Cyclosporine (CyA) has been used in orthotopic liver transplantation (OLTx) for over 10 years, while FK 506 has been in use for the last 2 years1 at the University of Pittsburgh. It has been shown that CyA dosage in pediatric OLTx is higher compared to adult OLTx, possibly owing to rapid metabolism in children and/or poor absorption from Roux-en-Y loop often used for biliary reconstruction.2 It is also known that absorption of CyA is dependent on the presence of bile in the gut.3 We have shown FK 506 absorption does not change with the presence or absence of bile both in humans4,5 and in animals.6 We have also observed that the half-life of FK 506 in children is about two times smaller, the clearance is nearly two to four times more rapid, and the volume of distribution is 1.8 times higher as compared to adults with comparable liver function in the immediate posttransplant period (unpublished data). The present study compares how the above differences reflect in actual clinical practice following OLTx in adults and children, both in terms of intravenous (IV) and oral (PO) therapy. In addition, we also compared the trough levels of FK 506 (in plasma) and CyA (whole blood TDx) in adults and children.
MATERIAL AND METHODS
We selected the first 20 consecutive adult primary OLTx (transplanted between August to October 1989) and the first 20 pediatric (age <6 years) OLTx (transplanted between November 1989 to July 1990) who were treated with FK 506 and were alive without retransplantation at the end of 1 year. We selected 20 adult and 20 pediatric OLTx (matched for their age and weight) treated with CyA for comparison. Mean age of the adults for the FK 506 group was 41.1 (SD 9.9) years and mean body weight was 74.3 (SD 15.4) kg. Mean age and weight for the CyA group was 42.3 years (SD 13.4) and 71.2 (SD 8.1) kg, respectively. Mean age of the pediatric FK 506 OLTx was 2.1 (SD 1.4) years and weight was 10.7 (SD 4.2) kg and the mean age and weight for the CyA group was 2.2 (SD 1.6) years and 11.3 (SD 4.0) kg, respectively. CyA was commenced as IV 5 mg/kg/d in two divided doses in adult OLTx and 6 mg/kg/d in three divided doses in pediatric OLTx. FK 506 was given as 0.15 mg/kg/d in two divided short infusions over 2 to 4 hours to both adults and children (current dose of FK 506 is 0.1 mg/kg/d as a continuous infusion). Subsequent adjustments in the dosage were made based on trough concentrations, hepatic function, renal function, side effects from the drug, history of rejection episodes, and presence of infection in all patient groups. Oral therapy was commenced once patients could tolerate oral fluids. Intravenous therapy was discontinued if sufficient level of the drug could be maintained in plasma/whole blood. Daily dose of FK 506 and cyclosporine were recorded for a period of 12 months in each patient: weight-adjusted doses were calculated for weeks 1, 2, 3, 4, 5–6, 7–8, 9–12, 13–16, 17–20, 21–24, 25–32, 33–40, and 41–52 posttransplant based on corresponding mean body weights. Median value of trough plasma levels of FK 506 and trough whole-blood CyA level for each patient was obtained for the above time interval. Further, median value for the level was obtained in each group of patients for comparison.
Statistical Analysis
Comparisons across time and comparisons between children and adult dose requirements were done by repeated measures analysis of variance. Friedman’s test was used to test changes in median trough plasma levels of FK 506 and trough whole-blood CyA levels over time. Comparison between children and adults median plasma levels of FK 506 and whole-blood levels for Cy A were carried out using Wilcoxon rank sum test. The mean number of days of IV CyA and IV FK 506 for both children and adults were compared using the standard two sample Student’s t test. Due to multiple testing, the level of significance was adjusted using Bonferroni’s correction.
RESULTS
Intravenous Treatment
As shown in Table 1. children required 1.6 to 6.0 times higher CyA in the first 4 weeks compared with adults while FK 506 requirement was 0.9 to 2.33 times higher in children. The overall mean dose of CyA in adults was 2.07 (SD 1.32) mg/kg/d for the first 4 weeks and that for children was 5.1 (SD 3.45) mg/kg/d. This difference is statistically significant (P < .001), Mean IV FK 506 dose for adults was 0.05 (SD 0.03) mg/kg/d and that for children was 0.04 (SD 0.03) mg/kg/d for the Similar period: the difference is not significant (P =.668).
