Recent studies from our laboratories have attempted to clarify the events of hyperacute heterograft and homograft rejection.1, 7, 15, 17, 18 These investigations, as well as those from other laboratories, 3, 5, 6, 14 have emphasized the complexity of this fulminating variety of rejection as well as the nearly simultaneous contributing roles played by preformed humoral antibodies, coagulation, formed blood elements, and complement.
It might be anticipated that therapy which interfered with the foregoing etiologic factors could mitigate the vigor of hyperacute rejection. Furthermore, if heterografts and homografts transplanted to naturally or deliberately presensitized recipients undergo rejection by analogous mechanisms, successful treatment under either of these experimental conditions should be applicable to both. In this communication, it will be shown that sodium citrate, a calcium-binding agent, has the same type of ameliorating effect upon heterograft rejection as has already been reported by Linn and associates11 upon hyperacute homograft rejection. Since free calcium ions are required for coagulation, for complement activation, and for a number of other biologic processes, it cannot be stated unequivocally by what means the protection occurred.
Methods
Heterotransplantation
Twenty-two pig-to-dog kidney heterotransplantations were performed while the animals were under sodium pentobarbital anesthesia; the renal artery was anastomosed end to end to the recipient right common iliac artery and the renal vein was anastomosed end to side to the right common iliac vein (Fig. 1, A). A Teflon catheter was introduced into the right hypogastric vein and its tip positioned opposite to the venous anastomosis. By temporarily clamping the iliac vein below and above the anastomosis, the investigators could collect and measure the total venous effluent of the homograft (per unit time) with a stopwatch and a graduated cylinder (Fig. 1, A).
Fig. 1.
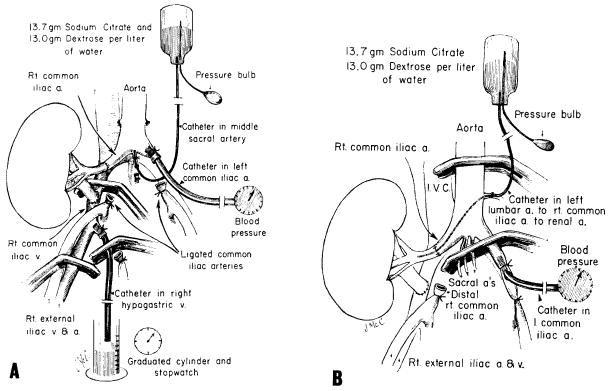
Procedures used for intra-arterial citrate infusion. A, Heterograft experiments and B, homograft experiments in sensitized recipients.
The experimental technique was designed to permit measurement of blood pressure in the distal aorta and to assure that fluid infused into the terminal aorta would pass mainly or exclusively into the graft renal artery (Fig. 1, A). This was done by inserting perfusion and pressure catheters proximally toward the orifices of the common iliac and middle sacral arteries with ligation of these vessels near their origin (Fig. 1, A). All smaller vessels originating at or near the aortic trifurcation were also ligated.
In 15 of the 22 dogs, the intra-arterial infusion was with a solution that contained 13.7 Gm. of sodium citrate and 13 Gm. of dextrose per liter of distilled water (sodium concentration, 140 mEq. per liter). The infusion was started one minute before heterograft revascularization and maintained at 0.75 ml. per kilogram per minute (10.3 mg. of citrate per kilogram per minute). The seven other animals served as control experiments and received: no infusion (one), normotonic saline infusion of the same fluid volume (one), heparinized normotonic saline solution delivering 2 mg. of heparin per kilogram per minute (two) or 40 mg. of heparin per kilogram per minute (two), and infusion with the above described sodium citrate solution to which 5 ml. of a ten percent calcium chloride solution were added per 100 ml. (one). In the four heparin experiments, an additional 5 mg. per kilogram (two experiments) or 100 mg. per kilogram (two experiments) of heparin were given intravenously before either graft revascularization or institution of the intra-arterial infusion.
