Abstract
Preventive immunization with whole inactivated Mycobacterium vaccae (MV) confers protection against HIV-associated tuberculosis (TB) in BCG-immunized adults with CD4 counts ≥ 200 cells/ul. We evaluated the immunogenicity of MV in the 2,013 subjects of the phase III DarDarTrial using an interferon gamma (IFN-γ) enzyme linked immunosorbent assay (ELISA), tritiated thymidine lymphocyte proliferation assay (LPA) and an ELISA for antibodies to the TB glycolipid lipoarabinomannan (LAM). MV immunization boosts IFN-γ and LPA responses to MV sonicate, and antibody responses to LAM. Post-immunization immune responses to MV correlated with baseline clinical factors, but the responses did not predict protection from HIV-associated TB.
Keywords: tuberculosis, HIV, vaccines, interferons, cell proliferation
INTRODUCTION
The current tuberculosis (TB) vaccine bacille Calmette-Guerin (BCG) reduces the incidence of severe TB disease and death during childhood [1-7]. Yet, the protection from TB disease elicited by BCG immunization is incomplete, may wane during adulthood [8-10], and BCG boosters are ineffective [11]. Therefore, multiple novel TB booster vaccines are now being tested for safety and immunogenicity [12-14]. Since infection with the human immunodeficiency virus (HIV) markedly increases the frequency and severity of TB disease [15-18], the development of a TB booster vaccine that is both safe and immunogenic for people with HIV is a leading global health priority [13, 14].
Whole inactivated Mycobacterium vaccae (MV) was shown to be safe and immunogenic in phase I and II studies in HIV-infected adults: five intradermal doses of MV elicited strong interferon gamma (IFN-γ) and lymphocyte proliferation assay (LPA) responses in HIV-infected subjects with prior BCG vaccination in both Finland and Zambia [19, 20]. The DarDar Trial was a phase III randomized double-blinded placebo-controlled clinical trial conducted in Dar es Salaam, Tanzania to determine the efficacy of MV for preventing TB in HIV-infected and BCG-vaccinated adults with CD4 counts ≥ 200 cells/ul. MV vaccination was safe, well-tolerated, and provided significant protection against laboratory-defined definite tuberculosis [21]. To characterize the immune response to MV and to identify clinical factors that impact the immunogenicity of MV, we assessed both cellular and humoral immune responses to mycobacterial antigens before and after vaccination using three methods: a five-day interferon gamma (IFN-γ) release assay, a standard tritiated-thymidine lymphocyte proliferation assay (LPA), and an enzyme-linked immunosorbent assay (ELISA) of antibody responses to lipoarabinomannan (LAM).
METHODS
Study subjects and design
The DarDar Trial was a phase III randomized placebo-controlled double-blind trial of a prime-boost vaccine strategy for the prevention of HIV-associated tuberculosis among adults in Dar es Salaam, Tanzania [21]. Subject enrollment occurred from 2001 to 2005, and study follow up continued through January 2008. Adults who gave informed consent were eligible for enrollment if they had two positive enzyme linked immunosorbent assay (ELISA) tests for HIV, a CD4 count ≥ 200/mm3, and a BCG scar. At baseline, subjects underwent history and physical examination, single view chest x-ray and provided sputum samples for acid-fast bacillus (AFB) smear and sputum mycobacterial culture. Subjects found to have active TB were excluded from enrollment. Subjects were randomized to receive either five intradermal doses of whole heat inactivated Mycobacterium vaccae (MV; representing 1×109 colony forming units) or matched saline placebo at 0, 2, 4, 6 and 12 months after enrollment. From blood collection through analysis of the assay results, all personnel were blinded to treatment allocation.
Human research conduct
Human experimentation guidelines of the United States Department of Health and Human Services and the Tanzania Food and Drug Authority, as well as that of the Committee for the Protection of Human Subjects at Dartmouth College and the Research Ethics Committee of the Muhimbili University of Health and Allied Sciences, were followed in the conduct of this research. This study was registered through the National Institutes of Health (NCT00052195).
Clinical surveillance for TB disease
We evaluated subjects for active TB disease via history and physical examination at baseline, months 2, 4, and 6, and every three months thereafter. Furthermore, subjects presenting at any time with two or more weeks of fever, cough or weight loss also underwent evaluation for active TB. Studies included a single-view chest x-ray, three collections of expectorated sputum for AFB smear and mycobacterial culture, plus phlebotomy for mycobacterial blood culture. We performed additional investigations as clinically indicated (e.g., cultures of other body fluids, tissue biopsies).
Definitions of TB
A three-person blinded adjudication panel reviewed all episodes of illness evaluated for active TB and designated subjects to have definite or probable TB according to rigorous study definitions ([21]).
Assays of mycobacterial immune responses
Subjects underwent phlebotomy prior to vaccination (referred to as “baseline”) and at fourteen months after enrollment, i.e, two months after the fifth (final) dose of vaccine (referred to as “post-dose 5”). PBMC were isolated by ficoll density gradient centrifugation, and IFN-γ and LPA assays were conducted on freshly isolated cells. Serum was centrifuged, frozen at -70C and sent to the United States for assessment of antibody responses to LAM.
