Abstract
A retrospective histopathologic review of all pathologic specimens from 394 adult liver transplant patients was undertaken with clinical correlation to determine if primary biliary cirrhosis has affected the posttransplant course compared to all other indications for liver transplantation and if recurrent primary biliary cirrhosis has occurred after liver transplantation. We also compared the histopathologic features seen in native livers with primary biliary cirrhosis to failed allografts with chronic rejection. One hundred six of the 394 adult patients transplanted during this time (1981 to July, 1986) fulfilled clinicopathologic criteria for a diagnosis of primary biliary cirrhosis. Neither the incidence nor any qualitative pathologic feature of histologically documented acute cellular rejection differentiated subjects transplanted for primary biliary cirrhosis vs. other diseases. No correlation between the titers of antimitochondrial antibody and the presence of posttransplant hepatic dysfunction based on liver enzyme profiles or the development of chronic rejection was seen in patients transplanted for primary biliary cirrhosis. Minor differences noted in the posttransplant course of primary biliary cirrhosis patients as compared to other conditions (higher incidence of chronic rejection as a cause of graft failure) was seen, but this did not significantly affect graft or patient survival. Recurrent primary biliary cirrhosis could not be diagnosed with certainty in any patient. A comparison of failed chronically rejected allografts vs. native hepatectomies obtained from patients with primary biliary cirrhosis revealed the presence of chronic obliterative vasculopathy, centrilobular cholestasis, and lack of granulomas, cirrhosis, cholangiolar proliferation, copper-associated protein deposition and Mallory’s hyalin in specimens with chronic rejection. In contrast, livers removed from patients with primary biliary cirrhosis demonstrated a mild vasculopathy, cirrhosis, granulomas, copper-associated protein deposition, Mallory’s hyalin and periportal cholestasis. Both conditions demonstrated a nonsuppurative destructive cholangitis with bile duct paucity.
Primary biliary cirrhosis (PBC) is a disorder of uncertain etiology that primarily affects middle-aged females and frequently results in chronic cholestatic liver disease. There is considerable circumstantial evidence to suggest that PBC is an autoimmune disorder. This evidence consists of the presence of antimitochondrial antibodies (AMA), in vivo complement activation, a nonsuppurative destructive cholangitis which can progress to cirrhosis and a variety of systemic manifestations which are also seen in graft-vs.-host disease (1–4). A diagnosis of PBC depends upon the combination of a consistent clinical, serologic and histopathologic profile. No effective medical therapy for this disorder has been fully established. Liver transplantation has become the treatment of choice for patients with advanced disease.
Histopathologic (5–7) and pathophysiologic (8, 9) similarities exist between PBC, graft-vs.-host disease and liver allograft rejection, particularly “chronic rejection.” Neuberger et al. (10) have suggested that recurrent PBC occurs in patients transplanted for this indication. This conclusion was based on the findings of a consistent hepatic histopathology, the presence of AMA and a posttransplant clinical course consistent with PBC. The documentation of recurrent disease is of paramount importance in organ transplantation. The ability to distinguish between these two processes (i.e. PBC and chronic liver allograft rejection) is essential for recognition of recurrent PBC. We undertook the following study to determine if, using criteria offered by Neuberger et al., we could document recurrent PBC in patients transplanted for PBC; to compare the histopathologic features of chronic liver allograft rejection and PBC, and to determine the effect, if any, of the primary disease on the posttransplantation course of patients with liver transplanted for PBC as compared to that of patients with transplantation for other indications.
MATERIALS AND METHODS
Source Material
A retrospective histopathologic review with clinical correlation of all adult liver specimens obtained from patients who underwent orthotopic liver transplantation between January, 1981, and July, 1986, at the University of Pittsburgh was carried out by a single pathologist (A.J.D.).
