Abstract
A reversed-phase high-performance liquid chromatography (RP-HPLC) method with diode array detection has been developed for analysis of the major polyphenols in the roots and rhizomes of black cohosh (Actaea racemosa), an important botanical dietary supplement for women's health, and three closely related American Actaea species, A. rubra, A. pachypoda and A. podocarpa. The method was validated with respect to sensitivity, linearity, precision, accuracy and recovery. The total content of eight major polyphenols in the dried root and rhizome of the four species was determined to be from 0.36 to 2.92% (w/w). The antioxidant activities of Actaea extracts and polyphenolic compounds isolated from A. racemosa were evaluated on 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radicals scavenging assay. The radical scavenging activity of the Actaea extracts correlates to their polyphenolic composition. This validated HPLC method can be used to distinguish A. racemosa from the other major American Actaea species based on this study.
Keywords: Actaea spp., black cohosh, polyphenols, HPLC, DPPH, antioxidants
INTRODUCTION
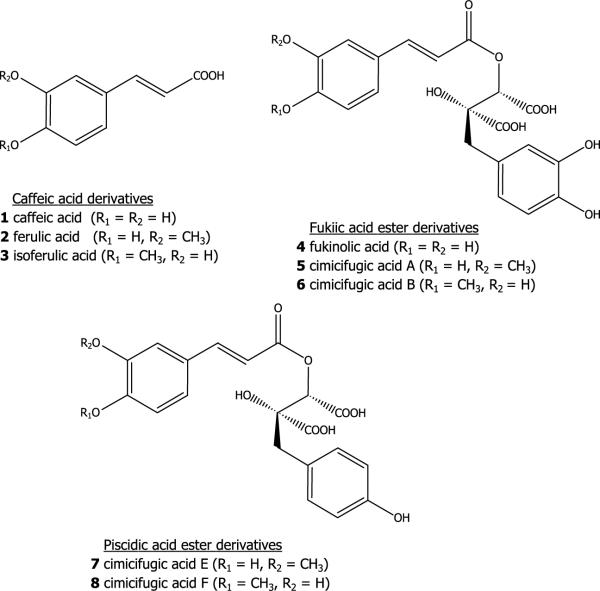
Black cohosh [Actaea racemosa L. (synonym: Cimicifuga racemosa)], a native North American plant in the buttercup family (Ranunculaceae), is the source of one of the most important botanical dietary supplements for the treatment of menopausal symptoms in the USA and Europe. Its rhizomes and roots have long been used by Native Americans to treat a variety of ailments, including malaise, gynecological disorders, diarrhea, sore throat and rheumatism (Rafinesque, 1828). Black cohosh has been used in Europe for more than 40 years to treat symptoms associated with menopause. In 2002, black cohosh ranked ninth among all herbal preparations in US sales (Blumenthal, 2003). Previous research on the chemical constituents of black cohosh has resulted in the isolation of two principal groups of compounds, triterpenoid glycosides and polyphenolic derivatives (Shao et al., 2000; Chen et al., 2002a,b; Kruse et al., 1999). To date, at least 43 triterpenoid glycosides have been reported, including actein, 23-epi-26-deoxyactein (previously known as 27-deoxyactein), cimicifugoside and cimiracemoside A. More than 20 polyphenolic derivatives have been isolated from the rhizomes and roots of black cohosh, including caffeic acid, isoferulic acid, fukinolic acid, as well as cimicifugic acids A, B, E and F (Kruse et al., 1999; Chen et al., 2002b; Stromeier et al., 2005; Nuntanakorn et al., 2006). These compounds have been reported to inhibit α-amylase, carboxypeptidase A and collagenase, and also display anti-inflammatory and antioxidant activities (Kusano et al., 1998, 2001; Loser et al., 2000; Burdette et al., 2002; Nuntanakorn et al., 2006).
Interest in black cohosh polyphenols has increased in recent years. Burdette et al. (2002) examined the antioxidant activity of a black cohosh methanolic extract and nine polyphenolic compounds subsequently isolated and analysed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. They found that the extract and all polyphenols exhibited antioxidant activity. The mechanism of action of black cohosh has not been determined yet, although it has been proposed that it could be the result of a complex synergistic action among its constituents (Borrelli and Ernst, 2002). The clinical effect of black cohosh may be, in part, related to its antioxidant activities. Owing to the strong UV absorption of polyphenols, high-performance liquid chromatography with diode array detection method (HPLC-DAD) is an appropriate analytical method (Ito et al., 1988). Numerous studies have been conducted on the qualification and quantification of polyphenolic compounds including flavonoids and caffeic acid derivatives (Sakakibara et al., 2003; Jiang et al., 2005; Li et al., 2003). Several analytical methods have been designed for qualitative and quantitative analysis of black cohosh (Li et al., 2003; Jiang et al., 2005, 2006; He et al., 2006). Jiang et al. reported an HPLC-DAD method to quantify polyphenols in black cohosh (Jiang et al., 2005, 2006), and HPLC methods with mass spectrometry to identify polyphenols in black cohosh extract have been reported as well (Li et al., 2003; He et al., 2006). However, no validated HPLC method has been published that enables black cohosh to be distinguished from its closest American relatives.
