Abstract
The present study examined whether a transient thyroid hormone (T4) deficit during infancy in male monkeys would compromise the arrest of luteinising hormone (LH) secretion during the infant–juvenile transition, and/or interfere with the pubertal resurgence of LH. Animals were orchidectomised and thyroidectomised (n = 3; Tx) or sham Tx (n = 3) within 5 days of birth. T4 replacement was initiated in two Tx monkeys at age 19 weeks to reestablish a euthyroid condition. Blood samples were drawn weekly for hormone assay. Body weight, crown–rump length, and bone age were assessed throughout the study. Within a week of Tx, plasma T4 declined to undetectable levels and, by 6–8 weeks of age, signs of hypothyroidism were evident. Transient hypothyroidism during infancy failed to prevent either arrest of LH secretion during the infant–juvenile transition or the pubertal resurgence of LH secretion, both of which occurred at similar ages to sham Tx animals. Although body weight exhibited complete catch-up with T4 replacement, crown–rump length and bone age did not. Thus, bone age at the time of the pubertal LH resurgence in Tx animals was less advanced than that in shams. Although Tx did not influence qualitatively the pattern of gonadotrophin secretion, LH levels during infancy and after pubertal LH resurgence were elevated in Tx monkeys. This was not associated with changes in LH pulse frequency and amplitude, but half-life (53 versus 65 min) of the slow second phase of LH clearance was greater in Tx animals. These results indicate that hypothalamic mechanisms dictating the pattern of gonadotrophin-releasing hormone release from birth to puberty are not dependent on T4 action during infancy, and fail to support the notion that onset of puberty is causally coupled to skeletal maturation. They also indicate that LH renal clearance mechanisms may be programmed in a T4 dependent manner during infancy.
Keywords: infancy, thyroid, primates, growth, puberty
Postnatal development of the hypothalamic–pituitary–gonadal axis in higher primates may be divided into three distinct phases: infantile, juvenile and pubertal, although, in man, the infancy and the juvenile phases of development are separated by childhood (1). Infancy in the male primate is characterised by an active period of gonadotrophin and testosterone secretion but, during the transition from infancy to childhood in boys, and directly to the juvenile phase of development in monkeys, gonadotrophin and testosterone secretion decline to low levels characteristic of subsequent prepubertal development (2, 3). This quiescent period in the activity of the pituitary–gonadal axis continues until there is a resurgence of gonadotrophin secretion that leads to the initiation of puberty at the end of juvenile development (2, 3). This postnatal pattern of activity in the pituitary–gonadal axis is considered to be dictated by parallel changes in pulsatile release of gonadotrophin-releasing hormone (GnRH) by the hypothalamus (2, 3).
Although our current understanding of the neurobiological control systems that regulate the postnatal ontogeny of pulsatile GnRH release in humans and other higher primates remains rudimentary, it is generally recognised that both structural and molecular remodeling within the developing hypothalamus are involved particularly during the infant–juvenile (on–off phase) and the juvenile–pubertal (off–on phase) transitions (3, 4). In regard to neuronal plasticity, thyroid hormone plays an important role in normal brain development and differentiation, particularly late brain development, which extends from the third trimester of gestation through the first 3 months of postnatal life in humans (5). Late brain development is characterised by neuronal cell migration, synaptogenesis, myelination, and proliferation of specific types of cells (e.g. glial); processes that are regulated by thyroid hormone either directly or indirectly (6). That structural remodeling within the hypothalamus may contribute to the on–off–on pattern of pulsatile GnRH release during postnatal development in primates is supported by findings in the male monkey, that embryonic neuronal cell adhesion molecule, a marker of neuronal plasticity (7), is expressed in the medial basal hypothalamus of prepubertal animals (8), and, synaptic input to GnRH perikarya declines in association with the pubertal resurgence of pulsatile GnRH release (9).
A GnRH dictated reversible hypogonadotrophic state reminiscent of the juvenile hiatus in GnRH release in primates is also observed during the nonbreeding season in animals such as the sheep (10, 11). Interestingly, the seasonal changes in pulsatile GnRH release in sheep are associated with plasticity in neuronal inputs to the GnRH neuronal network that is dependent on thyroid hormone (12, 13).
The foregoing considerations have led us to systematically examine the influence of thyroid hormone deficiency on the post-natal ontogeny of pulsatile GnRH release in the male monkey. The impact of a methimazole-induced thyroid hormone deficiency during juvenile development (post infantile) on the pubertal resurgence of gonadotrophin secretion was recently reported (14). The present study aimed to test the hypothesis that a transient thyroid hormone deficiency restricted to infancy would compromise the arrest of pulsatile GnRH release during the transition from infancy to the juvenile phase of development, and/or alter the characteristics of the pubertal resurgence in the release of this neuropeptide. Transient hypothyrodism during infancy was achieved by thyroidectomising the animals at 2 days of age and delaying thyroid hormone replacement until 4–5 months of age during juvenile development.
The agonadal model was again employed for several reasons. First, the hiatus in pulsatile GnRH release during juvenile development in primates occurs independently of the testis, and castration greatly amplifies the initiation and termination of this hiatus in neuroendocrine activity (15) enhancing the reliability of using circulating luteinising hormone (LH) to indirectly monitor developmental changers in pulsatile GnRH release (16). Second, gonadectomy eliminates any direct action of thyroid hormone on testicular steroid secretion and therefore the potential confounding action of testicular feedback on the developmental pattern of pulsatile GnRH release.
Materials and methods
Animals
Six neonatal male rhesus monkeys (Macaca mulatta) produced by the breeding programme of the Center for Research in Reproductive Physiology (CRRP) at the University of Pittsburgh School of Medicine, were employed in the present study. As neonates and infants, the males were housed with their mothers in individual cages at the Primate Core of the CRRP under controlled environments (usually lights on between 07.00 and 19.00 h at 20 °C; but see below). At 5–8 months of age, they were separated from their mothers and five of the six animals were housed in individual cages with another animal of comparable age. In the remaining animal (Tx), the experiment was terminated at the time of separation (18 weeks of age). Mothers and juveniles were fed a high protein monkey chow diet daily (between 10.00 and 12.00 h) supplemented with fruit in the afternoon. Water was available ad libitum. The juveniles remained paired for the duration of the study except during periods of remote venous sampling between 33 and 54 months of age (see below). The animals were maintained throughout this study according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals, and all protocols were approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
Surgical procedures and post-operative care
Thyroidectomy
Infants were Tx (n = 3) or sham Tx (n = 3) at 1 or 2 days of age (day of birth = day 0). Four animals (two Tx and two sham Tx) were anaesthetised with ketamine hydrochloride (Ketaject; Phoenix Scientific, Inc., St Joseph, MO, USA; 60–110 mg/kg body weight i.m. with supplemental doses of 20–40 mg/kg as required), and two animals were anaesthetised by inhalation via a mask of 1.5–2.5% isoflurane (Iso Flo; Abbott Laboratories, North Chicago, IL, USA) in oxygen. A local anaesthetic (0.5% bupivacaine HCl (Marcaine Hospira, Inc., Lake Forest, IL, USA) was applied to the incision site by irrigation in two of the animals that received ketamine hydrochloride (one Tx and one sham Tx). The two monkeys anaesthetised with isoflurane received atropine sulphate (0.2 mg i.m.; American Pharmaceutical Partners Inc., Shaunburg, IL, USA) and doxapram hydrochloride (one or two drops of 20 mg/ml sublingual, Doxapram; Bedford Laboratories, Bedford, OH, USA) immediately prior to surgery. A midline neck incision was made above the trachea and the sterno muscles were divided to reveal the thyroid gland. Using bipolar quartery, the two lobes of the thyroid gland and the connecting isthmus were dissected from the trachea taking care to preserve the laryngeal nerves. For sham Tx, the muscles were divided but the thyroid gland was not disturbed. Post-operatively, animals received Procaine Penicillin G (100000–150000 U i.m., Pen-G; Phoenix Pharmaceutical, Inc., St Joseph, MO, USA) and, in most cases, the analgesic, flunixin meglumine (2.5–5.0 mg i.m., Banamine; Schering-Plough Animal Health Corp., Union, NJ, USA).