All hepatectomy specimens (native and allograft) were sectioned according to a predefined protocol (11) so that similar areas of the liver were studied. All sections were stained with hematoxylin and eosin. Selected sections from each case were stained with trichrome and periodic acid-Schiff with diastase digestion. One section from 25 primary hepatectomy specimens with PBC, a section from all failed allografts because of chronic rejection and all posttransplant biopsies from patients with PBC as their original disease obtained at any time greater than 6 months posttransplantation were stained with orcein and/or rhodanine for the detection of copper-associated protein and copper, respectively.
Pathologic Features Examined
All posttransplant liver pathology specimens were evaluated for histopathologic features used in the evaluation of posttransplant liver specimens developed for the National Institute of Diabetes and Digestive and Kidney Diseases liver transplant data base (12). These criteria include the severity and composition of portal inflammation, the presence of subendothelial mononuclear infiltration of portal and/or central veins, the presence and percentage of damaged bile ducts, ductular and cholangiolar proliferation, piecemeal necrosis, lobular inflammation, disarray and hepatocyte necrosis and the presence and location of cholestasis. In addition to these features, the native hepatectomy specimens obtained from patients with PBC and failed allografts with chronic rejection were evaluated for the presence of several other findings. Specific additional features examined in these hepatectomy specimens included the approximate size of damaged bile ducts, presence of cirrhosis, piecemeal necrosis and type, extent and location of copper-associated protein deposition and the type and degree of arterial and venous pathology. A pretransplant wedge or needle biopsy obtained earlier in the course of their disease was available from 15 of the 106 patients transplanted for PBC.
Diagnostic Criteria
The criteria utilized to establish the diagnosis of PBC prior to transplantation were the presence of AMA (usually in titers greater than 1:40), a consistent clinical and biochemical profile (3) and a characteristic hepatic histopathology (4).
The pathologic criteria employed to diagnose hepatic rejection have been reported previously (11). For acute rejection these were the presence of a predominantly mononuclear portal inflammatory infiltrate, bile duct epithelial damage such as vacuolization, eosinophilia, pyknosis, dysplasia and/or luminal disruption and the absence of significant lobular alterations suggestive of any alternative diagnosis. Chronic rejection was diagnosed when two of the following three criteria (inclusive of criterion ii) were present: (i) mononuclear portal tract infiltrate (usually mild to moderate); (ii) duct damage in greater than 50% of the ducts with associated loss of bile ductules, and (iii) obliterative vasculopathy similar to that described in other solid organ allografts. All of the biopsy samples were obtained at the discretion of attending physicians who used clinical and/or biochemical indications of liver dysfunction as an indication for liver biopsy.
Patient Demographic Data
One hundred six of 394 patients transplanted during this period (1981 to July, 1986) satisfied the criteria for the pretransplant diagnosis of PBC (average age = 46.2 years; 93 females, 13 males). There were 209 posttransplant biopsies, 31 failed allografts and 11 autopsy specimens available for review in these 106 PBC patients.
The control samples consisted of all of the histopathologic material available on 288 patients, all of whom had a primary disease other than PBC and were transplanted during the same period of time. The average age of these individuals was 41.0 years; 123 were females and 162 were males. Significant AMA titers were detected in none of these patients. For this group, 431 biopsies, 74 failed allografts and 31 autopsy specimens were available for review. All patients had been maintained on an cyclosporin/steroid immunosuppressive regimen after transplantation with individual titration of dosages.
RESULTS
Analysis of the Postoperative Course
Comparison of the Incidence and Histologic Appearance of Acute Cellular Rejection
The incidence and pathologic appearance of histologically documented episodes of acute cellular rejection occurring in PBC patients were compared to those seen in all other patients (non-PBC). No attempt was made to evaluate histologic features of any nonrejection allograft syndromes because it was believed that recurrent PBC would appear more similar to rejection than other graft pathology. No significant difference in the incidence or qualitative histologic feature distinguished between these two groups (Table 1). However, more of the biopsies obtained from PBC patients with acute cellular rejection as the primary diagnosis had greater than 50% of the bile ducts damaged and demonstrated piecemeal necrosis as compared to patients transplanted for other indications (p < 0.05). It must be emphasized, however, that the presence of piecemeal necrosis was based on a subjective interpretation of each biopsy and as such may not be either reliable or reproducible.