Besides black cohosh, the genus Actaea includes more than 20 species. The North American species share many morphological similarities, and display minor differences. For example, the fruits of black cohosh are dried follicles, while A. pachypoda and A. rubra have fleshy berries (Compton et al., 1998). Since black cohosh is still wildcrafted in the USA, mistakenly collecting closely related species in overlapping ranges is a possible occurrence. Misidentification of black cohosh may lead to serious consequences, including altered therapeutic effects, and the risk of toxicity such as gastroenteritis. Although phytochemical investigations on Actaea species other than A. racemosa have been conducted, and many triterpenoid glycosides and polyphenolic derivatives similar to those of black cohosh have been isolated (Kusano et al., 1996; Takahira et al., 1998b; Chen et al., 2002a,b; Wang et al., 2005), phytochemical variation among wild populations of native North American Actaea species has not been thoroughly investigated. Zerega et al. (2002) used DNA fingerprinting to distinguish black cohosh from three related species, but they could not analyse all samples due to the instability of DNA and the absence of DNA in a typical alcoholic extract. He et al. (2006) recently reported an HPLC with mass spectrometry method to yield polyphenol fingerprints for Actaea species identification, but only three American Actaea species including, black cohosh, were studied qualitatively. Additional studies are necessary on the phytochemical and molecular genetic characteristics of black cohosh and its closely related American species.
HPLC is a practical method that can be used to assist in the identification of both plant materials and botanical products from black cohosh (Jiang et al., 2006). The present study consists of qualitative and quantitative analyses of the eight major antioxidant polyphenols isolated from four North American Actaea species, including black cohosh. An RP-HPLC method with DAD was developed and validated in this study. The antioxidant activities of the extract of black cohosh and three related species were also tested and compared.
EXPERIMENTAL
Chemicals and reagents
HPLC-grade acetonitrile and methanol (J. T. Baker, Phillipsburg, USA) were used for sample preparation and HPLC analysis. Reagent-grade methanol, hexane, n-butanol and acetic acid (VWR, Seattle, WA, USA) were used for the extraction and separation of standards 1–8. Dimethyl sulfoxide (Aldrich, Milwaukee, USA) and 1,1-diphenyl-2-picryhydrazyl (DPPH, Sigma Chemical Co., St. Louis, MO, USA) were used for the DPPH radical scavenging assay. The absorbents for open column chromatography were octadecyl (C18 40 μm; J. T. Baker, Phillips-burg, USA), Sephadex LH-20 (25–100 μm; Pharmacia Fine Chemicals, Piscataway, NJ, USA), and Diaion HP-20 (Supelco, Bellefonte, USA).
Plant materials
Dried rhizomes and roots of Actaea rubra (lot number SK200301), A. pachypoda (lot number DB727) and A. podocarpa (lot number DB710303) were purchased from Botanical Liaison (LLC, Boulder, CO, USA). Voucher specimens of A. rubra (4/16/04 SK200301), A. pachypoda (4/16/04 DB727) and A. podocarpa (8/19/03 DB710303) were deposited at the herbarium at Botanicals International, Botanical Liaison LLC (Boulder, CO, USA). Dried rhizomes and roots of A. racemosa (lot number 9–2677) and the standardised A. racemosa extract (lot number 9–2044) were prepared by PureWorld Botanicals (South Hackensack, NJ, USA).
Equipment
HPLC analyses were performed on a Waters 2695 Separations Module (Milford, MA, USA) equipped with a Waters 996 photodiode array detector and Waters Empower software, and a 250 × 4.6 mm i.d., 5 μm, Aqua C18 column (Phenomenex). Preparative HPLC was carried out in a 250 × 21.1 mm i.d., 10 μm, Nucleosil C18 column (Phenomenex) using a Waters 600 controller with a Waters 486 tunable absorbance detector and Waters Empower software. UV absorption spectra were measured on a Lambda 2 UV–vis spectrophotometer (Perkin-Elmer, Boston, MA, USA). 1H NMR and 13C NMR spectra were recorded using a Bruker AMX-300 MHz NMR spectrometer, operating at 300 and 75 MHz, respectively. All NMR spectra were obtained in CD3OD, with chemical shifts expressed in δ and coupling constant (J) in Hertz. MS was performed on a Thermo Finnigan LCQ instrument (San Jose, CA, USA) in the negative mode. The instrument was equipped with an electrospray ionization (ESI) source and controlled by Xcalibur software. Samples were dissolved in MeOH and introduced by direct injection.
The capillary voltage was 10 V, the spray voltage was 4.5 kV, and the tube lens offset was 0 V. The capillary temperature was 230°C.
Standards
Eight standards, caffeic acid (1), ferulic acid (2), isoferulic acid (3), fukinolic acid (4) and cimicifugic acids A (5), B (6), E (7) and F (8), were prepared by column chromatography and preparative HPLC as described below. A standardised black cohosh powdered extract (0.5 kg) was re-extracted with 80% MeOH–water at room temperature overnight (12 h). After the MeOH was removed in vacuo, the resulting aqueous portion was sequentially partitioned with hexane and n-BuOH. The hexane, n-BuOH, and aqueous portions were concentrated in vacuo at 40°C. A portion (30 g) of the residue from the n-BuOH extract was fractionated over Diaion HP-20 eluting sequentially with water–MeOH (1:1), MeOH and acetone. Four fractions were obtained and designated B1–4. Fraction B2 (3.2 g) was chromatographed over C18 and eluted with a gradient of MeCN (5–50%) in 0.1% aqueous acetic acid, producing 10 subfractions (B2a–j). Fraction B2d (390 mg) was chromatographed over Sephadex LH-20 eluting with MeOH–water (9:1) to yield 1 (290 mg). Fraction B2h (82 mg) was purified by preparative HPLC eluting with 0.1% aqueous acetic acid and MeOH (3:1) at a flow rate of 10 mL/min to give 2 (32.2 mg). Fraction B2i (100 mg) was chromatographed over Sephadex LH-20 with MeOH/H2O (9:1) as the eluant and yielding 3 (84.2 mg).