Because the parathyroid glands were also removed at the time of Tx, these animals received calcium and vitamin D supplementation for the entire duration of the study. From the time of Tx until weaning, this was provided orally with calcium glubionate syrup (Calcionate syrup; Rugby Laboratories Inc., Duluth, GA, USA or Neo-Calglucon; Sandoz Pharmaceuticals Co., East Hanover, NJ, USA) and dihydrotachysterol (0.2 mg/ml Intensol; Roxane Laboratories Inc., Columbus, OH, USA) administered daily or every other day. The calcium glubionate syrup (1.8 g/ml) was diluted 1 : 8 with a 10% aqueous sucrose solution. Tx animals received 50–400 mg calcium glubionate/day and between 40–140 μg/day of dihyrodrotachysterol (equivalent to 4800–17000 U vitamin D activity). Sham animals received a comparable volume of sugar water. Following weaning, vitamin D supplementation was provided with daily or every other day s.c. injections of ergocalciferol (vitamin D2) in oil (250000 U/ml; Heibers Pharmacy, Pittsburgh, PA, USA). The dose of ergocalciferol decreased with age and ranged from approximately 1000–17000 U/day. For calcium supplementation, the juvenile Tx animals were initially given twice daily s.c. injections of 2.3 ml calcium gluconate (100 mg/ml; Presbyterian University Hospital Pharmacy, Pittsburgh, PA, USA). After 3–4 weeks, calcium supplementation was provided in tablet form (½ to one Caltrate 600D tablet; Wyeth Consumer Healthcare, Madison, NJ, USA) every day or every other day. The calcium tablet, which also contained vitamin D activity (400 U/tablet) was powdered and placed into a piece of banana. Sham animals received control injections of saline and were given a piece of banana at the same time the Tx received their calcium supplementation.
Rectal temperature was usually monitored every other day. Classical signs of hypothyroidism began to appear in the Tx animals within 6–8 weeks after surgery. These included but were not limited to myxedema, lethargy, growth retardation and hypothermia. As a consequence of the hypothermia, two of the Tx animals and their mothers were moved into a humidified animal room with an elevated environmental temperature (25–28 °C). Because of their myxedemic condition, these two animals were also treated daily with a moisturiser cream.
Orchidectomy
Bilateral orchidectomy (Orx) was conducted at 2–5 days of age with two animals being castrated at the time of Tx or sham Tx. In the remaining animals, the second surgery was conducted within 4 days of Tx or sham Tx using ketamine hydrochloride (50–110 mg/kg b.w.) and irrigating with bupivacaine as necessary. Post-operatively, the animals received a second injection of the long acting penicillin and the analgesic, flunixin meglumine.
Venous catheterisation
The monkeys were first adapted to a jacket and tether system and then received an indwelling venous catheter (jugular or femoral; inner diameter 0.040, outer diameter 0.085; Stuart Bio-Sil, Sil-Med Corp., Tauton, MA, USA) following sedation with ketamine hydrochloride (8–10 mg/kg b.w. i.m.), and anaesthesia with 1–2.5% isoflurane as described on many previous occasions (17–19). The animals received standardised antibiotic and analgesia medication consisting of a single prophylactic injection of penicillin (300000 U i.m.; Phoenix Scientific Inc., St Joseph, MO, USA) on the day of surgery and for 3 days thereafter. Analgesia was achieved with ketoprofen (2 mg i.m. twice daily for 4 days; Fort Dodge Animal Health, Fort Dodge, IA, USA).
Thyroid hormone replacement
Thyroid hormone replacement was achieved with daily i.m. injections of thyroxine (T4, 2.0 μ/kg b.w., Levothyroxine sodium for injection; Bedford Laboratories, Bedford, OH, USA). Sham Tx animals received daily i.m. injections of saline.
Experimental protocol
There were three phases to the experimental protocol. The first addressed the question of whether hypothyroidism during infancy would interfere with the suppression of gonadotrophin secretion that in agonadal males is normally observed between 2 and 8 months of age in the euthyroid state (15). The second phase examined the influence of the transient hypothyroidism during infancy on the time-course of the subsequent pubertal resurgence in gonadotrophin secretion that is observed at approximately 26 months of age in control animals (range 24–30 months) (15). The last phase examined the effects of the transient hypothyroidism during infancy on pulsatile patterns of LH secretion and on LH clearance following completion of the pubertal resurgence in gonadotrophin secretion.
Phase 1: infant–juvenile transition
Starting at 1 week of age, blood samples were drawn weekly throughout the study by femoral venipuncture (between 09.00 and 11.00 h) when the animals were lightly sedated with ketamine hydrochloride (5–10 mg/kg b.w. i.m.) for calcium and hormone determinations. Crown–rump length was recorded monthly under additional ketamine hydrochloride sedation (40–60 mg/kg b.w. i.m.) as previously described (20). During phase 1, an initial bone age assessment was made for each animal. Radiographs of the wrist and hands were taken while animals were sedated for crown–rump length measurements. Bone age was assessed by comparing radiographs from each animal with similar images in a roentgenographic atlas of postnatal skeletal development in normal male rhesus monkeys (21), as previously described (14).
Phase 2: juvenile–pubertal transition
Phase two of the experiment began at 19 weeks of age when T4 replacement was initiated in two Tx animals and continued until 33 months of age. The three sham Tx monkeys received control injections of saline during this period. Animals were sedated with ketamine hydrochloride at approximately weekly intervals for venepuncture and determination of rectal temperature. Crown–rump length was recorded monthly and bone age was assessed every 3–4 months.
Phase 3: LH pulsatility and clearance
The last phase of the study began after the pubertal resurgence of gonadotrophin secretion had plateaued. T4 replacement was continued without interruption throughout the final phase of the study. Between 33 and 54 months of age, monkeys were implanted with an indwelling venous catheter, fitted with a jacket and tether and housed in specialised cages that allowed continuous access to the venous circulation without sedation and with minimal restraint as previously described (17–19).
Pulsatile LH secretion
To compare the characteristics of pulsatile LH secretion between Tx and sham Tx animals, a series of blood samples (0.4–0.6 ml) was collected at 10-min intervals beginning at 12.00 h and continuing until 24.00 h from each of the monkeys as previously described (17–19).