Table 1.
IV CyA/FK 506 Adults and Children
| Interval | CyA mg/kg/d Mean (SD) |
Fk 506 mg/kg/d mean (SD) |
||||
|---|---|---|---|---|---|---|
| Adults (a) | Children (C) | Ratio (C/a) | Adults (a) | Children (C) | Ratio (C/a) | |
| Week 1 | 4.7 (1.0) | 7.5 (2.6) | 157 | 0.13 (0.02) | 0.11 (0.03) | 0.88 |
| Week 2 | 2.4 (2.1 ) | 6.8 (43) | 2.8 | 0.015 (0.03) | 0.035 (0.05) | 2.33 |
| Week 3 | 75 (.95) | 3.9 (3.8) | 5.0 | 0.008 (.008) | .009 (0.031 | 1.13 |
| Week 4 | .376 (.913) | 2.26(2.8) | 6.0 | – | 0.009 (0.02) | |
Demonstrates IV requirement of CyA and FK 506 in adult and pediatric OL Tx from week 4. Ratio of CyA dose in children and adults and is much higher than that of FK 506 (P < 05).
Mean total number of days of IV CyA therapy in children was 25.4 (SD 11.3) days while that in adults was 15.2 (SD 5.9) days. The difference is Significant (P < .001). The mean days of IV FK 506 therapy in children was 9.0 (SD 5.3) days and that in adults was 7.5 (SD 2.3) days (p = .238).
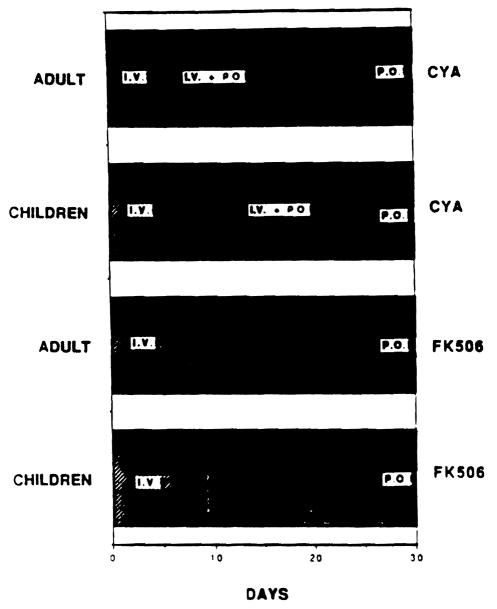
There was a period of overlap with oral and IV CyA therapy with a mean of 9.3 days in adults and mean of 18.3 days in children. This period of overlap was < 1 day in pediatric and adult patients receiving FK 506. as shown in Fig 1.
Fig 1.
Demonstrates prolonged use IV treatment in CyA group of patients with need to combine IV and oral treatment, whereas in FK 506 group of patients there is no need for combined IV and oral treatment.