A portion (30 g) of the residue from the water extract was fractionated over Diaion HP-20 eluting with water, MeOH–water (1:1), MeOH and acetone to give seven combined fractions (W1–7). Fraction W3 (2.12 g) was chromatographed over C18 eluted with a gradient of MeCN (5–35%) in 0.1% aqueous acetic acid to obtain eight combined fractions (W3a–h). Fractions W3c (170 mg), W3e (82.0 mg) and W3g (94.9 mg) were separated over preparative C18 HPLC eluting with a linear gradient of MeOH (33–50%) in 0.1% aqueous acetic acid (run time = 60 min) to yield crude fukinolic acid (102.2 mg), crude cimicifugic acid A (39.1 mg) and crude cimicifugic acid B (46.6 mg), respectively. The crude fukinolic acid, cimicifugic acid A and cimicifugic acid B fractions were further purified by preparative C18 HPLC, eluting with an isocratic solvent system consisting of 0.1% aqueous acetic acid and MeCN (8:2), yielding 4 (54.4 mg), 5 (19.4 mg) and 6 (30.1 mg), respectively. Fraction W4 (120.4 mg) was chromatographed over C18 and eluted with a gradient of MeCN (10–40%) in 0.1% aqueous acetic acid producing four combined fractions (W4a–d). Fraction W4d (20.2 mg) was separated by preparative C18 HPLC eluting with a linear gradient of MeOH (33–50%) in 0.1% aqueous acetic acid with a run time of 60 min, to obtain crude cimicifugic acid E (6.1 mg) and crude cimicifugic acid F (11.4 mg). The crude cimicifugic acids E and F were further purified by preparative C18 HPLC eluting with an isocratic solvent system of 0.1% aqueous acetic acid and MeCN (8:2), to yield 7 (3.8 mg) and 8 (8.7 mg).
The isolated compounds were identified by comparison of their UV–vis, MS, and NMR spectral data with published reports (Lee et al., 2001; Souliman et al., 1991; Takahira et al., 1998a, b). The chemical structures of standards are as shown, 1–8.
Validation of the HPLC method
In compliance with the Guidelines of the International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Humans (ICH, 1995), our HPLC method was validated with respect to linearity, precision, accuracy, recovery and sensitivity. Stock solutions (about 1 mg/mL) of the standards 1–8 were prepared independently in 80% MeOH. For calibration purposes, working solutions were freshly prepared by diluting each stock solution with 80% MeOH. Calibration curves were established on five data points covering a concentration range of 1–250 μg/mL. Each calibration curve was obtained by plotting peak areas (extracting at 320 nm) vs the concentration of the standard.
Inter-day precision and accuracy were evaluated by performing six injections of a standard mixture solution at three concentration levels for three consecutive days. Intra-day precision and accuracy were determined with six injections of a standard mixture solution at three concentration levels over one-day period. The precision at each concentration was expressed as the percentage relative standard deviation (RSD, %) of the measured concentration of standards, whereas the accuracy was assessed for each standard and expressed as percent relative error (RE, %) by comparing the nominal concentration with measured concentration.
For recovery studies, caffeic acid (1), fukinolic acid (4) and cimicifugic acid E (7) were chosen to represent the three main classes of polyphenolic constituents of black cohosh, namely caffeic acid, fukiic acid ester and piscidic acid ester derivatives. The recovery for the analytical method was evaluated by adding known amounts of the three representative standards (approximately 2 mg each) to ground rhizomes and roots of A. racemosa (1 g) prior to extraction. The sample was prepared as described in sample above. Recovery was determined by subtracting the values obtained for the control matrix preparation from the sample added with standards, and dividing the result by the amount of standards. Recovery was expressed as percentage.
The limits of detection (LOD) and limits of quantification (LOQ) were calculated as signal-to-noise ratios with minimal values of 3:1 and 10:1, respectively. LOD and LOQ experiments were evaluated by performing three injections of individual standard solution at the LOD and LOQ concentrations.
Sample preparation for HPLC analysis
Ground rhizomes and roots (1.0 g) were extracted with 15 mL 80% MeOH–water in a 20 mL PTFE-capped vial by sonication for 30 min. After cooling to room temperature, the supernatant solution was filtered, and the residue was re-extracted twice more as described above. The combined solution was evaporated to dryness in vacuo at 40°C. The dried extract was kept in the freezer at −20°C until use in HPLC analysis.
A portion of the dried extract was dissolved in 80% MeOH–water to generate a solution with concentration of 10 mg/mL and the solution was filtered through a 0.45 μm membrane filter prior to HPLC analysis.
Identification and quantification of polyphenols
Samples analyses were carried out on a Waters 2695 Separations Module equipped with a Waters 996 photodiode array detector and Waters Empower software using a Phenomenex Aqua C18 column. Injection volume was 10 μL. Chromatographic separation was achieved over 60 min using a gradient solvent system consisting of 5% aqueous acetic acid (A) and MeCN (B) at a flow rate of 1 mL/min. The gradient profile was as follows: 0–8 min, 5–15% B; 8–20 min, 15% B; 20–55 min, 15–38% B; and 56–60 min, 100% B. The gradient condition returned to initial conditions at 60 min and held for 5 min for the next injection. The UV–vis spectra were recorded from 200 to 400 nm, while 320 nm was used for quantification. Peaks were identified on the basis of their retention time (tR) values and UV spectra by comparison with the standard solution. Ambiguous peaks were confirmed using internal standards.
