Abstract
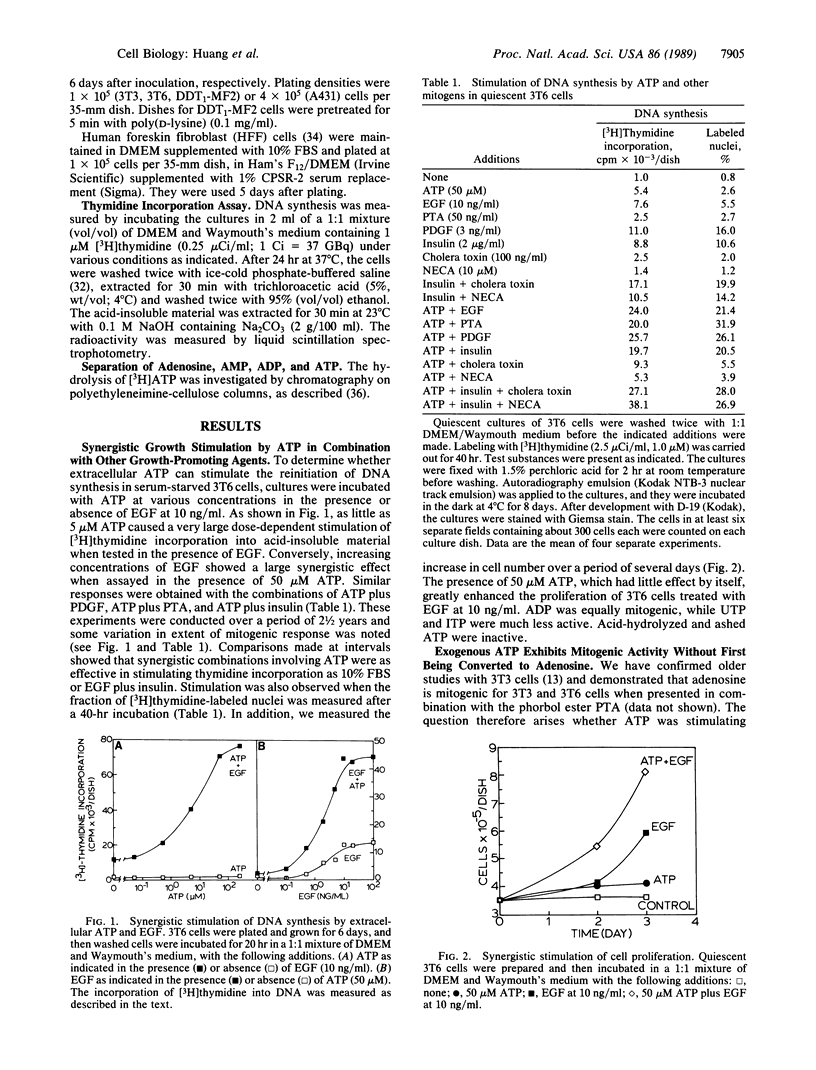
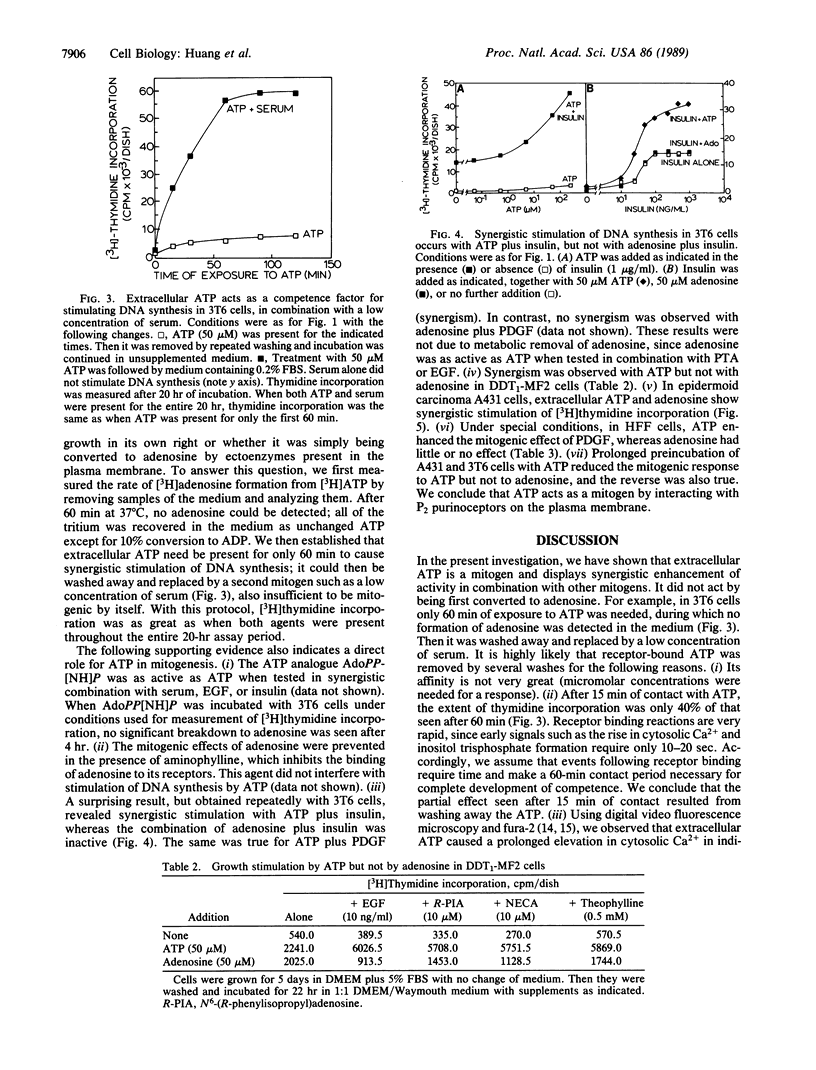
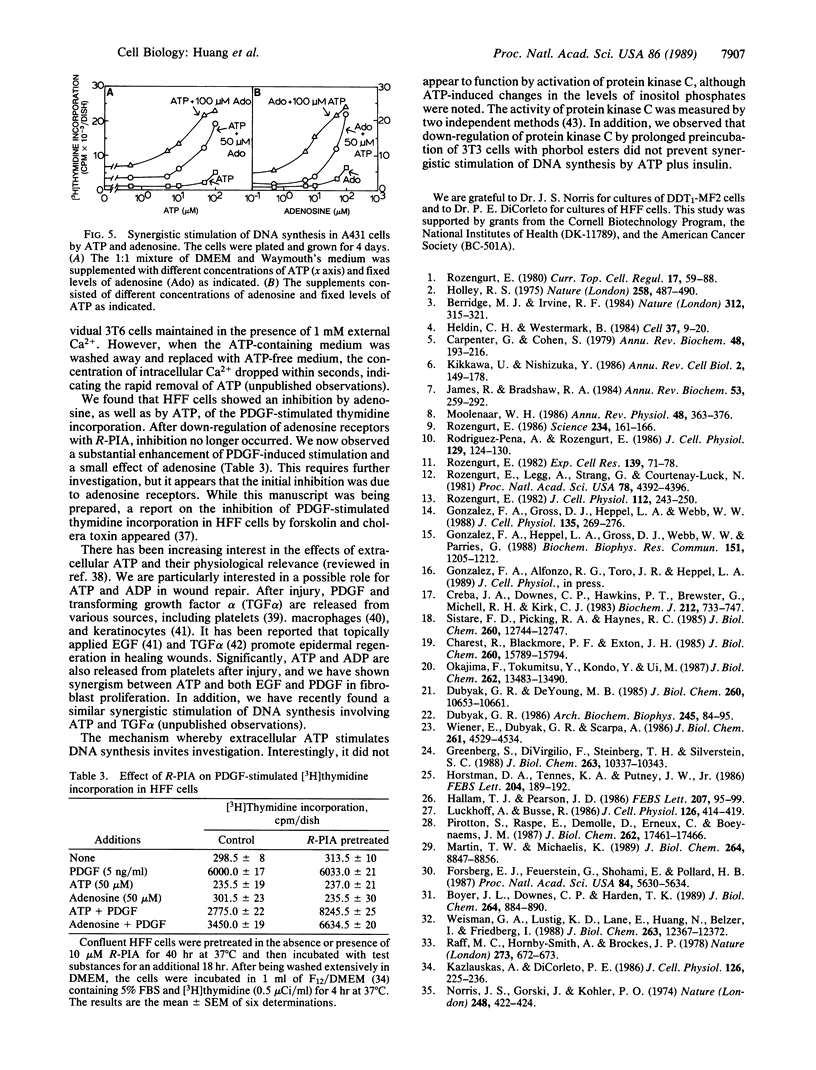
Extracellular ATP in concentrations of 5-50 microM displayed very little mitogenic activity by itself but it caused synergistic stimulation of [3H]thymidine incorporation in the presence of phorbol 12-tetradecanoate 13-acetate, epidermal growth factor, platelet-derived growth factor, insulin, adenosine, or 5'-(N-ethyl)carboxamidoadenosine. Cultures of Swiss 3T3, Swiss 3T6, A431, DDT1-MF2, and HFF cells were used. The percent of cell nuclei labeled with [3H]thymidine and cell number were also increased. ADP was equally mitogenic, while UTP and ITP were much less active. The effect of ATP was not due to hydrolysis by ectoenzymes to form adenosine, a known growth factor. Thus, the nonhydrolyzable analogue adenosine 5'-[beta, gamma-imido]triphosphate was mitogenic. In addition, it was found that ATP showed synergism in 3T6 and 3T3 cells when present for only the first hour of an incorporation assay, during which time no significant hydrolysis occurred. Furthermore, prolonged preincubation of cells with ATP reduced the mitogenic response to ATP but not to adenosine; preincubation with adenosine or N6-(R-phenylisopropyl)adenosine had the reverse effect. Finally, the effect of adenosine, but not of ATP, was inhibited by aminophylline. We conclude that extracellular ATP is a mitogen that interacts with P2 purinoceptors on the plasma membrane.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boyer J. L., Downes C. P., Harden T. K. Kinetics of activation of phospholipase C by P2Y purinergic receptor agonists and guanine nucleotides. J Biol Chem. 1989 Jan 15;264(2):884–890. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dubyak G. R. Extracellular ATP activates polyphosphoinositide breakdown and Ca2+ mobilization in Ehrlich ascites tumor cells. Arch Biochem Biophys. 1986 Feb 15;245(1):84–95. doi: 10.1016/0003-9861(86)90192-x. [DOI] [PubMed] [Google Scholar]