LH clearance
To examine LH clearance, endogenous LH secretion was first abolished by three daily i.m. injections of the GnRH receptor antagonist, acyline (60 μg/kg b.w./day). On the last day of acyline administration, a single i.v. bolus injection of recombinant monkey (rm) LH (2 μg/kg in 1 ml sterile DPBS) was administered at 09.00 h (time 0). Blood samples (1.0 ml) were collected via the indwelling venous catheter approximately 15 min before and at 1, 2.5, 5, 10, 20, 40, 60, 80, 120, 180, and 240 min after the injection of LH. Acyline was obtained from the Contraceptive and Reproductive Health Branch, Center for Reproductive Sciences, NICHD, and rmLH (AFP6936A) was obtained from the National Hormone and Pituitary Programme, NIDDK.
Hormone and calcium assays
Plasma or serum LH and follicle-stimulating hormone (FSH) were determined by radioimmunoassay using homologous reagents provided by the National Hormone and Peptide Programme as previously described (22, 23). rmLH (AFP6936A) and rcFSH (AFP6940A) were used as the respective standards. The sensitivities of the LH and FSH assays were routinely in the 0.1–0.2 ng/ml range, respectively. The intra- and inter-assay coefficients of variation for the LH and FSH assays, respectively, were 4.9% and 15.6%, and 8.5% and 22.8%. Total T4 levels in the plasma were measured by a commercially available radioimmunoassay kit from Diagnostic Products Corp. (Los Angeles, CA, USA). The sensitivity of the T4 assay was 0.4 μg/dl. The intra- and inter-assay coefficients of variation for the T4 assays were 5.9% and 8.4%, respectively. Serum calcium measurements were conducted by the Clinical Chemistry Laboratory (University of Pittsburgh Medical Center, Pittsburgh, PA, USA).
Statistical analysis
Data for hormone levels and body weight during early infancy (1–18 weeks of age), for hormone levels after the age of pubertal LH resurgence (28–33 months of age), and somatic parameters over the duration of the study (1 week–33 months of age) were analysed by two-way anova (treatment versus time) with repeated measures over time. A pubertal resurgence of LH secretion was identified as a sustained elevation in plasma LH levels to 1.0 ng/ml or greater, and age at the initiation of this elevation in circulating LH was designated the age of the pubertal LH resurgence as previously described (14). The juvenile phase of diminished gonadotrophin secretion was defined as the period during which LH concentrations were < 1.0 ng/ml. Data for the age of pubertal LH resurgence, duration of the juvenile phase of diminished gonadotrophin secretion, and body weight, crown–rump length and bone age at the time of pubertal LH resurgence were analysed by one-way anova. Episodes of LH secretion (pulses) during periods of sequential sampling were identified by Pulsar, an algorithm for assessing pulsatile hormone secretion as previously described (24, 25). The resulting values for mean LH levels, pulse amplitudes, pulse number, and inter-peak intervals were analysed by one-way anova.
To analyse LH clearance rates, data for circulating LH concentrations following administration of the i.v. pulse of rmLH were first plotted in semilog form (log time versus LH concentration). Linear regressions were performed on the plot for each animal for two periods: 0–10 min post-LH administration and 10–240 min post-LH administration. The two-period model was used since similar LH clearance patterns involving an initial fast half-life followed by a slow half-life have been observed in humans (26) and rats (27, 28). Using the slopes of the lines obtained from these regressions, LH half-life was computed for each monkey for each of the two periods. Differences in the resulting LH half-life values between control and Tx animals were analysed by one-way anova.
Results
Serum calcium
In Tx animals, serum calcium ranged between 6–12 mg/dl, which compared to a range of 9–11 mg/dl for sham Tx animals. Calcium levels in Tx animals only rarely fluctuated out of the range observed for the sham controls (data not shown).
Phase 1: infant–juvenile transition
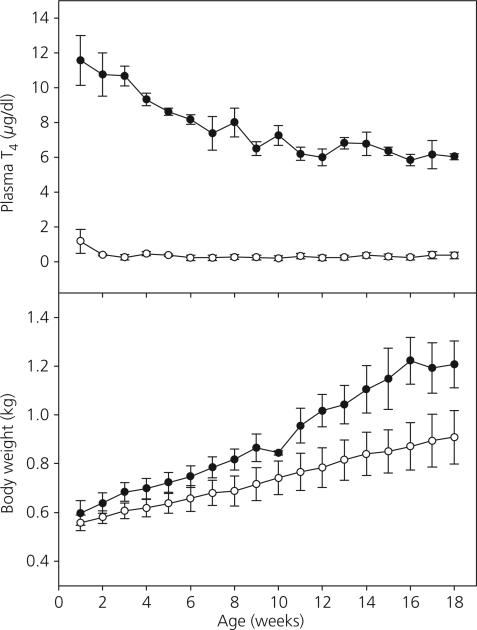
As shown in Fig. 1 (top panel), Tx during the first 3 days of postnatal life in male monkeys resulted in a profound decline in plasma T4 levels (first blood sample taken at 1 week of age) that was maintained until 18 weeks of age at which time this phase of the study was terminated. Within 2 weeks of removing the thyroid gland, circulating T4 levels were near the minimal detectable concentration (0.4 μg/dl) and were significantly lower than those in the sham Tx animals (P < 0.0001) during the 18-week period. In sham Tx animals, T4 levels declined significantly (P < 0.0001) from a high at 1 week of age (approximately 12 μg/dl) to values of 5–7 μg/dl by 18 weeks of age. Classical signs of hypothyroidism began to appear in the animals within 6–8 weeks after surgery. These included but were not limited to myxedema, lethargy, growth retardation and hypothermia. Over the first 18 weeks of postnatal life, there was a trend toward reduced somatic growth (reduced body weight gain) in the Tx animals (Fig. 1, bottom panel), although body weight differences between Tx and control animals were not statistically significant.
Fig. 1.
Mean ± SEM plasma total thyroxine (T4) concentrations (top panel) and body weight (bottom panel) in thyroidectomised (Tx, open circles) and sham Tx (solid circles) agonadal male monkeys from 1–18 weeks of age. T4 concentrations during this period were significantly reduced in Tx animals relative to shams (P < 0.0001).
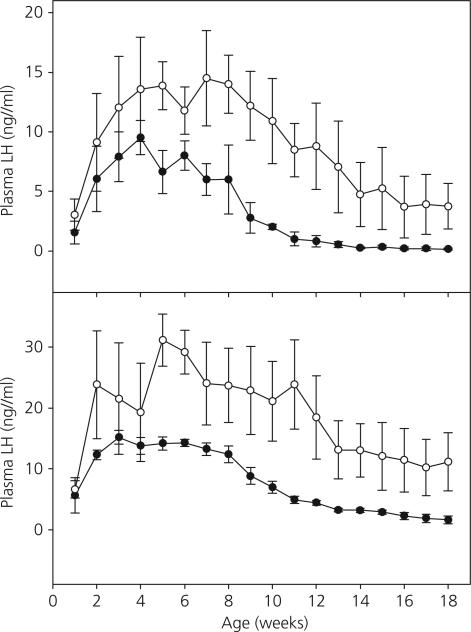
The time courses of changes in mean concentrations of circulating LH and FSH during the first 18 weeks of life in the Tx and sham Tx group were qualitatively similar (Fig. 2). As expected in both the Tx and sham Tx groups, mean concentrations of the gonadotrophins increased progressively over the first 4–5 weeks of postnatal life (Fig. 2). Plasma LH and FSH levels then plateaued in both groups before declining regardless of thyroid hormone status. Quantitative differences in the early developmental pattern of both LH and FSH levels, however, were observed between the two groups. Notably, plasma LH concentrations were higher overall during the infantile period in the Tx group compared to the controls (P < 0.05) and declined more slowly (interaction of treatment · time, P < 0.001) in the Tx group than in the controls during the later half of the infantile period. Circulating LH concentrations in sham Tx monkeys had declined to < 1 ng/ml by an average age of 11.67 ± 0.67 weeks (2.67 ± 0.17 months). On the other hand, in two out of the three Tx animals, LH levels had not fallen below 1.0 ng/ml at the termination of this phase of the study. Plasma FSH concentrations during infancy were also higher overall (P < 0.01) in the Tx group compared to the controls.