DPPH radical scavenging activity
DPPH assays were performed using extracts and purified isolates as previously described (Smith et al., 1987). Test samples were dissolved in DMSO and mixed with ethanolic solutions of DPPH (400 mm) in 96-well microtiter plates, following incubation at 37°C for 30 min. DPPH reduction was estimated at 515 nm. Final concentrations of test materials were typically in a range from 1 to 50 μg/mL. Percentage inhibition by the sample treatment was determined by comparison with a DMSO-treated control group. IC50 values denote the concentration of sample required to scavenge 50% DPPH free radicals. All experiments were carried out in triplicate.
RESULTS AND DISCUSSION
Owing to similarity in appearance and overlapping geographical distribution between black cohosh (Actaea racemosa L.) and other American Actaea species, we developed a polyphenolic fingerprinting technique using HPLC-DAD, which is a simple, reliable and convenient analytical method. The four American Actaea species in this study displayed qualitatively and quantitatively distinguishable chemical profiles for their polyphenolic constituents. These polyphenolic profiles appeared to be less complex than previously reported triterpene glycoside fingerprints (Wang et al., 2005), but are equally useful to distinguish among the four Actaea species based on this study.
Method development
Several RP-HPLC methods have been developed for the analysis of hydroxycinnamic acid derivatives such as caffeic acid and ferulic acid. (Hernanz et al., 2001; Jiang et al., 2005, 2006; Li et al., 2003; He et al., 2006). Jiang et al. (2005, 2006) used gradients of 10% aqueous formic acid and MeCN as mobile phase on an HPLC-DAD method, but gradients of 0.05–0.1% aqueous acetic acid and MeCN have also been used in HPLC and LC-MS methods for the identification of polyphenols in black cohosh (Li et al., 2003; He et al., 2006). Formic or acetic acids were used in these gradient systems because they reduce the peak tailing of polyphenolic derivatives. However, the chemical profiles of polyphenols of Actaea species may greatly vary from study to study when analytical conditions are changed. For example, He et al. described a separation method, using a Zorbax DBS column, that resulted in chromatograms which differed significantly in both the sequence and retention times of the corresponding eight major polyphenols that were found using the method we report here (Li et al., 2003; Jiang et al., 2006; He et al., 2006).
To develop a suitable solvent system for HPLC separation of the eight major polyphenols isolated from black cohosh, we tested a binary solvent mixture of formic acid (or acetic acid) and MeCN, in which we varied the concentration of the acids. We found that there is no significant difference between formic acid and acetic acid. Acid concentrations from 5 to 10% provided baseline separation. The lower concentration of acid (5%) was chosen to protect the stability of column in long-term use. In this study, two solvents were used in the mobile phase: 5% aqueous acetic acid and MeCN. To optimise the mobile phase for a binary gradient profile, different compositions of MeCN in 5% aqueous acetic acid were studied. The gradient conditions of MeCN and 5% aqueous acetic acid described in the materials and methods section gave baseline separation of all polyphenolic standard compounds and of all four Actaea species extracts.
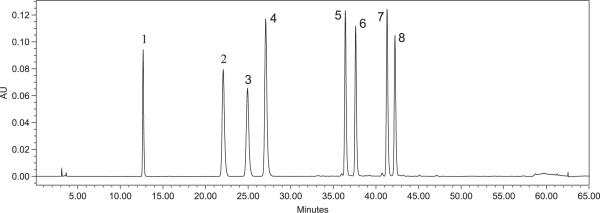
To select a wavelength for quantification, UV–vis spectra were recorded from 200 to 400 nm; monitoring at 320 nm provided the optimum absorbance for all quantified polyphenols. A typical HPLC-DAD chromatogram at 320 nm of a standard mixture is shown in Fig. 1. The retention times and UV spectra of the standards are reported in Table 1.
Figure 1.
HPLC-DAD chromatogram of standard polyphenols at 320 nm: caffeic acid (1), ferulic acid (2), isoferulic acid (3), fukinolic acid (4), cimicifugic acid A (5), cimicifugic acid B (6), cimicifugic acid E (7), and cimicifugic acid F (8).
Table 1.
Retention times, UV bands, calibration curves,a and limits of detection (LOD) of standards
| Compound | tR ± SD (min) | UV band (nm) | Slope a | Intercept b | Correlation coefficient, r2 | Linearity (μg/mL) | LOD (ng/mL)b | LOQ (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| Caffeic acid (1) | 12.26 ± 0.13 | 240, 295 sh, 324 max | 56,806 | +5350 | 0.9999 | 1.04–265 | 24.8 ± 0.15 | 82.7 ± 0.51 |
| Ferulic acid (2) | 21.18 ± 0.31 | 240, 295 sh, 323 max | 51,345 | −35,580 | 0.9970 | 0.98–250 | 51.6± 1.17 | 172.0 ± 3.89 |
| Isoferulic acid (3) | 23.89 ± 0.38 | 240, 295 sh, 323 max | 45,991 | −63,864 | 0.9984 | 1.02–260 | 52.9 ± 1.15 | 176.3 ± 3.82 |
| Fukinolic acid (4) | 25.77 ± 0.55 | 236, 288 sh, 330 max | 25,868 | −5786 | 0.9999 | 1.00–255 | 66.9 ± 0.58 | 222.9 ± 1.96 |
| Cimicifugic acid A (5) | 35.86 ± 0.27 | 236, 288 sh, 329 max | 23,099 | −74,804 | 0.9998 | 0.98–250 | 66.3 ± 1.01 | 220.9 ± 3.37 |
| Cimicifugic acid B (6) | 37.12 ± 0.25 | 236, 288 sh, 329 max | 25,258 | −2672 | 0.9999 | 0.98–250 | 69.9 ± 0.52 | 233.2 ± 1.73 |
| Cimicifugic acid E (7) | 40.86 ± 0.19 | 236, 288 sh, 329 max | 21,513 | −41,574 | 0.9999 | 1.02–260 | 72.8 ± 0.94 | 242.6 ± 3.08 |
| Cimicifugic acid F (8) | 41.81 ± 0.19 | 236, 288 sh, 329 max | 20,194 | +21,490 | 0.9991 | 1.03–260 | 78.3 ± 0.94 | 260.9 ± 3.13 |
For each curve the equation is y = ax + b, where y is the area under the peak, x is the concentration of the analyte (μg/mL), a is the slope, b is the intercept, and r2 is the correlation coefficient.