Arteriovenous gradients of formed blood elements, fibrinogen, and whole complement were determined across the heterotransplants by methods previously reported.7 In addition, arterial and venous calcium and total protein concentrations were measured with a spectrophotometer and a refractometer,* respectively. The arterial samples were taken from the abdominal aorta at some distance above the trifurcation, and the venous samples were collected during transient occlusion of the common and external iliac veins (Fig. 1, A).
In four of the 15 citrate experiments and in all four of the heparin experiments, serial biopsies were obtained from 5 to 75 and from 5 to 20 minutes, respectively. Biopsies were taken prior to and after discontinuation of the citrate infusion. A portion of the specimens was fixed in formalin and stained with hematoxylin and eosin and periodic acid–Schiff (PAS). The rest was snap-frozen and studied by direct immunofluorescence.21
Homotransplantation
Eleven mongrel dogs were grafted six to eight consecutive times with skin from an eventual kidney donor.1, 15 Then one of the donor kidneys was transplanted to the neck for 24 hours by means of anastomoses to the common carotid artery and external jugular vein. At a final experiment carried out an average of 61 days after beginning sensitization and 7 to 11 days after the first renal homotransplantation, the other donor kidney was transplanted to the iliac vessels of the pelvis of the recipient; urine was collected via a skin ureterostomy. At the sites of the rejected skin grafts or first kidney, all dogs developed severe infections. Four animals died before the investigation could be completed, leaving seven of which three and four were used for controls and for citrate evaluation, respectively. In all seven dogs, antidonor hemagglutinins, leukoagglutinins, lymphocytotoxins, and thromboagglutinins were measured in systemic venous blood throughout the sensitization period, as described previously.1, 15 Before and after the final renal transplantation, hematocrit, pH, total protein, sodium, chloride, and total calcium were determined in central venous samples; arterial samples were not obtained. Rejection was judged by gross appearance, cessation of urine excretion, and the state of bleeding from the incised organ. Blood urea nitrogen and creatinine were measured in animals living for one day or longer after the definitive renal transplantation. Biopsies were taken from the first kidneys at the time of removal 24 hours after transplantation and from the second kidneys after rejection had occurred or at the time of death. A portion of the specimens were fixed in formalin and stained with hematoxylin and eosin and periodic acid–Schiff (PAS). The rest was snap-frozen for study by direct immunofluorescence.21
At the time of the second renal homotransplantation, bilateral recipient nephrectomies were performed. The details of the homograft infusion procedure for both the control and treated dogs are shown in Fig. 1, B. A catheter was inserted through a left lumbar artery, advanced across the aorta and common iliac artery, until the iliac-renal arterial anastomosis could be performed around it. These manipulations were during temporary aortic cross-clamping. The citrate solution and its rate of infusion were the same as in the heterograft experiments, starting one minute before revascularization. The average water load was 1,200 ml. and the average sodium load was 168 mEq. during the two hours of treatment. The same infusion was repeated six hours after revascularization.
The procedure in the three control experiments differed in that approximately 400 ml. of citrate infusion was begun from 15 to 35 minutes after revascularization by which time the homografts had already been rejected. More than 400 ml. could not be given to these anuric control animals because of the production of hypotension, arrhythmia, pulmonary edema, and acidosis. In the three control as well as the four test animals, 3 Gm. of calcium chloride were given intravenously to prevent cardiac arrest.
Results
Heterotransplantation
Control studies
The seven control pig kidneys were rejected by the dogs in three to ten minutes with a mean urinary output of 6 ml. The events of the hyperacute rejection were the same as have been described in detail by Giles and associates7 in a previous paper. Five minutes after revascularization, arteriovenous gradients (Fig. 2) were observed for leukocytes (33 percent of the arterial value), platelets (62 percent), fibrinogen (33 percent), total protein (25 percent), total calcium (52 percent), and whole complement (65 percent). These gradients were essentially the same in the four heparin experiments as in the non-heparin control experiments, despite the massive doses. It was also noteworthy that the kidney underwent almost immediate rejection during perfusion with citrate solution to which calcium chloride had been added.