TABLE 1.
Comparison of histologic features of posttransplant pathology specimens with diagnosis of acute cellular rejection from patients with PBC and non-PBC prior to transplantation
| Non-PBC | PBC | χ2 probability (p value) |
|
|---|---|---|---|
| Total patients | 288 (100) | 106 (100) | |
| No. of patients having at least one specimen for review | 225 (78) | 85 (80) | |
| No. of patients with at least one histologically documented episode of acute cellular rejectiona | 115 (51) | 50 (58) | 0.30 |
| Total No. of specimens with ACRb as primary diagnosis | 174 (100) | 106 (100) | |
| No. of ACR specimens with >50% bile duct damage | 46 (26) | 44 (41) | 0.01 |
| No. of ACR specimens with ductular proliferation | 37 (21) | 19 (17) | 0.60 |
| No. of ACR specimens with arteritis | 5 (3) | 5 (5) | 0.60 |
| No. of ACR specimens with piecemeal necrosis | 3 (2) | 11 (10) | <0.01 |
Numbers of patients (percentages) are given.
Percentage is calculated using patients with pathology specimens for review as denominator.
ACR = Acute cellular rejection.
AMA Serology
The results of the AMA serologic screening studies and the clinical course of the first 76 patients transplanted for PBC after transplantation have been reported previously (13). No fractionation of AMA subtypes was performed (14). Of these patients 98% had elevated AMA titers prior to transplantation. In contrast, no significant AMA elevations were present in the patients transplanted for a disease other than PBC. Forty-three of the 45 survivors with PBC had elevated AMA titers postoperatively which, in general, remained at the same level or decreased slightly in titer following transplantation. There has been no apparent correlation between the presence of a postoperative elevated AMA titer and the presence of any liver dysfunction as assessed using standard liver injury parameters (13).
Two of the 10 patients with PBC as their original disease who lost their grafts because of chronic rejection experienced a decline in their AMA titers posttransplantation. Two others have shown an increase, and another two have had no change. Follow-up titers were not available for four subjects.
Causes of Allograft Failure
The major causes for graft failure in the PBC patients and those transplanted for other indications were quite similar, except for an apparent increase in hepatic vascular injury present in the patients transplanted for a condition other than PBC and a relative increase in the frequency of chronic rejection patients transplanted for PBC. Although a trend for these two differences between the two groups was noted, neither finding reached the level of being statistically significant (Table 2). All of the PBC patients who experienced graft loss because of chronic rejection did so less than 3 years after the date of transplantation.
TABLE 2.
Cause of graft failure by primary disease
| Primary disease | PBC (106 patients) No. (%) of grafts | Non-PBC (288 patients) No. (%) of grafts | χ2 probability (p value) | Total (394 patients) No. (%) of grafts |
|---|---|---|---|---|
| Primary nonfunctiona | 5 (16) | 11 (15) | 0.89 | 16 (15) |
| Vascular compromiseb | 10 (32) | 31 (42) | 0.48 | 41 (39) |
| Acute rejection | 4 (13) | 11 (15) | 0.97 | 15 (14) |
| Chronic rejection | 10c (32) | 12d (16) | 0.11 | 22 (21) |
| Othere | 2 (6) | 9 (12) | 0.6 | 11 (10) |
| Total | 31 (100) | 74 (100) | 105 (100) |
Includes preservation injury and antibody-mediated rejection. The vascular tree was patent.
Includes hepatic artery or portal vein thrombosis, vascular wall dissections and antibody-mediated rejection.
Ten grafts from nine patients (average graft survival 247 ± 302 days).
Twelve grafts from 10 patients (average graft survival 717 ± 843 days).