- Forsberg E. J., Feuerstein G., Shohami E., Pollard H. B. Adenosine triphosphate stimulates inositol phospholipid metabolism and prostacyclin formation in adrenal medullary endothelial cells by means of P2-purinergic receptors. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5630–5634. doi: 10.1073/pnas.84.16.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. A., Gross D. J., Heppel L. A., Webb W. W. Studies on the increase in cytosolic free calcium induced by epidermal growth factor, serum, and nucleotides in individual A431 cells. J Cell Physiol. 1988 May;135(2):269–276. doi: 10.1002/jcp.1041350214. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Heppel L. A., Gross D. J., Webb W. W., Parries G. The rapid desensitization of receptors for platelet derived growth factor, bradykinin and ATP: studies on individual cells using quantitative digital video fluorescence microscopy. Biochem Biophys Res Commun. 1988 Mar 30;151(3):1205–1212. doi: 10.1016/s0006-291x(88)80494-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Rozengurt E., Heppel L. A. Extracellular ATP induces the release of calcium from intracellular stores without the activation of protein kinase C in Swiss 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4530–4534. doi: 10.1073/pnas.86.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S., Di Virgilio F., Steinberg T. H., Silverstein S. C. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988 Jul 25;263(21):10337–10343. [PubMed] [Google Scholar]
- Hallam T. J., Pearson J. D. Exogenous ATP raises cytoplasmic free calcium in fura-2 loaded piglet aortic endothelial cells. FEBS Lett. 1986 Oct 20;207(1):95–99. doi: 10.1016/0014-5793(86)80019-9. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Heldin N. E., Paulsson Y., Forsberg K., Heldin C. H., Westermark B. Induction of cyclic AMP synthesis by forskolin is followed by a reduction in the expression of c-myc messenger RNA and inhibition of 3H-thymidine incorporation in human fibroblasts. J Cell Physiol. 1989 Jan;138(1):17–23. doi: 10.1002/jcp.1041380104. [DOI] [PubMed] [Google Scholar]
- Holley R. W. Control of growth of mammalian cells in cell culture. Nature. 1975 Dec 11;258(5535):487–490. doi: 10.1038/258487a0. [DOI] [PubMed] [Google Scholar]
