOI] [PubMed] [Google Scholar]
- Pusztai A., Clarke E. M., King T. P. The nutritional toxicity of Phaseolus vulgaris lectins. Proc Nutr Soc. 1979 May 1;38(1):115–120. doi: 10.1079/pns19790015. [DOI] [PubMed] [Google Scholar]
- Sturm A., Van Kuik J. A., Vliegenthart J. F., Chrispeels M. J. Structure, position, and biosynthesis of the high mannose and the complex oligosaccharide side chains of the bean storage protein phaseolin. J Biol Chem. 1987 Oct 5;262(28):13392–13403. [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Wilson K. A., Laskowski M., Sr Isolation of three isoinhibitors of trypsin from garden bean, Phaseolus vulgaris, having either lysine or arginine at the reactive site. J Biol Chem. 1973 Feb 10;248(3):756–762. [PubMed] [Google Scholar]