Antigens
Four mycobacterial antigens were used in IFN-γ and LPA assays: MV sonicate (2 mcg/ml), M. tuberculosis Ag85 (1 mcg/ml), M. tuberculosis ESAT-6 (2 mcg/ml), or M. tuberculosis whole cell lysate (WCL; 1 mcg/ml). Phytohemagglutinin (PHA, 2.5 mcg/mL; Sigma, St. Louis, MO) was used as a positive control; media alone as the negative control. MV sonicate was provided courtesy of John Stanford, University of London; Ag85, ESAT-6, and WCL were obtained via National Institute of Allergy and Infectious Diseases Contract No. HHSN266200400091C “Tuberculosis Vaccine Testing and Research Materials” awarded to Colorado State University.
IFN-γ responses
After five days of incubation, PBMC cultures were centrifuged, supernatants frozen at -70C, sent to the United States and assayed for IFN-γ using a standard ELISA (R&D Systems, Minneapolis, MN). Assays were considered valid if the IFN-γ response to PHA was greater than 300 pg/mL and not smaller than the response to the negative control condition.
LPA responses
After four days of incubation, 3H-thymidine was added to PBMC cultures, and then after 24 hours the cells were harvested onto filter paper and sent to the National Public Health Institute in Finland for data acquisition on a scintillation counter. Results were expressed as a proliferation index (PI; defined as counts per minute [CPM] of antigen stimulated cells divided by CPM of unstimulated cells). Assays were considered valid if the PHA PI was ≥3.
Antibody responses to LAM
Serum diluted 1:100 was assayed via ELISA for IgG antibody to LAM, a glycopeptide widely expressed on mycobacteria [22]. The positive control for this assay was serum from an MTB experienced individual which had been diluted 1/10 in 5% goat serum in phosphate buffered saline, aliquotted and frozen at -70°C. The color reaction in each assay was allowed to develop until the optical density (OD) of the positive control reached 2.0. All LAM assays were considered valid.
TST
Tuberculin (0.1 mL, RT-23, State Serum Institute, Copenhagen) was injected intradermally on the forearm in all subjects at baseline, and trained personnel measured the size of skin induration at the site after 48-72 hours. Subjects with reactions ≥ 5 mm (“positive”) were offered isoniazid 300 mg daily for 6 months.
HIV viral load assessment
As part of a pre-planned safety sub-study, baseline HIV viral load was assessed in 713 subjects. Plasma was collected, frozen, shipped to the United States and HIV viral load subsequently measured using the Bayer Versant™ HIV-1 RNA 3.0 (bDNA) assay.
Statistical analysis
We compared post-dose 5 immune responses between subjects who received MV or placebo using a two-tailed Mann-Whitney U test with a threshold for statistical significance of P<0.05. Baseline and post-dose 5 immune responses among MV recipients were compared using the sign-rank test. We assessed the correlation between immune responses using Spearman correlation coefficients. We used a multivariate Cox proportional hazards regression model adjusting for age, baseline CD4 count, previous TB and TST status to relate vaccine immune responses to TB outcomes. We confirmed the proportional hazards assumption was not violated using log-log plots and Schoenfeld residuals. We analyzed the data using STATA 9 (College Station, TX).
RESULTS
Study subjects
2,013 subjects were enrolled and randomized; 1,975 were given at least one dose of vaccine. Thirteen subjects were excluded from the immunogenicity analysis because they were found to have active tuberculosis at baseline [23]. The characteristics of the remaining 1,962 study subjects are shown in Table 1. 1,500 subjects received five doses of MV or placebo, 295 received four doses, and 167 received three or fewer doses. Valid IFN-γ results were available for 1,454 subjects at baseline, and 1,025 subjects after vaccine dose 5. Valid LPA results were available for 1,095 subjects at baseline, and 838 subjects after vaccine dose 5. Valid LAM results were available for 1,958 subjects at baseline, and 1,640 subjects after vaccine dose 5. Subject loss to follow up and the prevalence of valid results on immune assays were evenly balanced between MV and placebo recipients.
Table 1.