Includes biliary tract problems, recurrent disease and intrahepatic abscesses.
Graft and Patient Survival
There were no significant differences in the graft or patient survival of patients transplanted for PBC when compared to patients undergoing engraftment for all other indications. In fact, posttransplant patient survival for those with PBC was slightly better than for patients transplanted for other disorders (13). This was due to the inclusion of recipients with hepatitis B and hepatic malignancies, who have a high incidence of recurrent disease with an adverse outcome.
Pathologic Comparison of Native Hepatectomies with PBC and Failed Allografts with Chronic Rejection
Gross Pathology
The vast majority of native hepatectomy specimens with PBC were enlarged (average = 2,128 ± 380 gm), bile stained and cirrhotic. Enlarged hilar lymph nodes (up to 3.0 cm diameter) were often present. Focal atherosclerotic changes in the larger arteries were occasionally present. Sectioning revealed a well-developed cirrhosis with regenerative nodules.
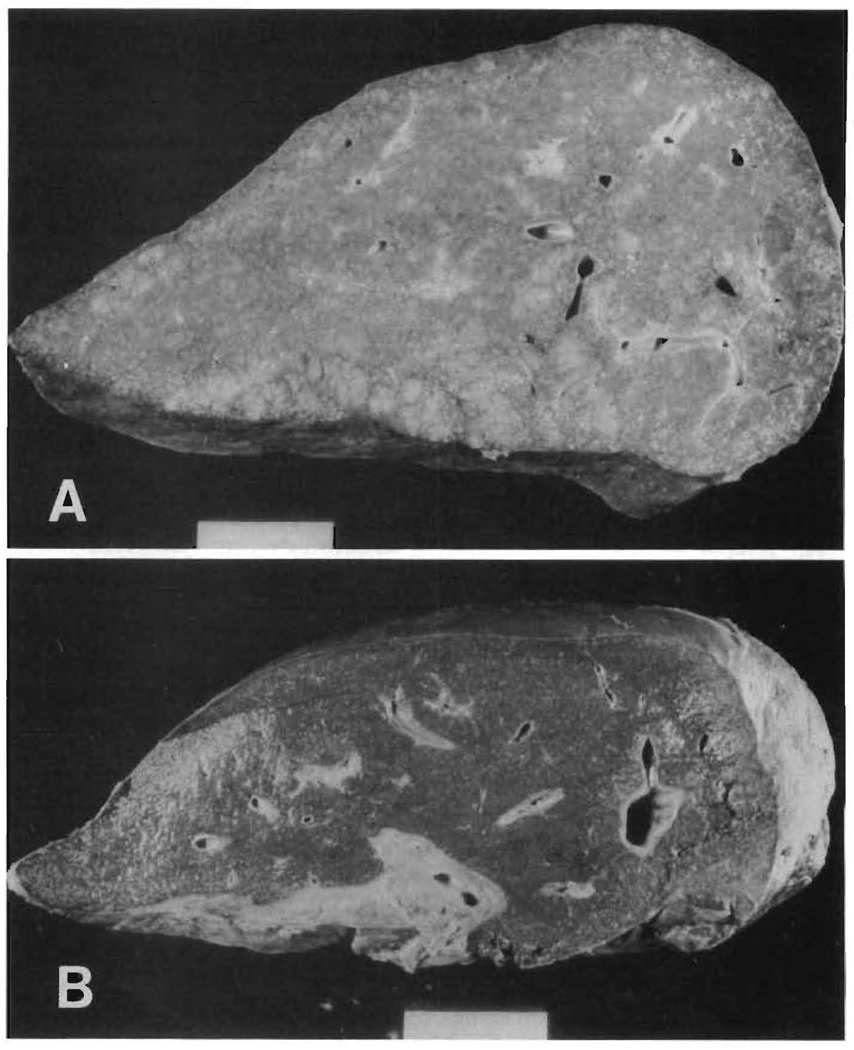
In contrast, liver grafts removed for chronic rejection, while often enlarged (average in PBC patients = 1,816 ± 481 gm and in non-PBC patients = 1,551 ± 370 gm), seldom if ever demonstrated nodularity (Figure 1). Moreover, dissection of the hepatic arterial system often revealed thickened arterial walls with focal occlusion.