Fig. 2.
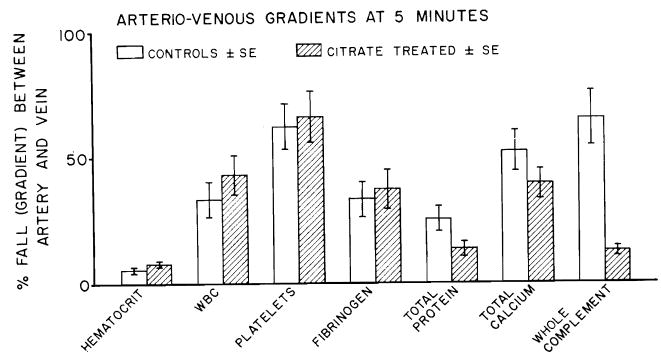
Pig-to-dog kidney heterotransplantation. Arteriovenous gradients ± S.E. in control and citrate-treated animals at five minutes after revascularization.
Citrate treatment
In contrast, the kidneys infused with citrate did not lose their initial pink color. These organs remained firm and continued to produce urine as long as the infusion was maintained (30 to 240 minutes). When the citrate infusion was discontinued, the kidneys were rejected within 5 to 25 minutes. Mean rejection time of the 15 citrate-treated kidneys was 85 minutes with a mean urinary output of 108 ml.
During the citrate infusion, the average arterial blood pressure decreased from an initial mean of 126 to 78 mm. Hg two hours later in animals treated that long. Part of the relative hypotension was probably explicable by blood loss since transfusions were not given, but the citrate itself undoubtedly also contributed.2 During this time, renal blood flow decreased from a mean of 74 to 36 ml. per minute. Renal vascular resistance, computed as an expression of mean blood pressure/renal blood flow was increased 30 percent over the original value.
The arterial samples were taken proximal to the site of citrate infusion. Because of the more distal infusion of significant volumes of the citrate solution (which accounted for almost 20 percent of the fluid flow through the kidney), decreases in the concentrations of the various measured substances in the venous effluent would be predicted on a dilutional basis unless a brisk urinary output were able to excrete all the infused solution, which was never the case. This dilutional factor apparently accounted for most of the declines in hematocrit (Fig. 2). In contrast, higher gradients not explicable by this mechanism developed in the levels of leukocytes, platelets, fibrinogen, calcium, protein, and whole complement (Figs. 2 and 3). Sequestration of the latter blood constituents apparently continued throughout the entire period of observation (Fig. 3). With calcium, part of the arteriovenous gradient could have been accounted for by urinary excretion of calcium, particularly since the average urine volume was 108 ml. However, the range of calcium in this urine was 6.4 to 23.8 mg. percent, a value not nearly high enough to account for all of the calcium gradients.
Fig. 3.
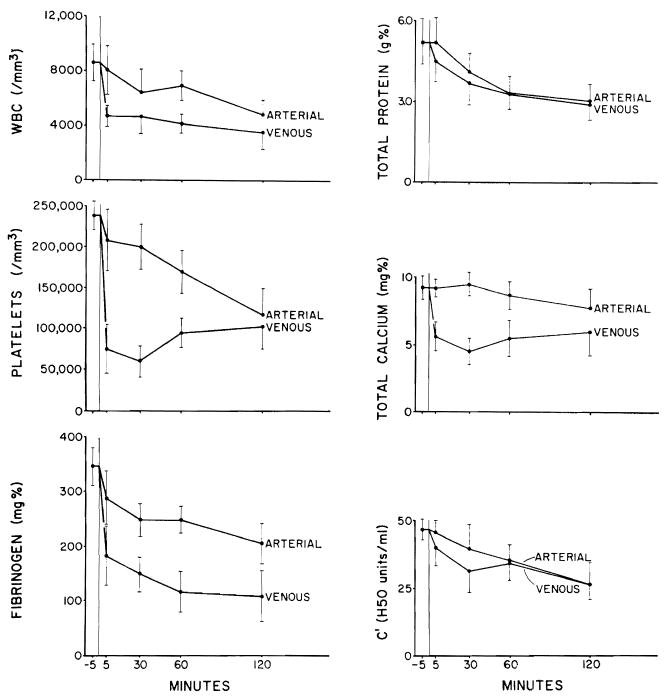
Arteriovenous gradients ± S.E. of hematocrit, white blood cell count, platelets, fibrinogen, total protein, total calcium, and whole complement before and at 5, 30, 60, and 120 minutes after pig-to-dog kidney heterotransplantation under citrate treatment.