- Horstman D. A., Tennes K. A., Putney J. W., Jr ATP-induced calcium mobilization and inositol 1,4,5-triphosphate formation in H-35 hepatoma cells. FEBS Lett. 1986 Aug 18;204(2):189–192. doi: 10.1016/0014-5793(86)80809-2. [DOI] [PubMed] [Google Scholar]
- James R., Bradshaw R. A. Polypeptide growth factors. Annu Rev Biochem. 1984;53:259–292. doi: 10.1146/annurev.bi.53.070184.001355. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., DiCorleto P. E. A comparison of the platelet-derived growth factor-dependent tyrosine kinase activity in sparse and confluent fibroblasts. J Cell Physiol. 1986 Feb;126(2):225–236. doi: 10.1002/jcp.1041260211. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Lückhoff A., Busse R. Increased free calcium in endothelial cells under stimulation with adenine nucleotides. J Cell Physiol. 1986 Mar;126(3):414–420. doi: 10.1002/jcp.1041260312. [DOI] [PubMed] [Google Scholar]
- Madtes D. K., Raines E. W., Sakariassen K. S., Assoian R. K., Sporn M. B., Bell G. I., Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988 Apr 22;53(2):285–293. doi: 10.1016/0092-8674(88)90390-x. [DOI] [PubMed] [Google Scholar]
- Magnusson R. P., Portis A. R., Jr, McCarty R. E. Quantitative, analytical separation of adenine nucleotides by column chromatography on polyethyleneimine-coated cellulose. Anal Biochem. 1976 May 7;72:653–657. doi: 10.1016/0003-2697(76)90580-7. [DOI] [PubMed] [Google Scholar]
- Martin T. W., Michaelis K. P2-purinergic agonists stimulate phosphodiesteratic cleavage of phosphatidylcholine in endothelial cells. Evidence for activation of phospholipase D. J Biol Chem. 1989 May 25;264(15):8847–8856. [PubMed] [Google Scholar]
- Moolenaar W. H. Effects of growth factors on intracellular pH regulation. Annu Rev Physiol. 1986;48:363–376. doi: 10.1146/annurev.ph.48.030186.002051. [DOI] [PubMed] [Google Scholar]
- Norris J. S., Gorski J., Kohler P. O. Androgen receptors in a Syrian hamster ductus deferens tumour cell line. Nature. 1974 Mar 29;248(447):422–424. doi: 10.1038/248422a0. [DOI] [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Pirotton S., Raspe E., Demolle D., Erneux C., Boeynaems J. M. Involvement of inositol 1,4,5-trisphosphate and calcium in the action of adenine nucleotides on aortic endothelial cells. J Biol Chem. 1987 Dec 25;262(36):17461–17466. [PubMed] [Google Scholar]