Data are the mean ± SD of triplicate determinations.
Validation of HPLC method
As previously mentioned, our method was validated with respect to linearity, precision, accuracy, recovery and sensitivity. Linear regression analysis for each standard was performed by the external standard method. Good linearity of five-point calibration curves was obtained for all standards between peaks area and concentration (r2 > 0.99) over the range test (ca. 1–250 μg/mL). The parameters of each calibration curve (slope, intercept and correlation coefficient) are reported in Table 1.
Intra- and inter-day analyses of the same solution containing all polyphenols in three different concentrations (1, 30 and 250 μg/mL) were used to validate the precision and accuracy of the method. The precision was calculated as RSD (%) at the 1, 30 and 250 μg/mL of the standard mixture solution; the RSD of all standards varied between 0.15 and 1.97% (n = 6) on the same day, but from 0.26 to 1.99% (n = 18) on different days. The accuracy was calculated as RE (%). The observed concentrations were in good agreement with the actual values. The RE (%) ranged from −1.91 to 1.83% (n = 6) on the same day and from −1.82 to 1.96% (n = 18) between days. These data corroborate the accuracy of the method established for the measurement of the polyphenols.
The average recovery (%) and RSD (%) for caffeic acid (1), fukinolic acid (4) and cimicifugic acid E (7) were 99.92 (0.08), 101.18 (0.22) and 101.33 (0.13), respectively. Similar recovery values for closely related compounds would be expected.
The limits of detection (LOD) and the limits of quantification (LOQ) of all eight polyphenols were established by means of the baseline-to-noise ratio. The LOD and LOQ for these compounds were found to be in the ranges 24.8–78.3 and 82.7–260.9 ng/mL (Table 1).
Quantification of polyphenols
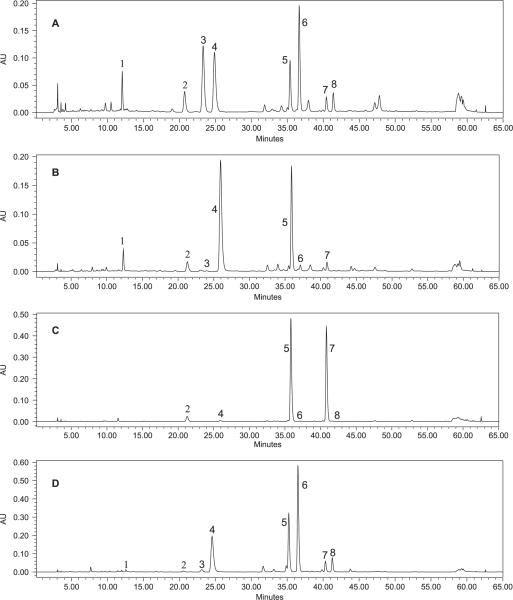
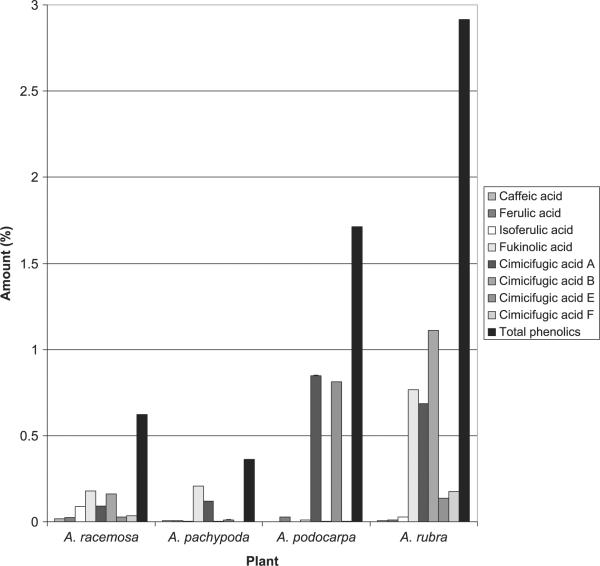
HPLC chromatograms of extracts from plants of four Actaea species are shown in Fig. 2, and the contents of the eight polyphenolic compounds in these plant materials are found in Table 2 and Fig. 3. The eight main polyphenolic compounds constitute 0.36% weight in the dried plant material for A. pachypoda, 1.71% for A. podocarpa, 0.62% for A. racemosa and 2.92% for A. rubra. Compared with A. racemosa, the related species A. rubra has a similar chemical profile, differing in the ratio of polyphenols. The other two Actaea species displayed polyphenolic profiles that are quite distinct from A. racemosa. Cimicifugic acid F was not detected in A. pachypoda, whereas isoferulic acid was not detected in A. podocarpa. Among the eight polyphenolic compounds, fukinolic acid is the most abundant polyphenol in A. pachypoda and A. racemosa, while the most abundant polyphenols in A. podocarpa and A. rubra are cimicifugic acids A and B, respectively. An A. pachypoda extract contained only two main polyphenols, fukinolic acid and cimicifugic acid A, which added up to 90.29% of the total polyphenols in the extract. A. podocarpa extract also contained two main polyphenols, cimicifugic acids A and E, which accounted for 97.24% of the total polyphenols in the extract.