Fig. 1.
Gross pathology. The gross appearance of a native liver with PBC (A) is different from an allograft failing from chronic rejection (B). Note the well-developed cirrhosis with capsular and regenerative nodularity in (A) and its absence in (B).
Inflammation
The intensity of the portal/septal inflammation in chronic rejection specimens was usually mild (no. mild = 18, 82%; no. moderate = 1, 4%; no. severe = 3, 14%). The composition of the portal/septal infiltrate in both PBC and chronic rejection consisted largely of lymphocytes, macrophages and plasma cells. Periductal or intralobular granulomas with granulomatous duct lesions were present in 25 (24%) of the native PBC livers but were not a feature of the chronic rejection specimens (no. = 0). Foamy cells could be seen around damaged intermediate-sized ducts in chronic rejection. Moreover, neutrophils were frequently present at the periphery of the nodules in areas of ongoing biliary and ductular type piecemeal necrosis in PBC specimens but were not prominent in cases of chronic rejection. Mononuclear inflammation in and around the central veins and central venous sclerosis was present in 12 (55%) of the chronically rejected livers but was not seen in any of the PBC livers.
Bile Duct Changes
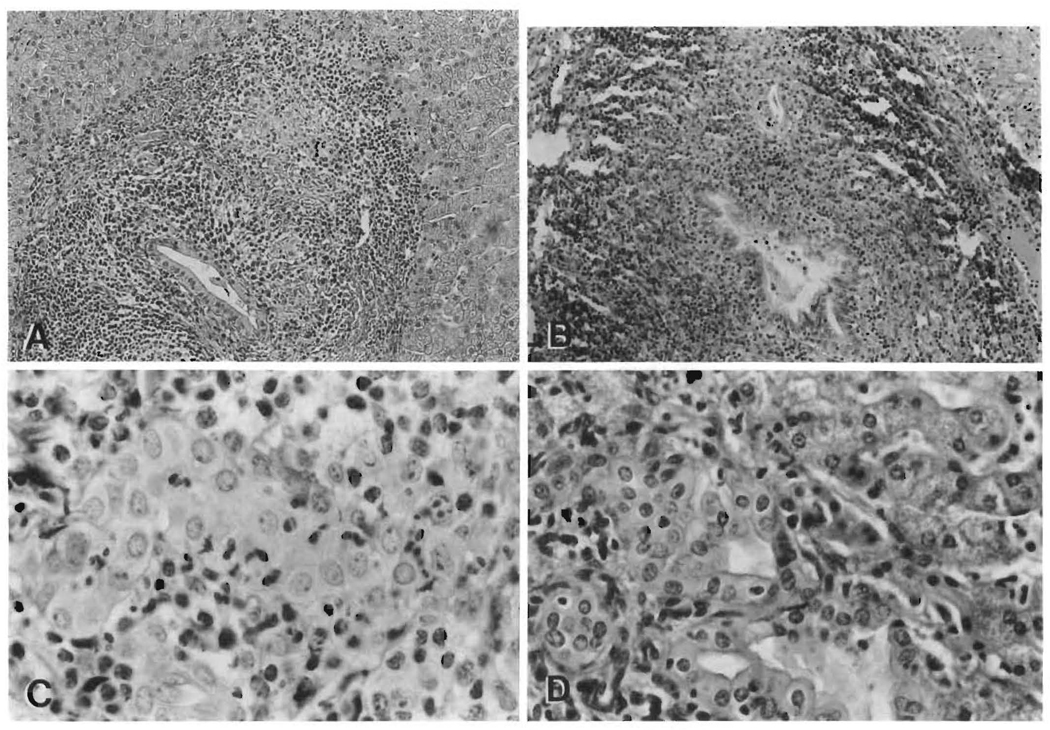
The small and intermediate-sized bile ducts were targets of the lymphocytic infiltration in both PBC and chronic liver allograft rejection, which presumably leads to bile duct damage, destruction and ultimately an apparent loss of bile ducts. Intermediate-sized ducts appeared to be the preferred targets in early PBC. In contrast, the smallest ducts and ductules were the preferred targets in acute and chronic rejection. Degenerative changes were seen in the biliary epithelium in both disorders and included cytoplasmic eosinophilia, vacuolization, syncytia formation, pyknosis and disruption of the basement membrane. Additional evidence of bile duct damage includes reactive changes in the epithelial cells such as enlargement, an increase in the nuclear/cytoplasmic ratio, stratification of the cells and the presence of nucleoli and mitosis (Figure 2).