Baseline characteristics of 1962 study subjects
| MV (N=983) | Placebo (N=979) | P value | |
|---|---|---|---|
| Age, mean years | 33.3 | 33.0 | 0.4821 |
| Male, n (%) | 230 (23.4) | 237 (24.2) | 0.6734 |
| CD4 T cell count, mean, cells/mm3 | 475.2 | 470.4 | 0.1838 |
| Antiretroviral therapy, n (%) | 24 (2.4) | 33 (3.4) | 0.2205 |
| Prior treatment for tuberculosis, n (%) | 87 (8.9) | 80 (8.2) | 0.5901 |
| Tuberculin skin test ≥ 5 mm, n (%) | 309 (32.2) | 312 (32.5) | 0.8961 |
P value by Mann-Whitney U test; MV, Mycobacterium vaccae
MV immunogenicity
IFN-γ and LPA responses to MV sonicate, as well as antibody responses to LAM, are shown in Figure 1. At baseline, immune responses were similar between subjects randomized to MV and to placebo. After dose 5, subjects who received MV had greater IFN-γ responses to MV sonicate, greater LPA responses to MV sonicate, and greater antibody responses to LAM, than subjects who received placebo. Among subjects who received MV, post-dose 5 LPA responses to MV sonicate and antibody responses to LAM were significantly increased compared to baseline, and there was a trend toward greater IFN-γ responses to MV sonicate. Among subjects who received MV, post-dose 5 immune responses were equivalent between those who received four or five doses: IFN-γ to MV sonicate (550 vs. 440 pg/ml, P=0.4240), LPA to MV sonicate (PI 2.8 vs 3.0, P=0.9272) and LAM (OD 0.61 vs. 0.65, P=0.9877). There were too few subjects who received three or fewer doses and who had valid assay data available for meaningful analyses. Compared to subjects who received placebo, subjects who received MV did not demonstrate greater post dose 5 IFN-γ responses to Ag85 (741 vs. 799 pg/ml, P=0.6525), ESAT-6 (1196 vs. 1219, P=0.7031) or WCL (2625 vs. 2910, P=0.5023). Similarly, post dose 5 LPA responses to these antigens were equivalent between subjects who received MV and those who received placebo: Ag85 (PI 2.9 vs. 3.0, P=0.6374), ESAT-6 (PI, 2.5 vs. 2.4, P=0.9792) and WCL (PI, 6.9 vs. 7.3, P=0.9491).
Figure 1.
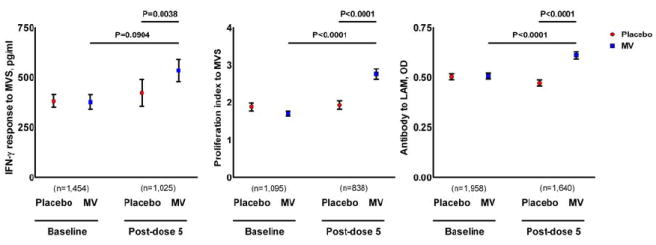
Interferon gamma (IFN-γ and lymphocyte proliferation assay LPA̤ responses to MV sonicate, and antibody responses to lipoarabinomannan (LAM) in MV and placebo recipients
IFN-γ, interferon gamma; LAM, lipoarabinomannan; LPA, lymphocyte proliferation assay; MV, Mycobacterium vaccae TB booster vaccine; MVS, Mycobacterium vaccae sonicate; OD, optical density; proliferation index is the ratio of counts per minute of the antigen over counts per minute of medium. Bars indicate the mean and standard error.
Impact of baseline CD4 count on MV immunogenicity
Figure 2A shows post-dose 5 immune responses stratified by baseline CD4 count. Among subjects with baseline CD4 counts ≥350/mm3, all three assays demonstrated significantly greater responses for MV recipients compared to placebo recipients. In contrast, among subjects with baseline CD4 counts < 350 cells/mm3, subjects who received MV only demonstrated significantly greater LPA responses to MV. Comparing MV recipients across CD4 strata, those with higher baseline CD4 counts had markedly greater IFN-γ responses; the two strata had similar LPA responses.
Figure 2.
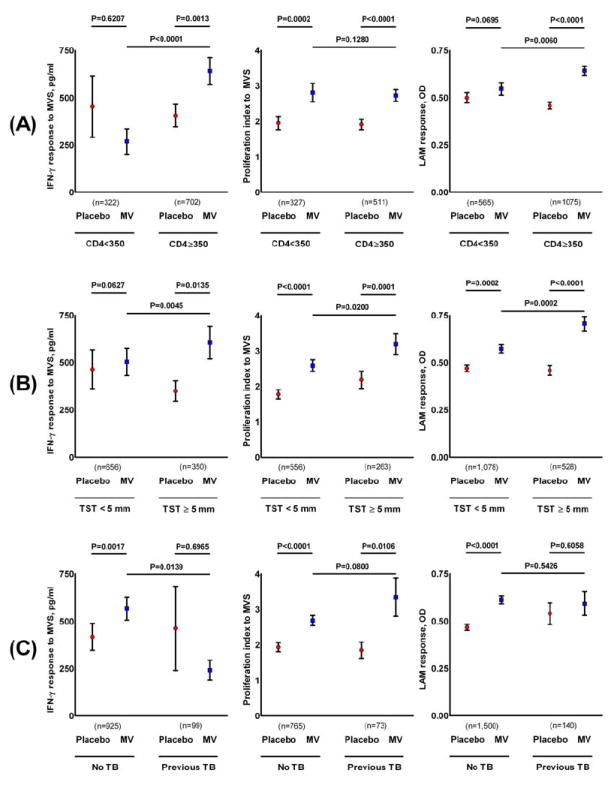
Post-dose 5 immune responses analyzed according to (A) baseline CD4 count, (B) baseline tuberculin skin test status, and (C) baseline TB treatment history.
IFN-γ, interferon gamma; LAM, lipoarabinomannan; LPA, lymphocyte proliferation assay; MV, Mycobacterium vaccae TB booster vaccine; MVS, Mycobacterium vaccae sonicate; OD, optical density; proliferation index is the ratio of counts per minute of the antigen over counts per minute of medium; TB, tuberculosis; TST, tuberculin skin test. Bars indicate the mean and standard error.