Fig. 2.
Mean ± SEM plasma luteinising hormone (LH) concentrations (top panel) and plasma follicle-stimulating hormone (FSH) concentrations (bottom panel) in thyroidectomised (Tx, open circles) and sham Tx (solid circles) agonadal male monkeys from 1–18 weeks of age. LH concentrations during this period were higher overall in Tx animals (P < 0.05) and declined more slowly (P < 0.001) than in the sham group. FSH concentrations during this period were also higher overall (P < 0.05) in the Tx animals than in shams.
Phase 2: juvenile–pubertal transition
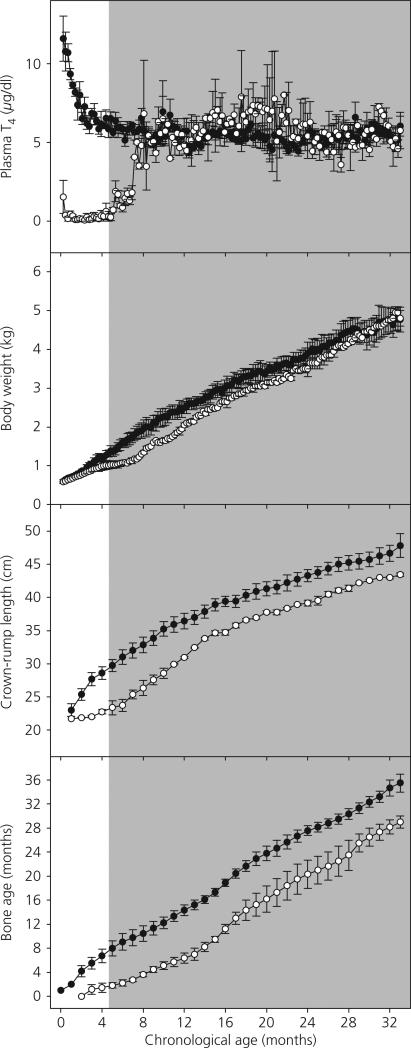
Following the initiation of T4 replacement in two Tx animals at 19 weeks of age, plasma T4 concentrations increased over a 3-month period to reach at approximately 8 months of age values similar to those of sham Tx animals (Fig. 3, top panel). Plasma T4 concentrations in Tx and sham Tx remained indistinguishable for the duration of this phase of the experiment, which terminated at 33 months of age. Body temperature in the Tx animals returned to the normal range as the T4 replacement took effect (data not shown). By 2 months of T4 replacement, myxedema was no longer apparent in the Tx animals and the use of moisturiser cream was discontinued.
Fig. 3.
Mean ± SEM plasma total thyroxine (T4) concentrations (top panel), body weight (second panel), crown–rump length (third panel) and bone age (bottom panel) in thyroidectomised (Tx, open circles) and sham Tx (solid circles) agonadal male monkeys from 1 week to 33 months of age. The shaded area represents the period of T4 replacement in Tx animals. Body weight and crown–rump in Tx animals exhibited accelerated growth rates (P < 0.01) once T4 replacement was initiated and, by the end of the study period, were not significantly lower than in controls. Throughout the entire study period, bone age remained significantly lower overall in Tx animals (P < 0.01) relative to shams.
Body weight (Fig. 3, second panel from top), which had begun to diverge between the two groups by the end of phase 1 of the study (i.e. 4–5 months of age), continued to be depressed in the Tx animals relative to the shams for the first several months of T4 replacement. The divergence in body weight between the groups appeared even greater for the initial months following the start of T4 replacement, and this probably reflected a reversal of the myxedematous state. After approximately 3 months of replacement, the increase in body weight accelerated in the Tx group (significant interaction effect on body weight of treatment and time, P < 0.01) and, by the end of the study period, body weight in the Tx animals was in the control range. Linear growth, as measured by crown–rump length (Fig. 3, third panel from top), showed a similar pattern (no overall effect of treatment but a significant interaction of treatment and time, P < 0.01). Bone age (Fig. 3, bottom panel) was significantly retarded in the Tx animals relative to the shams throughout the entire study period (P < 0.05).
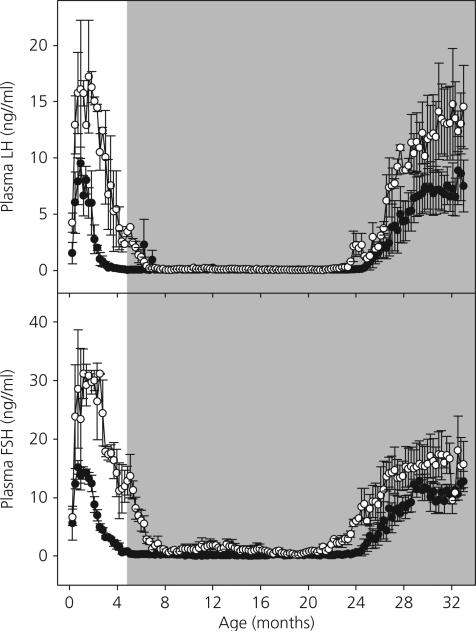
The changes in mean concentrations of circulating LH and FSH from the time of initiating T4 replacement until the termination of phase 2 at 33 months of age are shown in Fig. 4. The LH and FSH results obtained during phase 1 are again presented in Fig. 4 for overall perspective. During the first 2–3 months of T4 replacement, plasma LH and FSH concentrations continued to decline in the Tx monkeys reaching values similar to those in sham Tx animals by 7–8 months of age. Circulating LH levels in both groups were subsequently maintained at very low levels, frequently below the minimal detectable concentration, until the pubertal resurgence in LH secretion was initiated at 23.9 ± 0.1 months of age in the Tx monkeys and at 26.1 ± 0.8 months of age in the sham Tx animals (difference not statistically significant). The duration of the juvenile phase of diminished gonadotrophin secretion (LH concentrations < 1.0 ng/ml) was shorter (P < 0.05) in the Tx group than in the controls (17.8 ± 0.5 versus 23.4 ± 0.9 months, respectively). Interestingly, mean concentration of circulating LH levels in Tx animals appeared to attain a final plateau that was noticeably greater than that in the sham Tx group. This difference, however, was not statistically significant.
Fig. 4.
Mean ± SEM plasma luteinising hormone (LH) concentrations (top panel) and follicle-stimulating hormone (FSH) concentrations (bottom panel) in thyroidectomised (Tx) (open circles) and sham Tx (solid circles) agonadal male monkeys from 1 week to 33 months of age. The shaded area represents the period of T4 replacement in Tx animals. The duration of the juvenile phase of diminished gonadotrophin secretion (LH concentrations < 1.0 ng/ml) was significantly shorter in the Tx monkeys than in controls (P < 0.05).