FIG. 2.
Bile duct lesions. Both the intermediate- and small-sized bile ducts are the targets of putative lymphocytic mediated damage in PBC and allograft rejection. Note the similar appearance of the intermediate-sized duct lesions (except for the granulomatous reaction) in a pretransplant wedge biopsy from a PBC patient (A, H&E, 150×) and that seen posttransplantation in a failed allograft with chronic rejection (B, H&E, 150×). The smaller ducts are also targeted in both disorders, although somewhat more noticeably in allograft rejection (C, H&E, 450×). However, whereas prominent ductular proliferation is a hallmark of Stage 2 PBC (D, H&E, 400×), it is not a feature of allograft rejection.
The portal inflammation present in PBC cases insidiously extended into the lobule to involve the periportal hepatic parenchyma in stage II (4) (or greater) disease, whereas in chronic rejection, the periportal involvement was considerably less and appeared to be more of a “spillover” phenomenon, but nonetheless could be classified loosely as being a form of lymphocytic piecemeal necrosis.
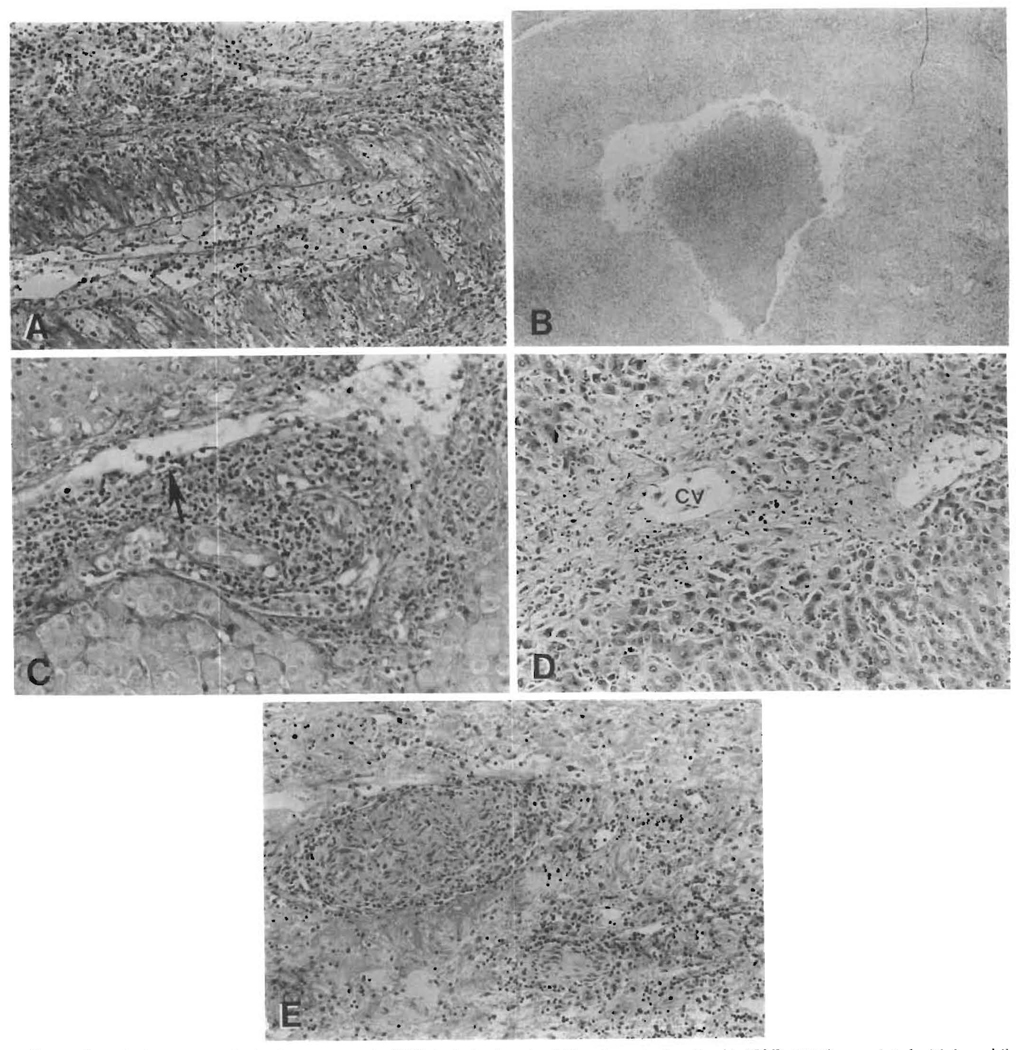
Structures which were targets of the cellular immune attack in allograft rejection other than bile ducts but not seen in PBC included hilar nerve trunks and the hepatic arterial and portal as well as hepatic venous systems (Figure 3).
FIG. 3.
Pathologic features of rejection, not seen in PBC, include chronic obliterative arteriopathy (A, H&E, 150×), associated with large bile duct damage such as mural necrosis, epithelial sloughing and acute cholangitis (B, H&E, 40×). Subendothelial infiltration of portal (C, arrow, H&E, 150×) and/or central veins, centrilobular ballooning, atrophy or necrosis with sclerosis and cholestasis (D, CV = central vein, H&E,100×) and mononuclear cell infiltration of hilar nerve trunks (E, H&E, 100×) are features of rejection but not of PBC.