Impact of baseline HIV viral load on MV immunogenicity
Among the 713 subjects in a pre-planned HIV viral load substudy, subjects who received MV and subjects who received placebo had equivalent baseline HIV viral loads (32,948 vs. 35,055 copies/ml, P=0.5858). Among subjects who received MV, baseline HIV viral was inversely correlated with the magnitude of IFN-γ responses to MV sonicate after dose 5 (N=180, Spearman’s rho, ρ -0.2243, P=0.0025), but did not correlate with LPA responses after dose 5 to MV sonicate (N=162, ρ -0.0963, P=0.2230) or with antibody responses to LAM (N=283, ρ 0.0059, P=0.9218).
Impact of antiretroviral therapy on MV immunogenicity
At baseline, 2.9% of subjects were on antiretroviral therapy. Baseline immune responses were not different in subjects on antiretroviral therapy compared to subjects who were not on antiretroviral therapy: IFN-γ responses to MV sonicate 643 vs. 371 pg/ml, P=0.0769; LPA responses to MV sonicate, PI 2.8 vs. 1.8, P=0.1461); and antibody responses to LAM, OD 0.48 vs. 0.51, P=0.7247. Post dose 5, 7.9% of MV recipients were on antiretroviral therapy. Among MV recipients, post dose 5 IFN-γ and LPA responses to MV sonicate were not different between subjects who were or were not on antiretroviral therapy at the year 1 study visit (IFN-γ: 243 vs. 557 pg/ml, P=0.5094; LPA: PI 2.6 vs. 2.8, P=0.7300). However, post dose 5 MV recipients who were on antiretroviral therapy had smaller magnitude antibody responses to LAM compared to subjects who were not on antiretroviral therapy (OD 0.51 vs. 0.62, P=0.0450).
Impact of TST status on MV immunogenicity
Figure 2B shows the post-dose 5 immune responses in each treatment group stratified by baseline TST status. Among subjects with a positive baseline TST, MV recipients demonstrated significantly greater responses than placebo recipients in all three assays. Among MV recipients, responses to MV sonicate in all three assays were greater in those whose baseline TST was positive rather than negative. Even among subjects with negative baseline TST, post dose 5 responses in all three assays were greater for MV recipients compared with placebo recipients, although the difference did not reach statistical significance for IFN-γ responses.
Impact of isoniazid administration on MV immunogenicity
Among MV recipients with a positive TST, immune responses in subjects who received isoniazid were equivalent to immune responses in subjects who did not receive isoniazid: IFN-γ responses to MV (619 vs. 478 pg/ml, P=0.3517), LPA responses to MV (PI 3.2 vs. 3.3, P=0.1508) and antibody responses to LAM (OD 0.69 vs. 0.84, P=0.8117). Similarly, there was no correlation between the magnitude of immune responses after MV immunization among MV recipients with a positive TST and the number of days of isoniazid received: IFN-γ responses to MV (N=156, ρ 0.1241, P=0.1228), LPA responses to MV (N=109, ρ -0.0119, P=0.9025), and antibody responses to LAM (N=236, ρ -0.0355, P=0.5870).
Impact of prior TB treatment history on MV immunogenicity
Figure 2C depicts immune responses after dose 5 in MV and placebo recipients according to whether subjects had a history of TB prior to trial enrollment. IFN-γ responses to MV sonicate and antibody responses to LAM were increased only in subjects who had no prior history of TB. LPA responses to MV sonicate were not impacted by prior TB history. Of note, among MV recipients, those with a history of prior TB had significantly weaker IFN-γ responses to MV sonicate than those with no prior TB history.
Correlation of immune responses after dose 5
After dose 5, subjects who received MV but not subjects who received placebo demonstrated statistically significant correlation between IFN-γ responses to MV sonicate and LPA responses to MV sonicate as well as with antibody responses to LAM (Table 2). This correlation was not seen in MV recipients who were treated for TB prior to trial enrollment (no previous TB, N=254, ρ 0.2538, P<0.0001; previous TB, N=30, ρ 0.0707, P=0.7106). MV vaccination did not alter the correlation between LPA responses to MV sonicate and antibody responses to LAM.
Table 2.
Correlation of immune responses to MV vaccination.
| MV | Placebo | |||
|---|---|---|---|---|
| IFN-γ response, pg/ml | Proliferation index | IFN-γ response, pg/ml | Proliferation index | |
| Proliferation index | ρ=0.2300 | --- | ρ=0.1015 | --- |
| P=0.0001 | P=0.0850 | |||
| LAM antibody response, OD | ρ=0.1861 | ρ=0.0889 | ρ=-0.0271 | ρ=0.0726 |
| P<0.0001 | P=0.0731 | P=0.5479 | P=0.1436 | |
IFN-γ, interferon gamma response to MV sonicate; LAM, lipoarabinomannan; MV, Mycobacterium vaccae; OD, optical density; proliferation index is defined as the ratio of counts per minute of the well stimulated with MV sonicate over the counts per minute in the medium control well.
Spearman’s rho statistic (ρ) and P value for the correlation are shown for all pairs of immune responses in subjects who received MV or placebo.