The developmental differences in plasma FSH levels (Fig. 4, bottom panel) between the two treatment groups were qualitatively and quantitatively similar to those in plasma LH (Fig. 4, top panel).
Somatic parameters at the time of the pubertal resurgence in LH levels are summarised in Table 1. Although chronological age at the time of this endocrine event did not differ between Tx and sham animals, bone age and linear growth were less advanced at the time of the pubertal resurgence in LH in Tx monkeys than in sham animals. Body weight at the time of the pubertal LH rise showed a similar trend in the two groups but did not reach significance.
Table 1.
Mean Age at Puberty, Body Weight at Puberty, Crown-Rump Length at Puberty and Bone Age at Puberty in Control and Thyroidectomised (Tx) Monkeys.
| Age at puberty (months) | Body weight at puberty (kg) | Crown-rump at puberty (cm) | Bone age at puberty (months) | |
|---|---|---|---|---|
| Sham | 26.1 ± 0.8 | 4.2 ± 0.3 | 44.3 ± 0.8 | 29.0 ± 1.3 |
| Tx | 23.9 ± 0.1 | 3.6 ± 0.2 | 39.2 ± 0.5* | 20.4 ± 2.4* |
Significantly lower than control value (P < 0.05).
Phase 3: LH pulsatility and clearance
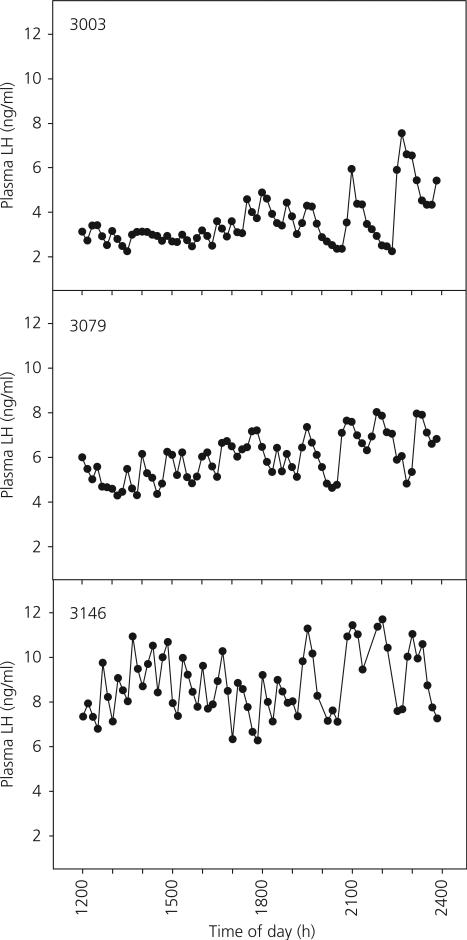
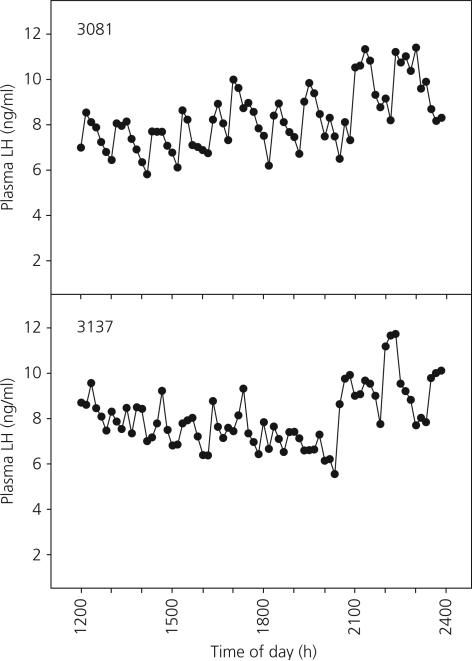
Pulsatile patterns of plasma LH levels over the 12-h experimental period in the two Tx and three shams are shown in Figs 5 and 6, respectively. There were no significant differences in mean plasma LH levels, pulse number, pulse amplitude or inter-peak interval between the sham and Tx monkeys (Table 2).
Fig. 5.
Moment to moment changes in plasma luteinising hormone (LH) concentrations in individual sham thyroidectomised agonadal male monkeys over a 12-h period of sequential sampling (sampling interval = 10 min) 8–26 months following the pubertal resurgence in LH secretion at 25–28 months of age. At the time of sampling, the ages of animals 3003, 3079 and 3146 were 54, 45 and 33 months, respectively.
Fig. 6.
Moment to moment changes in plasma luteinising hormone (LH) concentrations in two thyroidectomised agonadal male monkeys over a 12-h period of sequential sampling (sampling interval = 10 min) 12–20 months following the pubertal resurgence in LH secretion at 24 months of age. At the time of sampling, the ages of animals 3081 and 3137 were 44 and 35 months, respectively.
Table 2.
Mean Circulating Luteinising Hormone (LH) Levels, Numbers of Pulse Peaks, Amplitudes of Pulses and Inter-Peak Intervals in Control and Thyroidectomised (Tx) Postpubertal Monkeys Sampled Every 10 min Over a 12-h Period.
| Mean LH (ng/ml) | Number of pulses | Pulse amplitude (ng/ml) | Inter-peak interval (min) | |
|---|---|---|---|---|
| Sham | 6.07 ± 1.48 | 17.17 ± 0.73 | 2.05 ± 0.43 | 43.71 ± 2.31 |
| Tx | 8.30 ± 0.01 | 18.00 ± 1.00 | 2.11 ± 0.30 | 40.11 ± 1.77 |
Following an i.v. bolus injection of rmLH, LH concentrations declined in two phases: an initial period of rapid decline followed by a second period of slower decline. Linear regression analysis on the first four time points (time period 1; 0–10 min after the LH injection) and the last seven time points (time period 2; 10–240 min after the LH injection) for each individual animal provided the best overall fit (P < 0.05 confidence level in all cases) and these results were used to calculate the half-life of circulating LH (Table 3). Although there was no difference in LH half-life between sham and Tx animals during time period 1, during time period 2 the LH clearance was significantly greater in the Tx animals.
Table 3.
Mean (±SEM) Half-Lives of Circulating Luteinising Hormone (LH) in Agonadal Male Rhesus Monkeys Treated with LH Antagonist for 3 days Followed by a Single i.v. LH Pulse (2 μg/kg b.w.).
| Half-life (min) of plasma LH Time period 1 (0-10 min after LH administration) | Half-life (min) of plasma LH Time period 2 (10-240 min after LH administration) | |
|---|---|---|
| Sham | 4.02 ± 0.45 | 52.67 ± 2.67 |
| Tx | 4.75 ± 0.35 | 65.07 ± 0.20* |
Significantly higher than control value (P < 0.05).