It was of interest in the citrate experiments to compare the five-minute gradients with those of the control experiments at the same time. The gradients expressed as the percentage of the arterial values were not significantly different from those in the control experiments except for a strikingly less prominent complement consumption (Fig. 2). Comparisons of the calcium gradients across the citrate-protected heterografts as opposed to the control grafts were partly invalidated by the fact that the former kidneys excreted large amounts of urine, whereas the latter ones did not.
Immunofluorescence studies
The immunofluorescence findings in hyperacutely rejecting pig-to-dog heterografts have been described in detail by Giles and associates.7 The previous studies showed fibrin accumulation beginning within three minutes, becoming maximum in about ten minutes, and with localization in the lumens of glomerular and peritubular capillaries. Dog IgG and C3 were in a similar pattern.
In the present investigation, essentially the same findings were seen in the four heterografts that were transplanted to heparinized recipients. In contrast, fibrin, IgG, and complement deposition were retarded in three of the four grafts treated with citrate (Fig. 4). However, after discontinuance of the citrate infusion, deposits of these substances quickly developed as the organs underwent grossly visible rejection (Fig. 4, C).
Fig. 4.
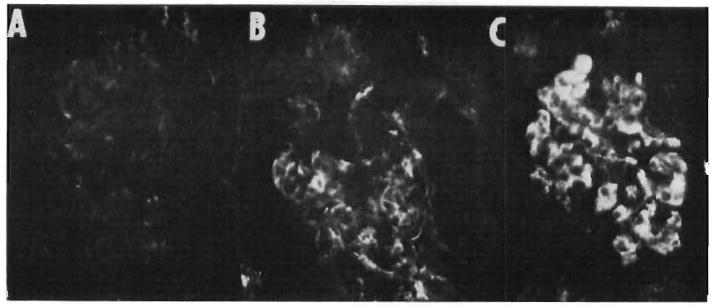
Fibrin deposits in the capillaries of a glomerulus and the peritubular area in a pig kidney transplanted to a citrate-treated dog. A and B, during infusion of citrate five and ten minutes after revascularization. C, five minutes after discontinuance of citrate and 40 minutes after revascularization. Heavy fibrin disposition occurred as the kidney underwent grossly visible rejection.
Homotransplantation
Control studies
By the time of the first renal homotransplantation, a high degree of sensitization had already been achieved since six of these seven kidneys were rejected in less than 24 hours. The three control animals which subsequently received the second kidneys without benefit of citrate protection rejected the homografts in 14, 20, and 35 minutes (Table I). The rapidity of homograft rejection in the control experiments was striking and considerably faster than in similar experimental conditions as reported by Simpson and associates, 15 MacDonald and associates, 12 and Boehmig and associates.1 This was apparently due to the very extensive and prolonged sensitization over the nine-week period. Additional sensitization may have occurred through cross-reacting bacterial antigens from the chronic infections at the sites of rejecting skin and kidney grafts.