Post-vaccination immune correlates of protection from TB
Among placebo subjects, post dose 5 IFN-γ responses to MV sonicate were significantly greater for those who did not develop TB compared to those who did develop TB (Table 3). Among the smaller number of MV recipients who developed TB, IFN-γ responses to MV sonicate were also lower compared to subjects who did not develop TB, but this difference did not reach statistical significance. LPA and LAM antibody responses showed no correlation to risk of TB in either treatment arm.
Table 3.
Post-dose 5 immune responses in subjects who received MV or placebo according to whether they developed definite TB during prospective follow up.
| MV | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Definite TB | No definite TB | Definite TB | No definite TB | |||||||
| N | Mean | N | Mean | P value | N | Mean | N | Mean | P value | |
| IFN-γ response, pg/ml | 14 | 191 | 498 | 545 | 0.3533 | 28 | 202.5 | 484 | 435.1 | 0.0080 |
| Proliferative index | 13 | 3.8 | 406 | 2.7 | 0.3557 | 27 | 2.7 | 392 | 1.9 | 0.9515 |
| Antibody response to LAM, OD | 28 | 0.53 | 797 | 0.61 | 0.5736 | 42 | 0.51 | 773 | 0.47 | 0.7482 |
P values by Mann-Whitney U test
IFN-γ response, interferon gamma response to MV soniciate; LAM, lipoarabinomannan; MV, Mycobacterium vaccae; OD, optical density; proliferation index is defined as the ratio of counts per minute in the well stimulated with MV sonicate over the counts per minute in the medium control well; TB, tuberculosis
Among MV recipients, a multivariate Cox regression model adjusting for age, baseline CD4 count, TST status and previous TB history showed no relation to protection from TB of IFN-γ responses to MV sonicate, LPA responses to MV sonicate, or antibody responses to LAM (data not shown). Similarly, among subjects who received MV, IFN-γ and LPA responses to Ag85, ESAT-6 and WCL did not correlate with protection from the subsequent development of TB (data not shown).
DISCUSSION
The development of a vaccine for the prevention of HIV-associated TB is a leading global health priority [13, 14]. A critical requirement for such a TB vaccine is that it be effective and immunogenic in persons with HIV infection. In a large, randomized, placebo-controlled, double-blind Phase III clinical trial in Tanzania, we administered five intradermal doses of whole inactivated Mycobacterium vaccae (MV) – a novel vaccine for preventing TB – to HIV-infected and BCG-vaccinated adults with CD4 counts ≥ 200 cells/mm3. Compared to placebo, MV recipients had increased mycobacterial immune responses in three in vitro assays – IFN-γ responses to MV sonicate, LPA responses to MV sonicate, and antibody responses to LAM. These results confirm our previous Phase II and III studies in Finland and Zambia [19, 20], and demonstrate consistent vaccine immunogenicity in a large cohort of BCG-vaccinated, HIV-infected subjects from a developing country with high rates of endemic TB. While we did not find a specific surrogate for vaccine efficacy for preventing TB, we found a trend toward higher IFN-γ responses in MV recipients who did not develop TB.
We identified multiple clinical factors related both to HIV status and TB experience that impacted immune responses to MV immunization. Subjects with baseline CD4 counts ≥ 350 cells/ul who received MV demonstrated greater IFN-γ responses to MV sonicate and antibody responses to LAM compared to those with baseline CD4 counts 200-349 cells/ul. This is concordant with data showing that HIV disease progression blunts the immunogenicity of BCG, influenza and hepatitis B immunization [24-26]. In subset analyses, baseline HIV viral load correlated negatively with the magnitude of IFN-γ responses to MV sonicate but did not correlate with LPA responses to MV sonicate or antibody responses to LAM in subjects who received MV. The difference may relate to the preferential deletion of MV-specific IFN-γ expressing memory T cell clones among HIV-infected subjects with frequent exposure to TB and other mycobacteria [27-29].
Subjects’ baseline TST status and prior TB treatment history also affected MV immunogenicity, but with notable differences. Among TST negative subjects, the difference in IFN-γ responses to MV sonicate between subjects who received MV and subjects who received placebo were smaller and did not reach statistical significance. Moreover, MV recipients who were TST positive at baseline had stronger post-dose 5 responses than those who were TST negative. The greater responses in MV recipients who were TST positive suggest either that prior exposure to mycobacteria enhances immune responses to MV, or that subjects with higher CD4 counts are more likely to respond to immunization with MV. MV recipients with a prior history of TB had significantly lower IFN-γ responses to MV sonicate than MV recipients without such a history. These data cannot resolve whether the poor IFN-γ responses among subjects with prior TB treatment is a consequence of prior TB disease, or of impaired immunity in general.
None of the immune parameters assessed in this study represent a clear surrogate marker for MV-mediated protection from TB disease. Possible explanations for this finding are (1) that our study lacked power to relate IFN-γ responses with protection from TB, (2) that we did not test responses to shared protective antigen(s), or (3) that we did not assess the immune mechanism through which MV immunization confers protection from TB disease.