Discussion
The major finding from the present study was that the qualitative pattern of gonadotrophin secretion during postnatal development in agonadal male monkeys was preserved in the face of a transient and profound hypothyroidism imposed during the first 4–5 months of postnatal life. This indicates that the hypothalamic mechanisms governing pulsatile GnRH release from infancy until puberty are largely independent of thyroid hormone action during early postnatal development. This is surprising because it is generally recognised that the absence of thyroid hormone during a critical period after birth leads to irreversible deficits in the development and differentiation of the brain (6). The transient absence of thyroid hormone during infancy in the monkey failed to interrupt either the suppression of pulsatile GnRH release during the infant–juvenile (on–off) transition or the pubertal resurgence of GnRH pulse generator activity during the subsequent juvenile-pubertal transition (off–on). As discussed in the Introduction, it has been suggested that structural and molecular remodeling within the developing hypothalamus play important roles in the control of both these profound developmental changes in pulsatile GnRH release. In the case of the on–off transition in GnRH pulse generator activity, which in the present study was initiated at 2–3 months of age in both sham and hypothyroid infant monkeys, it is reasonable to conclude that either plasticity in the hypothalamus during this phase of development is not obligatory for the ‘turn off’ in pulsatile GnRH release or is thyroid hormone independent. Similarly, the timing of the pubertal resurgence in pulsatile GnRH release was not perturbed by the transient hypothyroidism during infancy indicating that the initiation of the onset of puberty is independent of thyroid hormone dependent programming in the hypothalamus during infancy.
Because the thyroidectomised animals received thyroid hormone replacement during juvenile development, the present study does not address the role of thyroid hormone action during the juvenile–pubertal transition. In this regard, we have shown recently that a methimazole-induced profound hypothyroid state initiated at 15–19 months of age in the agonadal juvenile monkey leads to a pronounced delay in the pubertal resurgence of pulsatile GnRH secretion (14), and a thyroid hormone deficit in children is usually associated with retarded pubertal development (29–31). The present finding that a transient hypothyrodism during infancy had a minimal effect on the postnatal pattern of gonadotrophin secretion, including the pubertal resurgence in LH release, may be contrasted with the results of our earlier study in which methimazole induced hypothyroidism during juvenile development prevented or delayed the pubertal activation of gonadotrophin secretion. At the present time, however, we are reluctant to conclude that the hypothalamic network governing pulsatile GnRH release is more sensitive to the direct action of thyroid hormone at the time of puberty than at early stages of postnatal development. This is because it is not known whether the impact of thyroid hormone on GnRH pulse generator activity during peripubertal development is the result of a direct action of thyroid hormone on the hypothalamus or is mediated by a circulating signal reflecting thyroid hormone dependent growth and somatic development.
It is recognised that the number of animals employed in the present study was limited; a strategic decision that was based on the following considerations. First, the maintenance of hypothyroid infant monkeys in accord with accepted principles of animal care represented a major and unremitting challenge and, second, the major finding that thyroidectomy during infancy failed to arrest the decline in gonadotrophin secretion during the infant–juvenile transition, while robust, was negative. Thus, it was considered that examining additional animals to substantiate the negative finding was unjustified.
Although the qualitative pattern of developmental changes in LH and FSH secretion was unaltered by the transient thyroid hormone deficit during infancy, quantitative differences in LH levels in the circulation were demonstrated between sham controls and Tx monkeys. For example, the magnitude of the rise in both plasma LH and FSH levels during infancy was two-fold greater in Tx animals than in sham controls and the rate of decline in plasma LH concentrations during the infant–juvenile transition in Tx monkeys was less than that in sham Tx animals. Furthermore, the duration of the juvenile phase of diminished gonadotrophin secretion was significantly shorter in Tx animals versus sham controls. This was because the age at which circulating LH concentrations fell below 1 ng/ml, the threshold defining the initiation of the juvenile phase, was greater in Tx animals than that in the shams. Additionally, LH and FSH concentrations attained following the pubertal resurgence of pulsatile GnRH release were marginally higher in Tx animals.
That the quantitative differences in the developmental pattern of circulating LH levels reflect a thyroid dependent programming during infancy of mechanisms responsible for the clearance of circulating gonadotrophin rather than subtle changes in parameters of pulsatile GnRH release is suggested by the finding that LH pulse amplitude and inter-LH pulse interval following the pubertal resurgence of pulsatile GnRH release in Tx and sham Tx monkeys were indistinguishable. In the present study, it should be noted that the pulsatile pattern of LH secretion (number, duration and amplitude of peaks) in women were reported to be unaffected by a hypothyroid condition (32) and, in general, the nature of the pulsatile pattern of LH secretion did not differ between euthyroid and athyroid agonadal adult rats (33).
The clearance of LH from the circulation in humans and rodents has been shown to occur biexponentially with an initial fast half-life phase followed by a slow half-life phase (26–28). The rapid clearance phase has been attributed to hepatic function (34–36), although the kidney may also be involved (37). The hepatic clearance mechanism involves the binding of terminal sulphated oligosaccharides of the LH glycoprotein to receptors in endothelial cells (34–36). LH is then internalised and quickly degraded (34, 38). The slow component of LH clearance is by the kidney either through degradation or excretion in the urine, and the renal mechanism(s) accounts for the greater proportion (85% or more) of overall elimination of the gonadotrophin (37, 39). In the present study, exogenous rmLH was cleared from the circulation of monkeys in a biphasic pattern similar to that reported for humans and rodents. During the rapid phase, the half-life of LH in sham Tx animals was just 4 min and did not differ in Tx animals that had been exposed to a transient thyroid hormone deficit during infancy. By contrast, during the slow phase the half-life of LH in Tx monkeys was significantly longer (65 versus 53 min). These data suggest that the transient hypothyroid state may have permanently altered the ability of the kidney to process and clear LH during the slow phase of the clearance process.
Growth retardation, as demonstrated by a reduced rate of body weight gain, linear growth and skeletal maturation, was observed in Tx monkeys subjected to a transient thyroid hormone deficiency during infancy. Interestingly, the effects of the thyroid hormone deficit had a more pronounced effect on linear growth and bone maturation than on body weight gain and, although catch-up was evident for body weight gain with thyroid hormone replacement, linear growth and skeletal maturation showed little catch-up during the 2.5 years of hormone replacement. These data appear to be consistent with results from clinical studies. In children, the effects of hypothyroidism on body weight gain are usually less apparent than those on linear growth because the patients tend to be over-weight (40). In human infants in which congenital hypothyroidism is diagnosed early in postnatal life and treated appropriately, growth and skeletal maturation are appropriate for chronological age (41, 42). If, on the other hand, treatment of hypothyroid condition is delayed, catch-up growth may be compromised and adult height lower than predicted (43, 44).
The onset of puberty in man under both normal and pathophysiological conditions is generally recognised to be more closely associated with bone age than with chronological age (45). Thus, if bone age is advanced, puberty is likely to be premature and if bone age is retarded puberty is likely to be delayed. These clinical observations led to the notion that the onset of puberty may be timed by a central neural growth tracking device (somatometer) that is capable of registering a component of somatic development by monitoring a circulating signal produced for example by developing bone (46). In a previous study employing a limited number of agonadal male monkeys, treatment with either testosterone or oestradiol during the first year of life consistently led to advanced bone maturation, but a precocious pubertal resurgence of LH secretion was only observed following testosterone treatment. (20). The present study, in which bone maturation was perturbed by a steroid independent mechanism, provides a further example of a dissociation between bone maturation and the hypothalamic events that trigger puberty. Specifically, the pubertal resurgence of LH secretion in the Tx animals occurred at a dramatically younger bone age (20 months) than that observed in the sham Tx animals (29 months) or in untreated agonadal males (28 and 29 months) studied previously by us (14, 20). Together, these two sets of data appear to rule out a direct link between skeletal maturation and the timing of pubertal LH resurgence in higher primates, although it does not eliminate the possibility that a certain level of skeletal development needs to be achieved before puberty can occur. The view is consistent with a recent finding in normal boys that bone age at puberty was no less variable than that of chronological age at puberty (47).