Oral Treatment
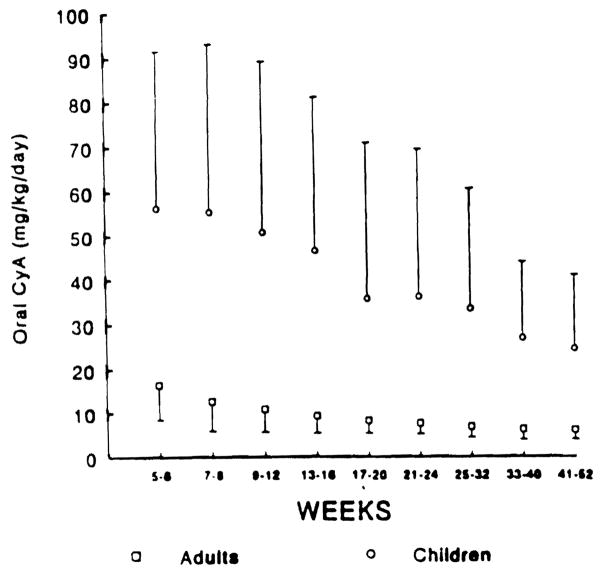
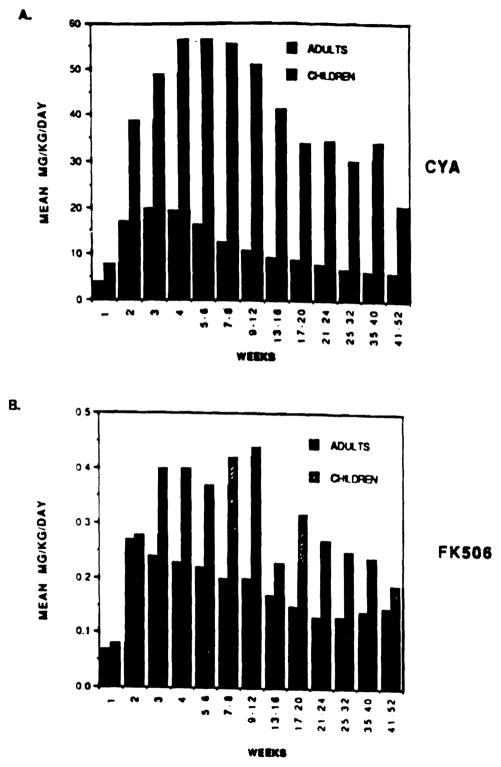
The mean dose requirement of oral CyA in all adults varied from 6.2 mg/kg/d to 16.5 mg/kg/d for week 5 to week 52 post-OLTx (mean 9.4. SD 4.5), respectively. The mean CyA requirements for children varied from 24.8 mg/kg/d to 56.4 mg/kg/d (mean 46.1. SD 30.8) over the same intervals (Fig 2). As shown in Fig 3A. children required 3.4 to 5.0 times (overall mean ratio 4.9. SE 0.9) the oral dose of CyA than that of adults.
Fig 2.
Mean ± SD oral dose of CyA in adults (□) and children (○) over weeks 5 to 52 posttransplant.
Fig 3.
(A) Direct comparison of mean oral dose of CyA in adults (solid bar) and children (hatched bar) from weeks 1 to 52 post-transplant. (B) Compares mean FK 506 oral dose in adults and pediatric OLTx for the same period.
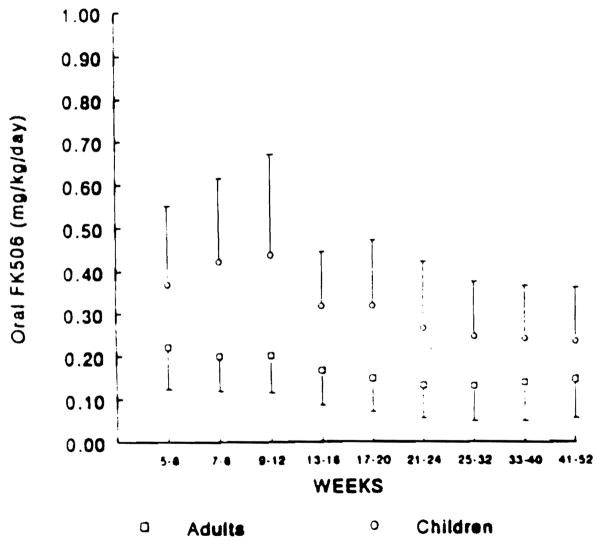
The mean requirement of FK 506 in adults varied from 0.15 mg/kg/d to 0.22 mg/kg/d for weeks 4 to 52 posttransplantation (mean 0.17. SD 0.09). The FK 506 requirements for children varied from 0.24 mg/kg/d to 0.37 mg/kg/d (mean 0.33. SD 0.17) for the same interval (Fig 4). Children required 1.60 to 2.14 times the oral dose of FK 506 than that of adults (overall mean ratio 1.95. SE 0.31) (Fig 3B). Ninety-five percent confidence intervals were generated for the overall mean ratio of CyA and FK 506 requirements. These intervals did not overlap, indicating that the oral requirements of CyA in children are Significantly higher (P < 0.05) than those of FK 506.
Fig 4.
Mean oral dose of FK 506 In adults (□) and pediatric OLTx (○) WIth SD over Weeks 5 to 52 posttransplant.