Table I. Outcome after renal transplantation to presensitized recipients at the time of the second renal homotransplantation, in relation to the preformed antibody titers (expressed as reciprocal of dilution).
| Experiment No. | Lymphocytotoxins | Hemagglutinins | Leukoagglutinins | Thromboagglutinins* | Rejection time |
|---|---|---|---|---|---|
| Control animals | |||||
| 2 | 128 | 4 | 32 | 8 | 14 min. |
| 7 | 256 | 2 | 0 | 4 | 20 min. |
| 8 | 0 | 0 | 0 | 2 | 35 min. |
| Citrate-treated animals | |||||
| 3 | 256 | 8 | 4 | 8 | 7 hr. |
| 6 | 2 | 32 | 8 | 32 | 24† hr. |
| 9 | 64 | 0 | 4 | 2 | 6.5 hr. |
| 10 | 64 | 32 | 0 | 2 | 48† hr. |
Thromboagglutinins were present even before sensitization in four of these seven dogs. In contrast, the lymphocytotoxins, hemagglutinins, and leukoagglutinins developed in response to the sensitization.
Rejection had not occurred when the homograft was removed.
Citrate treatment
In contrast, graft viability in the four dogs of the citrate group was very much prolonged and in two instances rejection had not occurred when the experiments were terminated after 24 and 48 hours (Table I). The difference in results between the control experiments was not due to any obvious difference in the preformed antibodies in the two groups (Table I).
The renal function from the citrate-protected renal homografts was not accurately assessed because the ureterostomy urine collections proved unreliable. However, urine excretion was obvious during the times shown in Table I, and in animals No. 6 and 10, the blood urea nitrogen and creatinine remained normal until death. A brisk diuresis during the infusions was probably responsible for the fact that citrate toxicity was less severe than in the control animals (see below).
Citrate toxicity
There was marked toxicity from the citrate infusion in both the control and test animals in spite of prophylactic treatment with 3 Gm. of calcium chloride. A pronounced bleeding tendency was invariably observed which usually subsided about an hour after stopping infusion. Profound hypotension, cardiac arrhythmias, and bradycardia were invariable. All three of the control animals and two of the four test animals died of the cardiovascular complications. The animals with poor or absent renal function were unable to correct the iatrogenic abnormalities and consequently were much more susceptible to the various side effects which included severe acidosis. In addition, they developed hemodilution and, during treatment with calcium chloride, hypercalcemia (to 17.3 mg. percent) was observed.
Immunofluorescence studies
Through an accident, all the frozen tissues from these experiments were thawed and were unsuitable for examination.
Discussion
It has been recently emphasized1, 7, 12, 14, 15, 17, 18 that hyperacute rejection is not necessarily due to the direct toxic action of antibodies upon parenchymal cells, but that it may be in part dependent upon antibody-mediated effector mechanisms that jeopardize the blood supply of the graft. In the present investigation, an attempt was made to interrupt these effector systems, particularly coagulation. The value of heparin for the latter purpose in presensitized recipients of renal homografts was previously described.12, 18 However, Giles and associates7 were unable to see any benefit from systemic heparin in the difficult pig-to-dog renal heterotransplantation. The same observations were made with the heterografts of the present study.
In contrast, intra-arterial infusions of large quantities of sodium citrate delayed or prevented hyperacute heterograft rejection in a very dramatic way as has been claimed by Linn and associates.11 Homograft rejection was similarly influenced. Profound coagulation defects were produced by this therapy, but it is by no means certain that the protection obtained was due solely or even principally to anticoagulation, since citrate has many other metabolic effects. It can inhibit complement, 9 deaggregate platelets, 13 prevent the vascular lesions of E. coli endotoxin, 8 slow the release of histamine from leukocytes, 10 paralyze a wide range of enzyme activity, 19 and decrease smooth muscle contraction.19
Of these actions, the effect on complement may be the most significant since the inhibition of complement has been shown to delay accelerated rejection under other experimental circumstances5, 16 not involving anticoagulation. It was noteworthy that complement uptake by the heterografts was greatly reduced by intra-arterial citrate infusion (Fig. 2) and that this was the most striking effect of citrate that could be identified by the measurement of a variety of gradients. Of course, there is no reason to exclude the possibility that the citrate effect was by more than one pathway. Thus, to anticoagulation could have been added a further benefit from complement inhibition, thereby preventing or slowing a variety of antigen-antibody reactions at or near the vascular endothelium and elsewhere within the graft.