Acknowledgements
We thank David S. Zorub, who demonstrated to one of us (T.M.P.) the procedure of thyroidectomy. We also thank Robert Beidler, Michael Cicco, Rachel Rosland and Lisa Neiman Vento of the Primate Core of the Pittsburgh Specialised Cooperative Centers Programme in Infertility and Reproduction (SCCPIR) for their dedication and commitment to maintaining the well being of the experimental animals during infancy. We also acknowledge the contribution of Carolyn Phalin (Assay Core, Pittsburgh SCCPIR), who conducted the radioimmunoassays, and Meloni DiPietro, who tracked endocrine and somatic parameters on the animals throughout some of the study. We also gratefully acknowledge the statistical support of Dr Lewis VanBrackle (Department of Mathematics, Kennesaw State University, Kennesaw, GA, USA). The study was supported by NIH grants HD41749, RR03034 and RR18386 to Morehouse School of Medicine and HD08610 and HD13254 to University of Pittsburgh School of Medicine and a preliminary report of this work was presented at the 87th Annual Meeting of the Endocrine Society, Abstract # P93-8, SanDiego, CA, USA.
References
- 1.Bogin B. Growth and development: recent evolutionary and biocultural research. In: Boaz N, Wolfe LD, editors. Biological Anthropology: The State of the Science. International Institute for Human Evolutionary Research; Bend, Oregon: 1995. pp. 49–70. [Google Scholar]
- 2.Witchel SF, Plant TM. Puberty: gonadarche and adrenarche. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe's Reproductive Endocrinology. 5th edn. Elsevier; Philadelphia: 2004. pp. 493–535. [Google Scholar]
- 3.Plant TM, Witchel SF. Puberty in nonhuman and human primates. In: Neill JD, Plant TM, Pfaff DW, Challis JRG, deKretser DM, Richards JS, Wassarman PM, editors. Knobil and Neill's Physiology of Reproduction. 3rd edn. Elsevier; Amsterdam: 2006. pp. 2177–2230. [Google Scholar]
- 4.Plant TM. Gonadotropin-releasing hormone neuron remodeling: causal for puberty onset? Trends Endocrinol Metab. 2007;18:50–51. doi: 10.1016/j.tem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Fisher DA, Brown RS. Thyroid physiology in the perinatal period and during childhood. In: Braverman LE, Utiger RD, editors. Werner Ingbar's The Thyroid: A Fundamental and Clinical Text. 8th edn. Lippincott Willams & Wilkins; Philadelphia: 2000. pp. 959–972. [Google Scholar]
- 6.Anderson GW. Thyroid hormone and the brain. Front Neuroendocrinol. 2001;22:1–17. doi: 10.1006/frne.2000.0208. [DOI] [PubMed] [Google Scholar]
- 7.Theodosius DT, Poulain DA. Neuronal-glial and synaptic remodeling in the adult hypothalamus in response to physiological stimuli. In: Chadwick DJ, Jarsh J, editors. Functional Anatomy of the Neuroendocrine Hypothalamus. Wiley, Ciba Foundation Symposium; Chichester: 1992. pp. 209–232. [DOI] [PubMed] [Google Scholar]
- 8.Perera AD, Lagenaur CF, Plant TM. Postnatal expression of polysialic acid-neural cell adhesion molecule in the hypothalamus of the male rhesus monkey (Macaca mulatta). Endocrinology. 1993;133:2729–2735. doi: 10.1210/endo.133.6.7694845. [DOI] [PubMed] [Google Scholar]
- 9.Perera AD, Plant TM. Ultrastructural studies of neuronal correlates of the pubertal reaumentation of hypothalamic gonadotropin-releasing hormone (GnRH) release in the rhesus monkey (Macaca mulatta). J Comp Neurol. 1997;385:71–82. doi: 10.1002/(sici)1096-9861(19970818)385:1<71::aid-cne4>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 10.Dahl GE, Evans NP, Thrun LA, Karsch FJ. Thyroxine is permissive to seasonal transitions in reproductive neuroendocrine activity in the ewe. Biol Reprod. 1995;52:690–696. doi: 10.1095/biolreprod52.3.690. [DOI] [PubMed] [Google Scholar]
- 11.Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Thyroid hormones mediate steroid-independent seasonal changes in luteinizing hormone pulsatility in the ewe. Biol Reprod. 2002;66:701–706. doi: 10.1095/biolreprod66.3.701. [DOI] [PubMed] [Google Scholar]
- 12.Xiong J, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in synaptic inputs into GnRH neurons. Endocrinology. 1997;138:1240–1250. doi: 10.1210/endo.138.3.5000. [DOI] [PubMed] [Google Scholar]
- 13.Janson HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144:3663–3676. doi: 10.1210/en.2002-0188. [DOI] [PubMed] [Google Scholar]
- 14.Mann DR, Bhat GK, Stah CD, Pohl CR, Plant TM. Induction of a hypothyroid state during juvenile development delays pubertal reactivation of the neuroendocrine system governing luteinizing hormone secretion in the male rhesus monkey (Macaca mulatta). J Neuroendocrinol. 2006;18:662–671. doi: 10.1111/j.1365-2826.2006.01460.x. [DOI] [PubMed] [Google Scholar]
- 15.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116:1345–1350. doi: 10.1210/endo-116-4-1341. [DOI] [PubMed] [Google Scholar]
- 16.Plant TM. Gonadal regulation of hypothalamic gonadotropin-releasing hormone release in primates. Endocr Rev. 1986;7:75–88. doi: 10.1210/edrv-7-1-75. [DOI] [PubMed] [Google Scholar]
- 17.Suter KJ, Pohl CR, Plant TM. The pattern and tempo of the pubertal reaugmentation of open-loop pulsatile gonadotropin-releasing hormone release assessed indirectly in the male rhesus monkey (Macaca mulatta). Endocrinology. 1998;139:2774–2783. doi: 10.1210/endo.139.6.6055. [DOI] [PubMed] [Google Scholar]
- 18.Barker-Gibb ML, Sahu A, Pohl CR, Plant TM. Elevating circulating leptin in prepubertal male rhesus monkeys (Macaca mulatta) does not elicit precocious gonadotropin-releasing hormone release, assessed indirectly. J Clin Endocrinol Metab. 2002;87:4976–4983. doi: 10.1210/jc.2002-020784. [DOI] [PubMed] [Google Scholar]
- 19.Ramaswamy S. Pubertal augmentation in juvenile rhesus monkey testosterone production induced by invariant gonadotropin stimulation is inhibited by estrogen. J Clin Endocrinol Metab. 2005;90:5866–5875. doi: 10.1210/jc.2005-0092. [DOI] [PubMed] [Google Scholar]
- 20.Fraser MO, Arslan M, Plant TM. Androgen and estrogen treatment, alone or in combination, differentially influences bone maturation and hypothalamic mechanisms that time puberty in the male rhesus monkey (Macaca mulatta). Pediatric Res. 2005;57:141–148. doi: 10.1203/01.PDR.0000148063.68338.A0. [DOI] [PubMed] [Google Scholar]
- 21.Michejda M. Skeletal Development of the Wrist and Hand in Macaca mulatta and Man. Karger; Basel: 1987. [Google Scholar]