Measurements of Drug Level
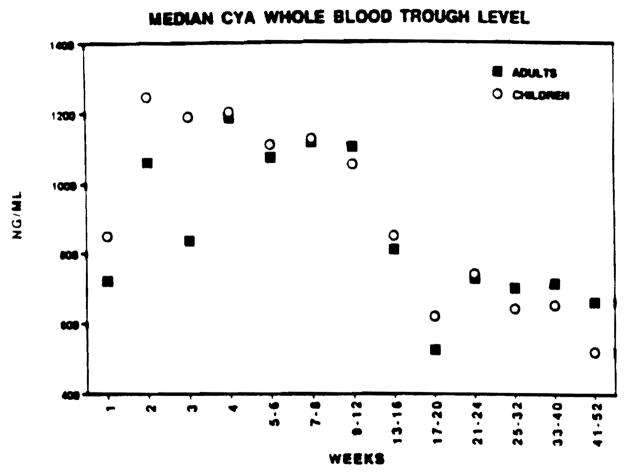
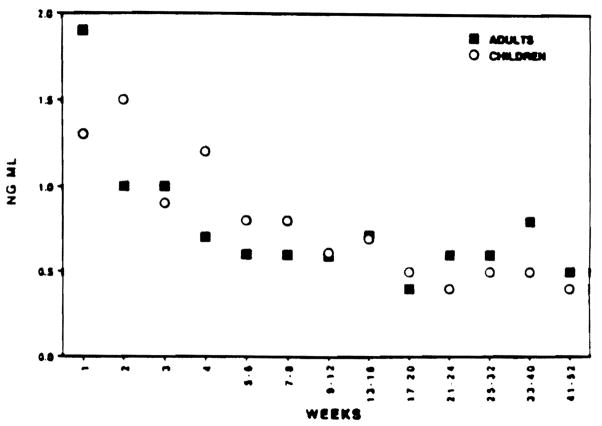
All patients were monitored with frequent trough level measurement of drug in plasma (FK 506) or whole blood (CyA). A total of approximately 4.000 observations in drug levels were made in the follow-up period of 1 year for all four groups of patients. These levels were grouped together for the similar time period for each patient as described earlier. The median value for each patient for the given interval was obtained. Median value from 20 patients in each group showed no difference despite the fact that children received much higher dosages of the drug (Figs 5 and 6)
Fig 5.
Median CyA whole-blood trough levels for adults and pediatric OLTx from weeks 1 to 52 posttransplant.
Fig 6.
Median FK 506 plasma trough levels for adults and children OLTx from weeks 1 to 52 posttransplant.
DISCUSSION AND CONCLUSIONS
Because of rapid metabolism of the drugs in children. the dose requirements (mg/kg/d) for CyA and FK 506 are higher than in adults. Prolonged IV therapy of CyA both in children and in adults often is required because of poor absorption of the orally administered CyA during the Immediate posttransplant period and the dependence of this absorption upon bile salts. Children also require more FK 506 than adults. but the drug is less dependent on bile for good oral absorption. Partly for this reason. doubleroute administration for prolonged periods is not required when oral FK 506 administration is resumed.
Acknowledgments
Aided by research grants from the Veterans Allairs and prolect grant DK 29961 from the National Institutes of Health.
References
- 1.Todo S, Fung JJ, Tzakis A, et al. Transplant Proc. 1991;23(1):1397. [PubMed] [Google Scholar]
- 2.Burckart GJ, Starzl T, Williams L, et al. Transplant Proc. 1985;17:1172. [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta M, Venkataramanan R, Burckart G, et al. Br J Clin Pharmacol. 1988;25:579. doi: 10.1111/j.1365-2125.1988.tb03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain A, Venkataramanan R, Cadoff E, et al. Transplant Proc. 1990;22:57. [PMC free article] [PubMed] [Google Scholar]
- 5.Venkataramanan R, Jain A, Warty VW, et al. Transplant Proc. 1991;23:931. [PMC free article] [PubMed] [Google Scholar]
- 6.Furukawa H, Imventarza O, Venkataramanan R, et al. Transplantation. doi: 10.1097/00007890-199204000-00002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]