Several pieces of evidence support the hypothesis that IFN-γ responses to MV sonicate contribute to the prevention of TB disease. First, there was a trend toward greater IFN-γ responses to MV sonicate in MV recipients who did not develop TB disease compared to MV recipients who did develop TB disease. Second, placebo recipients who developed TB had significantly lower magnitude IFN-γ responses to MV. Third, among placebo recipients, detectable baseline IFN-γ responses to mycobacterial antigens were also associated with lower risk of TB [30].
Our study had limitations that may have prevented the detection of a relationship between IFN-γ responses to MV sonicate and protection from TB disease. We plausibly lacked sufficient power since subjects who received MV were less likely to develop definite TB: only 14 subjects who received MV, and developed TB, had IFN-γ data available for analysis [31]. Immunological assay choice, too, may have impacted our ability to identify a surrogate marker of vaccine-mediated protection from HIV-associated TB. The baseline IFN-γ responses to mycobacterial antigens measured in our five-day assay are the only immunological responses shown to correlate prospectively with the subsequent risk of HIV-associated TB in the placebo recipients from the DarDar Trial [30]. However, among vaccine recipients the results of the post-vaccine IFN-γ release assay to MV sonicate, which presumably measured both central memory and effector memory responses [32, 33], were near or below the lower limits of detection. An assay more likely to measure rapid effector memory responses, such as commercially available IFN-γ release assays, or an assay with greater sensitivity for IFN-γ responses, such as for intracellular IFN-γ expression or polyfunctional T cell function, might correlate more definitively with MV-mediated protection [34, 35]. The laboratory infrastructure required for these assays, however, was not available in Tanzania at the 2001 outset of the DarDar Trial, and such assays recently failed to identify an immune surrogate for BCG-mediated protection from TB disease in healthy infants [36].
Given the multifaceted immune impact of MV immunization in animal models [37-42], and the complex nature of the human immune response to TB, it seems likely that immune responses other than IFN-γ responses contribute to MV-mediated protection from TB disease. Other potential immunological surrogates that will be worth assessing in future studies of MV immunogenicity include alternate cytokine and chemokine responses to MV immunization as well as non-classically-restricted T cell responses and the impact of MV immunization on assays of immune cell mycobactericidal function [43, 44]. Regrettably, none of these potential surrogate markers have been validated prospectively, which represents a major obstacle to rapid TB vaccine development [45].
Our choice of MV as the vaccine antigen was based on data from animal and human studies demonstrating that natural or vaccine-induced infection with non-tuberculous mycobacteria confers protection against the development of TB [46-48], presumably thru responses to shared mycobacterial antigens. Consistent with the prior observation that murine MV immunization does not generate cross-reactive responses to the M. tuberculosis RD1 antigens ESAT-6 or CFP-10 [49], immunization with MV conferred protection from TB and elicited clear immune responses to the vaccine mycobacterial antigen but not to the TB-specific antigen ESAT-6, or to Ag85. We suspect that MV immunization elicited protective immune responses against other as yet undefined shared mycobacterial antigens, or that protection was mediated via an immune response mechanism that we did not measure.
Five intradermal doses of whole inactivated Mycobacterium vaccae, a vaccine that protects against tuberculosis, boost IFN-γ and LPA responses to MV sonicate, as well as antibody responses to the TB glycolipid LAM, in HIV-infected and BCG-vaccinated adults with CD4 counts ≥ 200 cells/mm3. Baseline CD4 count, HIV viral load, TST status, and TB treatment history are important predictors of IFN-γ and antibody responses. Future prospective studies should test the hypothesis that IFN-γ responses to shared mycobacterial antigens correlate with vaccine-mediated protection from TB disease.
Acknowledgments
We thank Wendy Wieland-Alter, Outi Rautio, and Betty Mchaki for their skillful conduct of the immunological assays in this study, and Sue Tvaroha for excellent database management.
Support: National Institutes of Health, DAIDS, AI 45407 (C.F.vR) and Fogarty International Center, D43-TW006807 (C.F.vR, C.R.H.); National Institutes of Health, National Center for Research Resources, Centers for Biomedical Research Excellence 5P20RR016437-08 (T.L.).
