Abstract
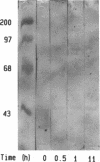
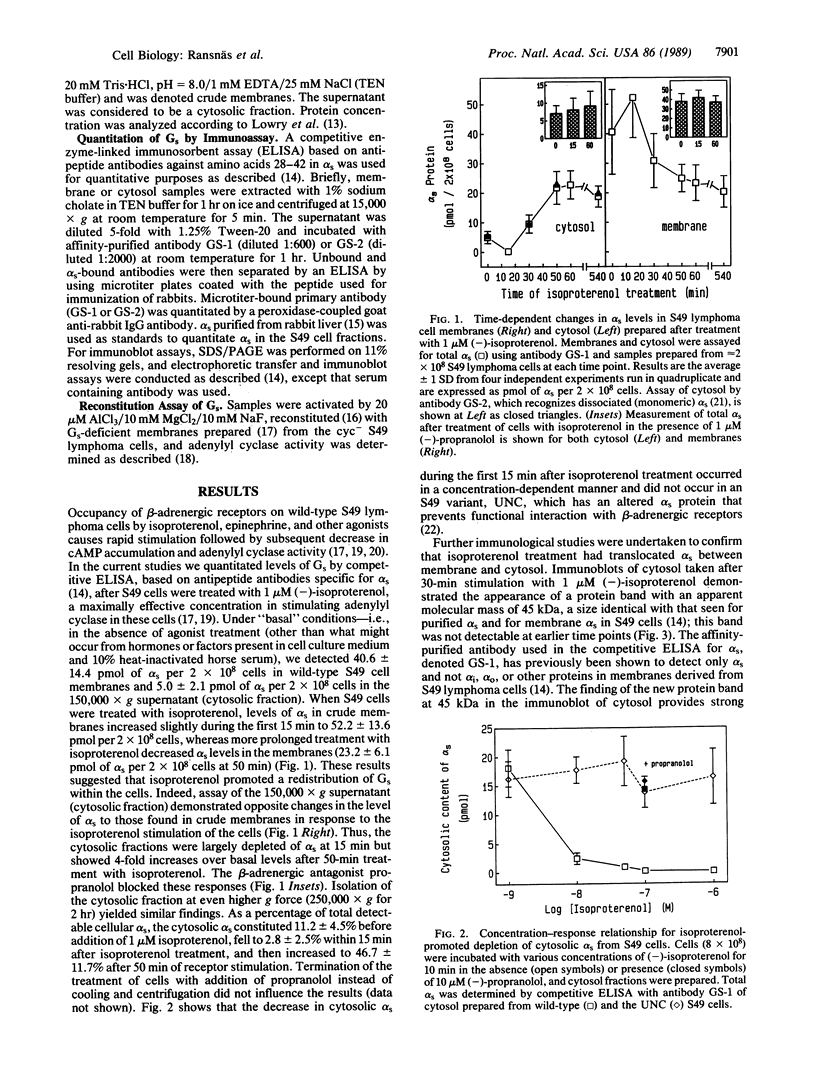
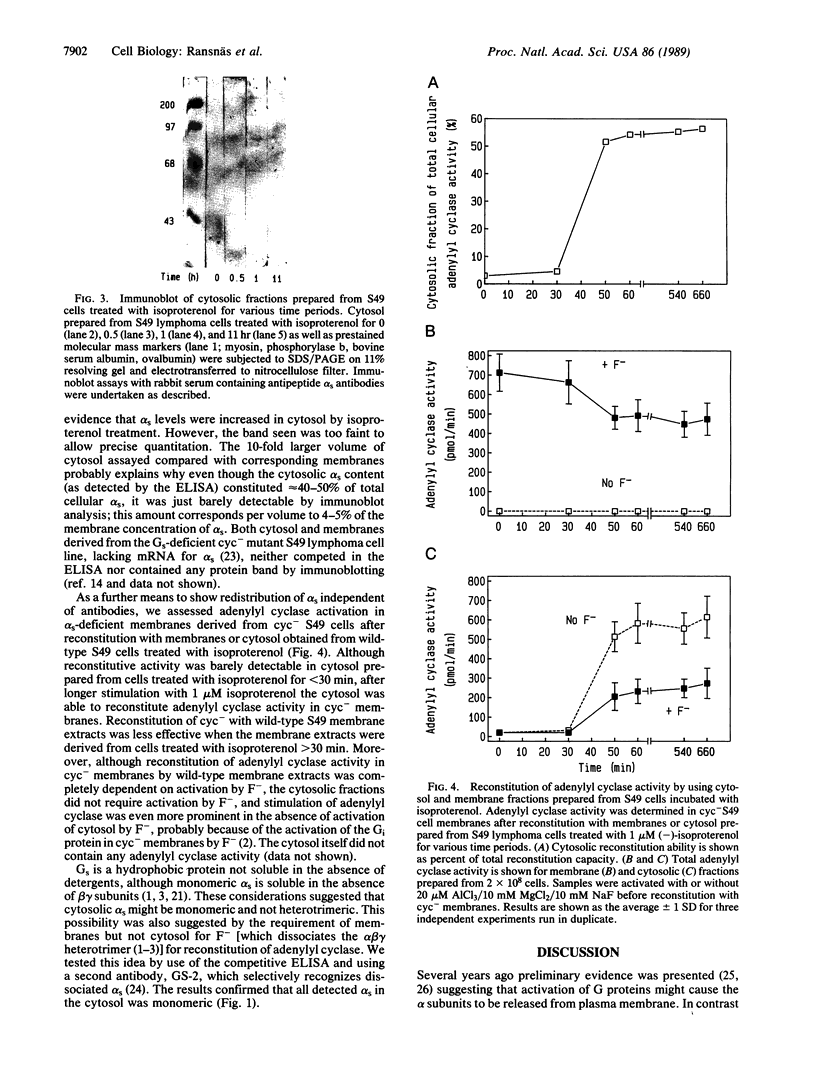
The stimulatory guanine nucleotide-binding protein (Gs), which links cell-surface receptors to second-messenger effector systems, is assumed to be confined to plasma membranes. In the current studies we tested whether Gs redistributes within cells by treating S49 lymphoma cells with the beta-adrenergic agonist isoproterenol, then separating cytosol and crude membrane fractions (defined as pellet and supernatant, respectively, after centrifugation for 1 hr at 150,000 x g), and assaying fractions for the alpha subunit of Gs (alpha s) using a competitive ELISA and reconstitution techniques. Under basal conditions, a small (10%) pool of alpha s was identified in supernatant fractions of S49 cells. The size of this pool decreased in the first 15 min after agonist treatment of cells. This decrease was blocked by a beta-adrenergic receptor antagonist and did not occur in an S49 variant, UNC, which lacks functional interaction between receptors and Gs. The size of the alpha s pool in supernatant fractions increased to almost 50% of total cellular alpha s during a 1-hr incubation of cells with isoproterenol. Before isoproterenol treatment only the competitive ELISA was sensitive enough to detect cytosolic alpha s, whereas at later time points (greater than or equal to 30 min) the presence of alpha s in the cytosol was confirmed by both immunoblotting and by reconstitution of adenylyl cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] in Gs-deficient membranes derived from cyc-S49 cells. In contrast to membrane alpha s, cytosolic alpha s did not require activation (e.g., by AlF4-) in the reconstitution assay to stimulate adenylyl cyclase. Use of an antibody that selectively recognizes monomeric dissociated alpha s, but not heterotrimeric alpha s, indicated that cytosolic alpha s is monomeric. These data indicate that alpha s is not exclusively localized to the plasma membrane and that agonist treatment redistributes this protein within target cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhat M. K., Iyengar R., Abramowitz J., Bordelon-Riser M. E., Birnbaumer L. Naturally soluble component(s) that confer(s) guanine nucleotide and fluoride sensitivity to adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3836–3840. doi: 10.1073/pnas.77.7.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokoch G. M., Katada T., Northup J. K., Ui M., Gilman A. G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J Biol Chem. 1984 Mar 25;259(6):3560–3567. [PubMed] [Google Scholar]