In a recent publication, 18 attention was drawn to the similarity of hyperacute kidney rejection to the renal findings of the generalized Shwartzman reaction that can be induced in rabbits by the intravenous injection of E. coli endotoxin. Since then, there have been new developments in an understanding of the Shwartzman reaction, indicating that desquamation of vascular endothelium in multiple areas may be an important lesion caused by endotoxin and that these areas could be the site of consumption of platelets, white cells, clotting factors, and other substances.4 Weymouth and associates20 have described the same kind of endothelial denudation in human renal homografts within one hour of revascularization. The onset of the damage could be correlated with the onset and severity of acute rejection.
It is by no means established that endothelial damage is the essential lesion of either the Shwartzman reaction or hyperacute rejection. However, the point seems clearer with each new set of observations that morphologic changes in both models are similar. This is particularly noteworthy since citrate treatment has been shown to minimize organ damage from the infusion of bacterial endotoxin8 in much the same way as it protects from hyperacute rejection.
As a practical therapeutic tool, citrate has significant limitations. After pig-to-dog heterotransplantation, the discontinuance of citrate treatment was followed by prompt destruction of the transplants. The results were somewhat more encouraging in the homotransplantation experiments in which the kidneys functioned for as long as two days after initial citrate infusions. Since all the control homografts transplanted to sensitized recipients were rejected within a few minutes, this finding holds some hope that citrate treatment could be of clinical value. A holdover effect would be a particularly important finding because of the formidable hazards citrate infusion presents when used even for short times. Those included bleeding, hypotension, and cardiac arrhythmias, all of which were only partially controlled by calcium infusion.
Summary
Hyperacute rejection was studied in seven presensitized canine recipients of renal homografts and in 22 canine recipients of pig kidneys. Under both experimental circumstances, untreated animals rejected the grafts within a few minutes. With the intra-arterial infusion of sodium citrate, hyperacute heterograft rejection was forestalled for the duration of therapy, and with homografts the benefit outlasted treatment by many hours. The protection of heterografts was not due solely to anticoagulation by the citrate, since heparin in large doses did not accomplish the same thing. A contributory factor may have been complement inhibition since removal of complement by the grafts was greatly reduced in the citrate-treated animals.
Acknowledgments
This work was supported by United States Public Health Service Grants No. AI-04152, AI-07007, AI-AM-08898, AM-12148, AM-06344, AM-07772, RR-00051, RR-00069, and HE-09110; by United States Public Health Service Contract PH-43-68-621, and by Atomic Energy Commission Contract AT (04-3)-410.
Footnotes
Presented at the Thirty-second Annual Meeting of the Society of University Surgeons, New Haven, Conn., Feb. 11 to 13, 1971.
Terris-Consolidated Industries, Brooklyn, N. Y.