Figure 2.
Comparison of HPLC-DAD chromatograms at 320 nm for the extracts of A. racemosa (A), A. pachypoda (B), A. podocarpa (C) and A. rubra (D).
Table 2.
Content of eight main polyphenols in four American Actaea species (dried root and rhizome)
| Compound | A. racemosa (% w/plant) | A. pachypoda (% w/plant)a | A. podocarpa (% w/plant) | A. rubra (% w/plant) |
|---|---|---|---|---|
| Caffeic acid (1) | 0.019 ± 3.6 × 10−5 | 0.007 ± 6.0 × 10−5 | Traceb | 0.007 ± 1.7 × 10−4 |
| Ferulic acid (2) | 0.023 ± 3.9 × 10−5 | 0.008 ± 3.3 × 10−5 | 0.029 ± 4.0 × 10−5 | 0.011 ± 8.1 × 10−4 |
| Isoferulic acid (3) | 0.088 ± 9.6 × 10−5 | 0.002 ± 1.2 × 10−5 | Trace | 0.028 ± 2.6 × 10−4 |
| Fukinolic acid (4) | 0.179 ± 1.7 × 10−4 | 0.207 ± 9.5 × 10−4 | 0.011 ± 8.6 × 10−5 | 0.767 ± 1.0 × 10−3 |
| Cimicifugic acid A (5) | 0.091 ± 2.9 × 10−4 | 0.118 ± 5.5 × 10−4 | 0.849 ± 1.3 × 10−3 | 0.686 ± 3.5 × 10−4 |
| Cimicifugic acid B (6) | 0.161 ± 1.1 × 10−3 | 0.005 ± 1.5 × 10−4 | 0.003 ± 2.6 × 10−3 | 1.11 ± 2.1 × 10−3 |
| Cimicifugic acid E (7) | 0.029 ± 1.1 × 10−4 | 0.012 ± 9.9 × 10−4 | 0.814 ± 1.5 × 10−3 | 0.137 ± 1.3 × 10−4 |
| Cimicifugic acid F (8) | 0.034 ± 5.1 × 10−5 | Trace | 0.003 ± 1.5 × 10−4 | 0.175 ± 2.8 × 10−4 |
| Total phenolics | 0.623 | 0.361 | 1.712 | 2.916 |
Data are the mean ± SD of triplicate determination.
Numeric value is smaller than 0.001.
Figure 3.
Content of eight main polyphenols in four American Actaea species (dried root and rhizome).
Four Actaea species, A. rubra, A. cordifolia, A. pachypoda and A. podocarpa, have geographical distributions areas which overlap with black cohosh. The possibility exists to misidentify similar Actaea species as black cohosh because the North American species share many morphological similarities. According to this study, our phytochemical method can be used to distinguish A. racemosa from A. pachypoda and A. podocarpa. On the basis of our HPLC chromatograms, A. racemosa extract contains all eight major polyphenols, while A. pachypoda contains only two major polyphenols, fukinolic acid and cimicifugic acid A. A. podocarpa contains two major polyphenols, cimicifugic acids A and E. We also examined eight populations of black cohosh from the eastern USA, from New York to North Carolina and Tennessee. We found that all populations displayed similar chemical profiles of phenolic constituents. Our study indicated that black cohosh can be distinguished from its closely related American Actaea species by the analysis of polyphenolic compounds.
DPPH radical scavenging activity
The DPPH radical scavenging activities of the extract of Actaea species and standard compounds are reported in Table 3. Fukinolic acid (4) has the highest activity with an IC50 of 12.9 μM, while isoferulic acid (3) has the lowest activity with IC50 = 289.1 μM, at a concentration comparable to ascorbic acid (105.5 μM). With regard to caffeic acid derivatives (1–3), the radical scavenging activity in the DPPH assay decreases in the order caffeic acid (1) > ferulic acid (2) > isoferulic acid (3). The order of radical scavenging activity in fukiic acid ester derivatives is fukinolic acid (4) > cimicifugic acid A (5) > cimicifugic acid B (6). For piscidic acid ester derivatives the order is cimicifugic acid E (7) > cimicifugic acid F (8). These results are in agreement with previous reports of structure–activity relationship of antioxidant hydroxycinnamic acid derivatives. For example, a single hydroxyl group substitution in para on an aromatic ring gave higher activity than those in ortho and meta on cinnamic acid; the presence of a second o-hydroxyl group in 4-hydroxycinnamic acid increases the antioxidant activity by further stabilizing the phenoxyl radical; and the presence of an ortho-electron-donating methoxyl group p-hydroxycinnamic acid also increases the antioxidant activity, but it is less effective than an o-hydroxyl group (Kikuzaki et al., 2002). A comparison among derivatives clearly shows that fukiic acid ester derivatives (4–6) have a higher radical scavenging activity than piscidic acid ester derivatives (7–8) and caffeic acid derivatives (1–3), due to the additional a catechol ring (o-dihydroxyl aromatic ring) on their structure. Thus, the radical scavenging activity on DPPH assay decreases in the following order: fukinolic acid (4) > cimicifugic acid A (5) > cimicifugic acid B (6) > caffeic acid (1) > cimicifugic acid E (7) > ferulic acid (2) > cimicifugic acid F (8) > isoferulic acid (3).
Table 3.