- Raff M. C., Hornby-Smith A., Brockes J. P. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature. 1978 Jun 22;273(5664):672–673. doi: 10.1038/273672a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Vasopressin rapidly stimulates protein kinase C in quiescent Swiss 3T3 cells. J Cell Physiol. 1986 Oct;129(1):124–130. doi: 10.1002/jcp.1041290117. [DOI] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Adenosine receptor activation in quiescent Swiss 3T3 cells. Enhancement of cAMP levels, DNA synthesis and cell division. Exp Cell Res. 1982 May;139(1):71–78. doi: 10.1016/0014-4827(82)90319-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Stimulation of DNA synthesis in quiescent cultured cells: exogenous agents, internal signals, and early events. Curr Top Cell Regul. 1980;17:59–88. doi: 10.1016/b978-0-12-152817-1.50007-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Synergistic stimulation of DNA synthesis by cyclic AMP derivatives and growth factors in mouse 3T3 cells. J Cell Physiol. 1982 Aug;112(2):243–250. doi: 10.1002/jcp.1041120213. [DOI] [PubMed] [Google Scholar]
- Schultz G. S., White M., Mitchell R., Brown G., Lynch J., Twardzik D. R., Todaro G. J. Epithelial wound healing enhanced by transforming growth factor-alpha and vaccinia growth factor. Science. 1987 Jan 16;235(4786):350–352. doi: 10.1126/science.3492044. [DOI] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Weisman G. A., Lustig K. D., Lane E., Huang N. N., Belzer I., Friedberg I. Growth inhibition of transformed mouse fibroblasts by adenine nucleotides occurs via generation of extracellular adenosine. J Biol Chem. 1988 Sep 5;263(25):12367–12372. [PubMed] [Google Scholar]
- Wiener E., Dubyak G., Scarpa A. Na+/H+ exchange in Ehrlich ascites tumor cells. Regulation by extracellular ATP and 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem. 1986 Apr 5;261(10):4529–4534. [PubMed] [Google Scholar]