Footnotes
Conflicts of interest: None to declare
Presented at: 16th Conference on Retroviruses and Opportunistic Infections, Montreal, Canada, February 2009
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
ClinicalTrials.gov Identifier: NCT0052195
References
- 1.Aronson JD. Protective vaccination against tuberculosis with special reference to BCG vaccination. Am Rev Tuberc. 1948;58:255–81. doi: 10.1164/art.1948.58.3.255. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson RG, Simes AB. BCG vaccination of infant Indians in Saskatchewan. Tubercle. 1949;30:5–11. doi: 10.1016/s0041-3879(49)80055-9. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal SR, Loewinsohn E, Graham ML, Liveright D, Thorne MG, Johnson V. BCG vaccination in tuberculous households. Am Rev Respir Dis. 1960;84:690–704. doi: 10.1164/arrd.1961.84.5P1.690. [DOI] [PubMed] [Google Scholar]
- 4.Rosenthal SR, Loewinsohn E, Graham ML, Liveright D, Thorne MG, Johnson V, et al. BCG vaccination against tuberculosis in Chicago: a twenty year study statistically analyzed. Pediatrics. 1961;28:622–41. [PubMed] [Google Scholar]
- 5.Levine MI, Sackett MF. Results of BCG immunization in New York City. Am Rev Tuberc. 1948;53:517–32. doi: 10.1164/art.1946.53.6.517. [DOI] [PubMed] [Google Scholar]
- 6.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, et al. The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics. 1995;96:29–35. [PubMed] [Google Scholar]
- 7.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 8.Sterne JA, Rodrigues LC, Guedes IN. Does the efficacy of BCG decline with time since vaccination? Int J Tuberc Lung Dis. 1998 Mar;2(3):200–7. [PubMed] [Google Scholar]
- 9.Packe GE, Innes JA. Duration of protection against tuberculosis conferred by BCG vaccination in infancy. Arch Dis Child. 1989 Apr;64(4):634–5. doi: 10.1136/adc.64.4.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, et al. Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study. Jama. 2004 May 5;291(17):2086–91. doi: 10.1001/jama.291.17.2086. [DOI] [PubMed] [Google Scholar]
- 11.Karonga Trial Prevention Group. Randomised controlled trial of single BCG, repeated BCG, or combined BCG and killed Mycobacterium leprae vaccine for prevention of leprosy and tuberculosis in Malawi. Lancet. 1996;348:17–24. [PubMed] [Google Scholar]
- 12.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006 Jun;4(6):469–76. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 13.Hoft DF. Tuberculosis vaccine development: goals, immunological design, and evaluation. Lancet. 2008 Jul 12;372(9633):164–75. doi: 10.1016/S0140-6736(08)61036-3. [DOI] [PubMed] [Google Scholar]
- 14.von Reyn CF, Vuola JM. New vaccines for the prevention of tuberculosis. Clin Infect Dis. 2002 Aug 15;35(4):465–74. doi: 10.1086/341901. [DOI] [PubMed] [Google Scholar]
- 15.Seng R, Gustafson P, Gomes VF, Vieira CS, Rabna P, Larsen O, et al. Community study of the relative impact of HIV-1 and HIV-2 on intrathoracic tuberculosis. Aids. 2002 May 3;16(7):1059–66. doi: 10.1097/00002030-200205030-00013. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: World Health Organization; 2008. [Google Scholar]
- 17.Dye C. Global epidemiology of tuberculosis. Lancet. 2006 Mar 18;367(9514):938–40. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 18.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999 Feb 4;340(5):367–73. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 19.Waddell RD, Chintu C, Lein AD, Zumla A, Karagas MR, Baboo KS, et al. Safety and immunogenicity of a five-dose series of inactivated Mycobacterium vaccae vaccination for the prevention of HIV-associated tuberculosis. Clin Infect Dis. 2000 Jun;30(Suppl 3):S309–15. doi: 10.1086/313880. [DOI] [PubMed] [Google Scholar]
- 20.Vuola JM, Ristola MA, Cole B, Jarviluoma A, Tvaroha S, Ronkko T, et al. Immunogenicity of an inactivated mycobacterial vaccine for the prevention of HIV-associated tuberculosis: a randomized, controlled trial. Aids. 2003 Nov 7;17(16):2351–5. doi: 10.1097/00002030-200311070-00010. [DOI] [PubMed] [Google Scholar]
- 21.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids. 2010 Jan 28;13(24(5)):675–85. doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigou J, Gilleron M, Puzo G. Lipoarabinomannans: from structure to biosynthesis. Biochimie. 2003 Jan-Feb;85(1-2):153–66. doi: 10.1016/s0300-9084(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 23.Lahey T, Matee M, Mtei L, Bakari M, Pallangyo K, von Reyn CF. Lymphocyte proliferation to mycobacterial antigens is detectable across a spectrum of HIV-associated tuberculosis. BMC Infect Dis. 2009;9:21. doi: 10.1186/1471-2334-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iorio AM, Alatri A, Francisci D, Preziosi R, Neri M, Donatelli I, et al. Immunogenicity of influenza vaccine (1993-94 winter season) in HIV-seropositive and -seronegative ex-intravenous drug users. Vaccine. 1997 Jan;15(1):97–102. doi: 10.1016/s0264-410x(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca MO, Pang LW, de Paula Cavalheiro N, Barone AA, Heloisa Lopes M. Randomized trial of recombinant hepatitis B vaccine in HIV-infected adult patients comparing a standard dose to a double dose. Vaccine. 2005 Apr 22;23(22):2902–8. doi: 10.1016/j.vaccine.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Mansoor N, Scriba TJ, de Kock M, Tameris M, Abel B, Keyser A, et al. HIV-1 infection in infants severely impairs the immune response induced by Bacille Calmette-Guerin vaccine. J Infect Dis. 2009 Apr 1;199(7):982–90. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004 Feb;78(3):1160–8. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002 May 2;417(6884):95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 29.Geldmacher C, Schuetz A, Ngwenyama N, Casazza JP, Sanga E, Saathoff E, et al. Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV-1 infection. J Infect Dis. 2008 Dec 1;198(11):1590–8. doi: 10.1086/593017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahey T, Sheth S, Matee M, Arbeit R, Horsburgh CR, Mtei L, et al. Interferon gamma Responses to Mycobacterial Antigens Protect against Subsequent HIV-Associated Tuberculosis. J Infect Dis. Sep 2; doi: 10.1086/656332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Reyn CF, Mtei L, Arbeit RD, Waddell R, Cole B, Mackenzie T, et al. Prevention of tuberculosis in Bacille Calmette-Guerin-primed, HIV-infected adults boosted with an inactivated whole-cell mycobacterial vaccine. Aids. Jan 28; doi: 10.1097/QAD.0b013e3283350f1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lalvani A. Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin Infect Dis. 2004 Mar 1;38(5):757–9. doi: 10.1086/381763. [DOI] [PubMed] [Google Scholar]
- 33.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004 Mar 1;38(5):754–6. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 34.Hanekom WA, Dockrell HM, Ottenhoff TH, Doherty TM, Fletcher H, McShane H, et al. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med. 2008 Jul 1;5(7):e145. doi: 10.1371/journal.pmed.0050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beveridge NE, Fletcher HA, Hughes J, Pathan AA, Scriba TJ, Minassian A, et al. A comparison of IFNgamma detection methods used in tuberculosis vaccine trials. Tuberculosis (Edinb) 2008 Nov;88(6):631–40. doi: 10.1016/j.tube.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, et al. Specific T Cell Frequency and Cytokine Expression Profile do not Correlate with Protection against Tuberculosis, Following BCG Vaccination of Newborns. Am J Respir Crit Care Med. Jun 17; doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abou-Zeid C, Gares MP, Inwald J, Janssen R, Zhang Y, Young DB, et al. Induction of a type 1 immune response to a recombinant antigen from Mycobacterium tuberculosis expressed in Mycobacterium vaccae. Infect Immun. 1997 May;65(5):1856–62. doi: 10.1128/iai.65.5.1856-1862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998 Mar;93(3):307–13. doi: 10.1046/j.1365-2567.1998.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grembiale RD, Camporota L, Naty S, Tranfa CM, Djukanovic R, Marsico SA. Effects of specific immunotherapy in allergic rhinitic individuals with bronchial hyperresponsiveness. Am J Respir Crit Care Med. 2000 Dec;162(6):2048–52. doi: 10.1164/ajrccm.162.6.9909087. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez-Pando R, Pavon L, Arriaga K, Orozco H, Madrid-Marina V, Rook G. Pathogenesis of tuberculosis in mice exposed to low and high doses of an environmental mycobacterial saprophyte before infection. Infect Immun. 1997 Aug;65(8):3317–27. doi: 10.1128/iai.65.8.3317-3327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janssen R, Kruisselbrink A, Hoogteijling L, Lamb JR, Young DB, Thole JE. Analysis of recombinant mycobacteria as T helper type 1 vaccines in an allergy challenge model. Immunology. 2001 Apr;102(4):441–9. doi: 10.1046/j.1365-2567.2001.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Camporota L, Corkhill A, Long H, Lordan J, Stanciu L, Tuckwell N, et al. The effects of Mycobacterium vaccae on allergen-induced airway responses in atopic asthma. Eur Respir J. 2003 Feb;21(2):287–93. doi: 10.1183/09031936.03.00042103. [DOI] [PubMed] [Google Scholar]
- 43.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, et al. A new recombinant bacille Calmette-Guerin vaccine safely induces significantly enhanced tuberculosis-specific immunity in human volunteers. J Infect Dis. 2008 Nov 15;198(10):1491–501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheon SH, Kampmann B, Hise AG, Phillips M, Song HY, Landen K, et al. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol. 2002 Jul;9(4):901–7. doi: 10.1128/CDLI.9.4.901-907.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallis RS, Pai M, Menzies D, Doherty TM, Walzl G, Perkins MD, et al. Biomarkers and diagnostics for tuberculosis: progress, needs, and translation into practice. Lancet. May 18; doi: 10.1016/S0140-6736(10)60359-5. [DOI] [PubMed] [Google Scholar]
- 46.Edwards LB, Palmer CE. Identification of the tuberculous-infected by skin tests. Ann NY Acad Sci. 1968;154:140–8. doi: 10.1111/j.1749-6632.1968.tb16704.x. [DOI] [PubMed] [Google Scholar]
- 47.Fine PEM. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346:1339–45. doi: 10.1016/s0140-6736(95)92348-9. [DOI] [PubMed] [Google Scholar]
- 48.Edwards ML, Goodrich JM, Muller D, Pollack A, Ziegler JE, Smith DW. Infection with Mycobacterium avium-intracellulare and the protective effects of Bacille Calmette-Guerin. J Infect Dis. 1982;145:733–41. doi: 10.1093/infdis/145.2.733. [DOI] [PubMed] [Google Scholar]
- 49.Demangel C, Garnier T, Rosenkrands I, Cole ST. Differential effects of prior exposure to environmental mycobacteria on vaccination with Mycobacterium bovis BCG or a recombinant BCG strain expressing RD1 antigens. Infect Immun. 2005 Apr;73(4):2190–6. doi: 10.1128/IAI.73.4.2190-2196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