DPPH free radical scavenging activity of Actaea species extracts and standardsa
| Extract | IC50 (μg/mL) | Compound | IC50 (μM) |
|---|---|---|---|
| A. pachypoda | 191.6 ± 3.7 | Caffeic acid (1) | 58.3 ± 0.3 |
| A. podocarpa | 111.4 ± 1.1 | Ferulic acid (2) | 121.4 ± 1.3 |
| A. racemosa | 144.6 ± 1.8 | Isoferulic acid (3) | 289.1 ± 4.2 |
| A. rubra | 79.3 ± 2.7 | Fukinolic acid (4) | 12.9 ± 0.3 |
| Cimicifugic acid A (5) | 21.9 ± 0.6 | ||
| Cimicifugic acid B (6) | 23.1 ± 0.1 | ||
| Cimicifugic acid E (7) | 65.5 ± 0.5 | ||
| Cimicifugic acid F (8) | 151.5 ± 6.6 | ||
| Ascorbic acidb | 105.5 ± 2.7 |
Values are the mean ± SD of triplicate determination.
Positive control.
The average IC50 values of radical scavenging activity for A. pachypoda, A. podocarpa, A. racemosa and A. rubra are 191.6, 111.1, 144.6 and 79.3 μg/mL, respectively. The antioxidant activity of the extracts correlates with their polyphenolic content. Of the four species, A. rubra is the richest in total polyphenols (Table 2) and its extracts displayed the highest DPPH radical scavenging activity (Table 3), whereas A. pachypoda, with the lowest content of total polyphenols, displayed the lowest values for DPPH radical scavenging activity in its extracts.
A new RP-HPLC method with DAD was developed and validated to quantify eight major polyphenol compounds in four North American Actaea species. A baseline separation of all eight polyphenols has been achieved in the extracts of the four species. This study provides initial phytochemical profiles of polyphenols for four North American Actaea species. However, more studies are needed to examine the differences in polyphenolic profiles among various wild populations of the American Actaea species other than black cohosh.
Acknowledgements
We wish to thank Dr. Kan He and Naturex/PureWorld Botanicals Inc. for providing the black cohosh extract. Part of this work was supported by NIH-SCORE award S06GM08225 and by a grant from the National Institute of Health National Center for Complementary and Alternative Medicine (NIH-NCCAM #P50-AT00090 and R21 AT002930). The contents of this paper are solely our responsibility and do not necessarily reflect the official views of NIH-NCCAM.
Contract/grant sponsor: NIH-SCORE award; Contract/grant number: S06GM08225.
Contract/grant sponsor: National Institute of Health National Center for Complementary and Alternative Medicine; Contract/grant number: NIH-NCCAM #P50-AT00090 and R21 AT002930.
REFERENCES
- Blumenthal M. The ABC Clinical Guide to Herbs. American Botanical Council; Austin, TX: 2003. pp. 13–409. [Google Scholar]
- Borrelli F, Ernst E. Cimicifuga racemosa: a systematic review of its clinical efficacy. Eur J Clin Pharmac. 2002;58:235–241. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- Burdette JE, Chen SN, Lu ZZ, Xu H, White BE, Fabricant DS, Liu J, Fong HH, Farnsworth NR, Constantinou AI, Van Breemen RB, Pezzuto JM, Bolton JL. Black cohosh (Cimicifuga racemosa L.) protects against menadione-induced DNA damage through scavenging of reactive oxygen species: bioassay-directed isolation and characterization of active principles. J Agric Food Chem. 2002;50:7022–7028. doi: 10.1021/jf020725h. [DOI] [PubMed] [Google Scholar]
- Chen SN, Fabricant DS, Lu ZZ, Fong HH, Farnsworth NR. Cimiracemosides I–P, new 9,19-cyclolanostane triterpene glycosides from Cimicifuga racemosa. J Nat Prod. 2002a;65:1391–1397. doi: 10.1021/np0200818. [DOI] [PubMed] [Google Scholar]
- Chen SN, Fabricant DS, Lu ZZ, Zhang H, Fong HH, Farnsworth NR. Cimiracemates A–D, phenylpropanoid esters from the rhizomes of Cimicifuga racemosa. Phytochemistry. 2002b;61:409–413. doi: 10.1016/s0031-9422(02)00209-1. [DOI] [PubMed] [Google Scholar]
- Compton JA, Culham AC, Jury SL. Reclassification of Actaea to include Cimicifuga and Soulinea (Ranunculaceae): phylogeny inferred from morphology, nrdna its, and cpdna trnl-f sequence variation. Taxon. 1998;47:593–634. [Google Scholar]
- He K, Pauli GF, Zheng BL, Wang HK, Bai NS, Peng TS, Roller M, Zheng QY. Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass pectrometric/evaporative light scattering detection for quality control of black cohosh products. J Chromatogr. 2006;1112:241–254. doi: 10.1016/j.chroma.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernanz D, Nunez V, Sancho AI, Faulds CB, Williamson G, Bartolome B, Gomez-Cordoves C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J Agric Food Chem. 2001;49:4884–4888. doi: 10.1021/jf010530u. [DOI] [PubMed] [Google Scholar]
- ICH Fed Regist; International Conference on Harmonization (ICH).1995. p. 11260. [Google Scholar]
- Ito Y, Hirayama F, Suto K, Oshima T, Sakara K, Yoshida T. Determination of flavonol glycosides in epimedii herba by high-performance liquid chromatography. J Chromatogr. 1988;456:392–397. [Google Scholar]