- 22.ElMajdoubi M, Sahu A, Ramaswamy S, Plant TM, Neuropeptide Y. A hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA. 2000;97:6179–6184. doi: 10.1073/pnas.090099697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramaswamy S, Pohl CR, McNeilly AS, Winters SJ, Plant TM. The time course of follicle-stimulating hormone suppression by recombinant human inhibin A in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 1998;139:3409–3415. doi: 10.1210/endo.139.8.6125. [DOI] [PubMed] [Google Scholar]
- 24.Merriam GR, Wachter KW. Algorithms for the study of episodic hormone secretion. Am J Physiol. 1982;243:E310–E318. doi: 10.1152/ajpendo.1982.243.4.E310. [DOI] [PubMed] [Google Scholar]
- 25.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Plant TM. Effect of continuous iv administration of human metastin 45-54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta). Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 26.le Cotonnec J-Y, Porchet HC, Beltrami V, Munafo A. Clinical pharmacology of recombinant human luteinizing hormone: Part I. Pharmacokinetics after intravenous administration to healthy female volunteers and comparison with urinary human luteinizing hormone. Fertil Steril. 1998;69:189–194. doi: 10.1016/s0015-0282(97)00501-3. [DOI] [PubMed] [Google Scholar]
- 27.Baenziger JU, Kumar S, Brodbeck RM, Smith P, Beranek MC. Circulatory half-life but not interaction with the lutropin/chorionic gonadotropin receptor is modulated by sulfation of bovine lutropin oligosaccharides. Proc Natl Acad Sci USA. 1992;89:334–338. doi: 10.1073/pnas.89.1.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgon PG, Stanton PG, Robertson DM. In vivo bioactivities and clearance patterns of highly purified human luteinizing hormone isoforms. Endocrinology. 1996;137:4827–4836. doi: 10.1210/endo.137.11.8895353. [DOI] [PubMed] [Google Scholar]
- 29.Larsen PR, Ingbar SR. The thyroid gland. In: Wilson JD, Poster DW, editors. Williams Textbook of Endocrinology. 8th edn. Saunders; Philadelphia: 1992. pp. 336–487. [Google Scholar]
- 30.Jannini EA, Ulisse S, D'Armiento M. Thyroid hormone and male gonadal function. Endocr Rev. 1995;16:443–459. doi: 10.1210/edrv-16-4-443. [DOI] [PubMed] [Google Scholar]
- 31.Longcope C. The male and female reproductive systems in hypothyroidism. In: Braverman LE, Utiger RD, editors. Werner Ingbar's the Thyroid: A Fundamental and Clinical Text. 8th edn. Lippincott Willams & Wilkins; Philadelphia: 2000. pp. 824–827. [Google Scholar]
- 32.Tomasi PA, Fanciulli G, Zini M, Demontis MA, Dettori A, Delitala G. Pulsatile gonadotrophin secretion in hypothyroid women of reproductive age. Eur J Endocrinol. 1997;136:406–409. doi: 10.1530/eje.0.1360406. [DOI] [PubMed] [Google Scholar]
- 33.Freeman ME, LaRochelle FT, Jr, Moore RB. Thyroid hormone regulation of the pulsatile discharges of luteinising hormone in ovariectomized rats. Endocrinology. 1975;97:738–743. doi: 10.1210/endo-97-3-738. [DOI] [PubMed] [Google Scholar]
- 34.Fiete D, Srivastava V, Hindsgaul O, Baenziger JU. A hepatic reticuloendothelial cell receptor specific for SO4-4GalNAcβ1,4GlcNAcβ1,2Manα that mediates rapid clearance of lutropin. Cell. 1991;67:1103–1110. doi: 10.1016/0092-8674(91)90287-9. [DOI] [PubMed] [Google Scholar]
- 35.Stanton PG, Burgon PG, Hearn MTW, Robertson DM. Structural and functional characterization of hFSH and hLH isoforms. Mol Cell Endocrinol. 1996;125:133–141. doi: 10.1016/s0303-7207(96)03958-5. [DOI] [PubMed] [Google Scholar]
- 36.Baenziger JU. Glycoprotein hormone GalNAc-4-sulphotransferase. Biochem Soc Trans. 2003;31:326–330. doi: 10.1042/bst0310326. [DOI] [PubMed] [Google Scholar]
- 37.Klett D, Bernard S, Lecompte F, Leroux H, Magallon T, Locatelli A, Lepape A, Combarnous Y. Fast renal trapping of porcine luteinizing hormone (pLH) shown by 123I-scintigraphic imaging in rats explains its short circulatory half-life. Reprod Biol Endocrinol. 2003;1:64–71. doi: 10.1186/1477-7827-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiete D, Baenziger JU. Isolation of the SO4-4-GalNAcβ1,4Glc-NAcβ1,2Manα-specific receptor from rat liver. J Biol Chem. 1997;272:14629–14637. doi: 10.1074/jbc.272.23.14629. [DOI] [PubMed] [Google Scholar]
- 39.Emmanouel DS, Stavropoulos T, Katz AI. Role of the kidney in metabolism of gonadotropins in rats. Am J Physiol. 1984;247:E786–E792. doi: 10.1152/ajpendo.1984.247.6.E786. [DOI] [PubMed] [Google Scholar]
- 40.Reiter EO, Rosenfeld RG. Normal and aberrant growth. In: Larsen PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. 10th edn. Saunders; Philadelphia: 2002. pp. 1003–1114. [Google Scholar]
- 41.Chiesa A, de Papendieck G, Keselman A, Heinrich JJ, Bergada C. Growth follow-up in 100 children with congenital hypothyroidism before and during treatment. J Pediatr Endocrinol. 1994;7:211–217. doi: 10.1515/jpem.1994.7.3.211. [DOI] [PubMed] [Google Scholar]
- 42.Grant DB. Growth in early treated congenital hypothyroidism. Arch Dis Child. 1994;6:464–468. doi: 10.1136/adc.70.6.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivkees SA, Bode HH, Crawford JD. Long-term growth in juvenile acquired hypothyroidism: the failure to achieve normal adult stature. N Engl J Med. 1988;318:599–602. doi: 10.1056/NEJM198803103181003. [DOI] [PubMed] [Google Scholar]
- 44.Pantsiouou S, Stanhope R, Uruena M, Preece MA, Grant DB. Growth prognosis and growth after menarche in primary hypothyroidism. Arch Dis Child. 1991;66:838–840. doi: 10.1136/adc.66.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall WA. Interrelationships of skeletal maturation, sexual development and somatic growth in man. Ann Hum Biol. 1974;1:29–40. doi: 10.1080/03014467400000031. [DOI] [PubMed] [Google Scholar]
- 46.Plant TM, Fraser MO, Medhamurthy R, Gay VL. Somatogenic control of GnRH neuronal synchronization during development in primates: a speculation. In: Delamarre-van de Waal HA, Plant TM, van Rees GP, Schoemaker J, editors. Control of the Onset of Puberty III. Excerpta Medica; Amsterdam: 1989. pp. 111–121. [Google Scholar]
- 47.Flor-Cisneros A, Roemmich JN, Rogol AD, Baron J. Bone age and onset of puberty in normal boys. Mol Cell Endocrinol. 2006;254/255:202–206. doi: 10.1016/j.mce.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]