- Buss J. E., Mumby S. M., Casey P. J., Gilman A. G., Sefton B. M. Myristoylated alpha subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7493–7497. doi: 10.1073/pnas.84.21.7493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B. Desensitization of hormonal stimuli coupled to regulation of cyclic AMP levels. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:151–209. [PubMed] [Google Scholar]
- Clark R. B., Kunkel M. W., Friedman J., Goka T. J., Johnson J. A. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wild-type lymphoma cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codina J., Kimura S., Kraus-Friedmann N. Demonstration of the presence of G-proteins in hepatic microsomal fraction. Biochem Biophys Res Commun. 1988 Jan 29;150(2):848–852. doi: 10.1016/0006-291x(88)90469-x. [DOI] [PubMed] [Google Scholar]
- Domínguez P., Velasco G., Barros F., Lazo P. S. Intestinal brush border membranes contain regulatory subunits of adenylyl cyclase. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6965–6969. doi: 10.1073/pnas.84.20.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Mahan L. C., Motulsky H. J., Stoolman L. M., Koachman A. M. Time-dependent decreases in binding affinity of agonists for beta-adrenergic receptors of intact S49 lymphoma cells. A mechanism of desensitization. J Biol Chem. 1983 Nov 25;258(22):13597–13605. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lochrie M. A., Simon M. I. G protein multiplicity in eukaryotic signal transduction systems. Biochemistry. 1988 Jul 12;27(14):4957–4965. doi: 10.1021/bi00414a001. [DOI] [PubMed] [Google Scholar]
- Lynch C. J., Morbach L., Blackmore P. F., Exton J. H. Alpha-subunits of Ns are released from the plasma membrane following cholera toxin activation. FEBS Lett. 1986 May 12;200(2):333–336. doi: 10.1016/0014-5793(86)81163-2. [DOI] [PubMed] [Google Scholar]
- Mahan L. C., Insel P. A. Use of superoxide dismutase and catalase to protect catecholamines from oxidation in tissue culture studies. Anal Biochem. 1984 Jan;136(1):208–216. doi: 10.1016/0003-2697(84)90327-0. [DOI] [PubMed] [Google Scholar]
- McArdle H., Mullaney I., Magee A., Unson C., Milligan G. GTP analogues cause release of the alpha subunit of the GTP binding protein, GO, from the plasma membrane of NG108-15 cells. Biochem Biophys Res Commun. 1988 Apr 15;152(1):243–251. doi: 10.1016/s0006-291x(88)80706-x. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Clapham D. E. Roles of G protein subunits in transmembrane signalling. Nature. 1988 May 12;333(6169):129–134. doi: 10.1038/333129a0. [DOI] [PubMed] [Google Scholar]