- Jiang B, Yang H, Nuntanakorn P, Balick MJ, Kronenberg F, Kennelly EJ. The value of plant collections in ethnopharmacology: A case study of an 85-year-old black cohosh (Actaea racemosa L.) sample. J Ethnopharmac. 2005;96:521–528. doi: 10.1016/j.jep.2004.09.049. [DOI] [PubMed] [Google Scholar]
- Jiang B, Kronenberg F, Nuntanakorn P, Qiu MH, Kennelly EJ. Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high-performance liquid chromatography with selected ion monitoring liquid chromatography-mass spectrometry. J Agric Food Chem. 2006;54:3242–3253. doi: 10.1021/jf0606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuzaki H, Hisamoto M, Hirose K, Akiyama K, Taniguchi H. Antioxidant properties of ferulic acid and its related compounds. J Agric Food Chem. 2002;50:2161–2168. doi: 10.1021/jf011348w. [DOI] [PubMed] [Google Scholar]
- Kruse SO, Lohning A, Pauli GF, Winterhoff H, Nahrstedt A. Fukiic and piscidic acid esters from the rhizome of Cimicifuga racemosa and the in vitro estrogenic activity of fukinolic acid. Planta Med. 1999;65:763–764. doi: 10.1055/s-2006-960862. [DOI] [PubMed] [Google Scholar]
- Kusano A, Shibano M, Kusano G, Miyase T. Studies on the constituents of Cimicifuga species. XIX. Eight new glycosides from Cimicifuga simplex Wormsk. Chem Pharm Bull. 1996;44:2078–2085. doi: 10.1248/cpb.44.2078. [DOI] [PubMed] [Google Scholar]
- Kusano G, Takahira M, Shibano M, Kusano A, Okamoto Y, Tsujibo H, Numata A, Inamori Y. Studies on inhibitory activities of fukiic acid esters on germination, α-amylase and carboxypeptidase A. Biol Pharm Bull. 1998;21:997–999. doi: 10.1248/bpb.21.997. [DOI] [PubMed] [Google Scholar]
- Kusano A, Seyama Y, Nagai M, Shibano M, Kusano G. Effects of fukinolic acid and cimicifugic acids from Cimicifuga species on collagenolytic activity. Biol Pharm Bull. 2001;24:1198–1201. doi: 10.1248/bpb.24.1198. [DOI] [PubMed] [Google Scholar]
- Lee HS, Beon MS, Kim MK. Selective growth inhibitor toward human intestinal bacteria derived from Pulsatilla cernua root. J Agric Food Chem. 2001;49:4656–4661. doi: 10.1021/jf010609z. [DOI] [PubMed] [Google Scholar]
- Li W, Sun Y, Liang W, Fitzloff JF, van Breemen RB. Identification of caffeic acid derivatives in Actaea racemosa (Cimicifuga racemosa, black cohosh) by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17:978–982. doi: 10.1002/rcm.1008. [DOI] [PubMed] [Google Scholar]
- Loser B, Kruse SO, Melzig MF, Nahrstedt A. Inhibition of neutrophil elastase activity by cinnamic acid derivatives from Cimicifuga racemosa. Planta Med. 2000;66:751–753. doi: 10.1055/s-2000-9563. [DOI] [PubMed] [Google Scholar]
- Nuntanakorn P, Jiang B, Einbond LS, Yang H, Kronenberg F, Weinstein IB, Kennelly EJ. Polyphenolic constituents of Actaea racemosa. J Nat Prod. 2006;69:314–318. doi: 10.1021/np0501031. [DOI] [PubMed] [Google Scholar]
- Rafinesque CS. Medical Flora or Manual of the Medical Botany of the United States of North America. Atkinson & Alexander; Philadelphia, PA: 1828. p. 88. [Google Scholar]
- Sakakibara H, Honda Y, Nakagawa S, Ashida H, Kanazawa K. Simultaneous determination of all polyphenols in vegetables, fruits, and teas. J Agric Food Chem. 2003;51:571–581. doi: 10.1021/jf020926l. [DOI] [PubMed] [Google Scholar]
- Shao Y, Harris A, Wang M, Zhang H, Cordell GA, Bowman M, Lemmo E. Triterpene glycosides from Cimicifuga racemosa. J Nat Prod. 2000;63:905–910. doi: 10.1021/np000047y. [DOI] [PubMed] [Google Scholar]
- Smith RC, Reeves JC, Dage RC, Schuettler RA. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochem Pharmac. 1987;36:1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- Souliman A, Barakat H, El-Mousallamy A, Marzouk M, Nawwar M. Phenolics from the bark of Tamarix aphylla. Phytochemistry. 1991;30:3763–3766. [Google Scholar]
- Stromeier S, Petereit F, Nahrstedt A. Phenolic esters from the rhizomes of Cimicifuga racemosa do not cause proliferation effects in MCF-7 cells. Planta Med. 2005;71:495–500. doi: 10.1055/s-2005-864148. [DOI] [PubMed] [Google Scholar]
- Takahira M, Kusano A, Shibano M, Kusano G, Miyase T. Piscidic acid and fukiic acid esters from Cimicifuga simplex. Phytochemistry. 1998a;49:215–219. [Google Scholar]
- Takahira M, Kusano A, Shibano M, Kusano G, Sakuri N, Nagai M, Miyase T. Three new fukiic acid esters, cimicifugic acids A, B and C, from Cimicifuga simplex Wormsk. Chem Pharm Bull. 1998b;46:362–365. [Google Scholar]
- Wang HK, Sakurai N, Shih CY, Lee KH. LC/TIS-MS fingerprint profiling of Cimicifuga species and analysis of 23-epi-26-deoxyactein in Cimicifuga racemosa commercial products. J Agric Food Chem. 2005;53:1379–1386. doi: 10.1021/jf048300d. [DOI] [PubMed] [Google Scholar]
- Zerega NJC, Mori S, Lindqvist C, Zhemg Q, Motley TJ. Using amplified fragment length polymorphisms (AFLP) to identify black cohosh (Actaea racemosa) Econ Bot. 2002;56:154–164. [Google Scholar]