References
- 1.Boehmig HJ, Giles GR, Amemiya H, Wilson CB, Coburg AJ, Genton E, Bunch DL, Dixon FJ, Starzl TE. Hyperacute rejection of renal homografts: With particular reference to coagulation changes, humoral antibodies, and formed blood elements. Transplant Proc. In press. [PMC free article] [PubMed] [Google Scholar]
- 2.Corbascio AN, Smith NT. Hemodynamic effects of experimental hypercitremia. Anesthesiology. 1967;28:510. [PubMed] [Google Scholar]
- 3.Clark DS, Gewurz H, Good RA, Varco RL. Complement fixation during homograft rejection. Surg Forum. 1964;15:144. [PubMed] [Google Scholar]
- 4.Gaynor E, Bouvier C, Spaet TH. Vascular lesions: Possible pathogenetic basis of the generalized Shwartzman reaction. Science. 1970;170:986. doi: 10.1126/science.170.3961.986. [DOI] [PubMed] [Google Scholar]
- 5.Gewurz H, Clark DS, Finstad J, Kelly WD, Varco RL, Good RA, Gabrielsen AE. Role of complement system in graft rejections in experimental animals and man. Ann N Y Acad Sci. 1966;129:673. [Google Scholar]
- 6.Gewurz H, Clark DS, Cooper MD, Varco RL, Good RA. Effect of cobra venom-induced inhibition of complement activity on allograft and xenograft rejection reactions. Transplantation. 1967;5:1296. doi: 10.1097/00007890-196709000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Giles GR, Boehmig HJ, Lilly J, Amemiya H, Takagi H, Coburg AJ, Hathaway WE, Wilson CB, Dixon FJ, Starzl TE. The mechanism and modification of rejection of heterografts between divergent species. Transplant Proc. 1971;2:522. [PMC free article] [PubMed] [Google Scholar]
- 8.Kux M, Coalson JJ, Massion WH, Guenter CA. Pulmonary effects of E. coli endotoxin: Role of leukocytes and platelets. J Appl Physiol. doi: 10.1097/00000658-197201000-00005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine L, Osier AG, Mayer MM. The respective roles of Ca++ and Mg++ in immune hemolysis. J Immunol. 1953;71:374. [PubMed] [Google Scholar]
- 10.Levy DA. Studies of histamine release from human leukocytes. Ann Allerg. 1969;27:511. [PubMed] [Google Scholar]
- 11.Linn BS, Jensen J, Pardo V, Levi DF, Hudson DG. Prolongation of renal xenografts caused by citrate. J A M A. 1970;212:864. [Google Scholar]
- 12.MacDonald A, Busch GJ, Alexander JL, Pheteplace EA, Menzoian J, Murray JE. Heparin and aspirin in the treatment of hyperacute rejection of renal allografts in presensitized dogs. Transplantation. 1970;9:1. doi: 10.1097/00007890-197001000-00001. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien JR, Shoobridge SM, Finch WJ. Comparison of the effect of heparin and citrate on platelet aggregation. J Clin Path. 1969;22:28. doi: 10.1136/jcp.22.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenberg JC, Broersma RJ, Bullemer G, Mammen EF, Lenaghan R, Rosenberg BF. Relationship of platelets, blood coagulation, and fibrinolysis to hyperacute rejection of renal xenografts. Transplantation. 1969;8:152. doi: 10.1097/00007890-196908000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Simpson K, Bunch DL, Amemiya H, Boehmig HJ, Wilson CB, Dixon FJ, Coburg AJ, Hathaway WE, Giles GR, Starzl TE. Humoral antibodies and coagulation mechanisms in the accelerated or hyperacute rejection of renal homografts in sensitized canine recipients. Surgery. 1970;68:77. [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder GB, Ballesteros E, Zarco RM, Linn BS. Prolongation of renal xenografts by complement suppression. Surg Forum. 1966;17:478. [PubMed] [Google Scholar]
- 17.Starzl TE, Boehmig HJ, Amemiya H, Wilson CB, Dixon FJ, Giles GR, Simpson KM, Halgrimson CG. Clotting changes including disseminated intravascular coagulation during rapid renal homograft rejection. New Eng J Med. 1970;283:383. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starzl TE, Lerner RA, Dixon FJ, Groth CG, Brettschneider L, Terasaki PI. Shwartzman reaction after human renal homotransplantation. New Eng J Med. 1968;278:642. doi: 10.1056/NEJM196803212781202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Geertruyden J. Ions alcalino-terreux. In: Eichler O, Farah A, editors. Handbuch der Experimentallen Pharmakologie. part 2. Vol. 17. Springer-Verlag; Berlin: 1964. [Google Scholar]
- 20.Weymouth RJ, Seibel HR, Lee HM, Hume DM, Williams GM. The glomerulus in man one hour after transplantation. Amer J Path. 1970;58:85. [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson CB, Dixon FJ. Antigen quantitation in experimental immune complex glomerulonephritis. I. Acute serum sickness. J Immunol. 1970;105:279. [PubMed] [Google Scholar]