- Nielsen T. B., Lad P. M., Preston M. S., Rodbell M. Characteristics of the guanine nucleotide regulatory component of adenylate cyclase in human erythrocyte membranes. Biochim Biophys Acta. 1980 Apr 17;629(1):143–155. doi: 10.1016/0304-4165(80)90273-1. [DOI] [PubMed] [Google Scholar]
- Peters R. Lateral mobility of proteins and lipids in the red cell membrane and the activation of adenylate cyclase by beta-adrenergic receptors. FEBS Lett. 1988 Jul 4;234(1):1–7. doi: 10.1016/0014-5793(88)81290-0. [DOI] [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Quantitation of the guanine nucleotide binding regulatory protein Gs in S49 cell membranes using antipeptide antibodies to alpha s. J Biol Chem. 1988 Jul 5;263(19):9482–9485. [PubMed] [Google Scholar]
- Ransnäs L. A., Insel P. A. Subunit dissociation is the mechanism for hormonal activation of the Gs protein in native membranes. J Biol Chem. 1988 Nov 25;263(33):17239–17242. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- Ross E., Schatz G. Cytochrome c1 of bakers' yeast. I. Isolation and properties. J Biol Chem. 1976 Apr 10;251(7):1991–1996. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scherer N. M., Toro M. J., Entman M. L., Birnbaumer L. G-protein distribution in canine cardiac sarcoplasmic reticulum and sarcolemma: comparison to rabbit skeletal muscle membranes and to brain and erythrocyte G-proteins. Arch Biochem Biophys. 1987 Dec;259(2):431–440. doi: 10.1016/0003-9861(87)90509-1. [DOI] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Gilman A. G. Reconstitution of catecholamine-sensitive adenylate cyclase. Reconstitution of the uncoupled variant of the S40 lymphoma cell. J Biol Chem. 1979 May 10;254(9):3333–3340. [PubMed] [Google Scholar]
- Sternweis P. C. The purified alpha subunits of Go and Gi from bovine brain require beta gamma for association with phospholipid vesicles. J Biol Chem. 1986 Jan 15;261(2):631–637. [PubMed] [Google Scholar]
- Strasser R. H., Benovic J. L., Caron M. G., Lefkowitz R. J. Beta-agonist- and prostaglandin E1-induced translocation of the beta-adrenergic receptor kinase: evidence that the kinase may act on multiple adenylate cyclase-coupled receptors. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6362–6366. doi: 10.1073/pnas.83.17.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Miller R. T., Masters S. B., Beiderman B., Heideman W., Bourne H. R. Identification of receptor contact site involved in receptor-G protein coupling. Nature. 1987 Dec 24;330(6150):758–760. doi: 10.1038/330758a0. [DOI] [PubMed] [Google Scholar]