Vascular Pathology
A lymphocytic subendothelial mononuclear cell infiltration of veins (portal and/or central) was present and characteristic of acute rejection (40%). A similar lesion was not seen in either the chronic rejection specimens or in the PBC specimens. Hepatic venular sclerosis with surrounding inflammation was present in 12 (55%) of the chronically rejected livers (Figure 3). The venous pathology present in native PBC livers appeared to be limited to the portal venous system and consisted of thrombi which were at various stages of organization.
A spectrum of arterial lesions was observed in chronic rejection specimens. These changes were present in nearly all of the chronically rejected allografts (no. = 21, 95%) and included: a deposition of subintimal foam cells (most common); a mild lymphocytic and/or neutrophilic arteritis; myointimal hyperplasia and intimal sclerosis (Figure 3). All of the arterial lesions described in allograft livers can be found in association with immunoglobulin and complement deposition (15). Subintimal foam cells were seen occasionally in venous channels and around bile ducts in failed allografts.
These arterial lesions were not a feature of PBC. However, a mild to moderate intimal sclerosis was often observed in hilar arterial vessels of native livers removed for PBC. Small and medium-sized arteries present in the fibrous septae of cirrhotic PBC specimens demonstrated focal medial thickening and an intimal sclerosis involving only a partial circumference of the vessel. Two PBC cases showed a focal necrotizing arteritis. Subintimal foam cells were not found in any PBC cases.
Parenchymal Changes
Reflective of their gross appearance, the majority (no. = 101, 95%) of native hepatectomy specimens removed from patients with PBC displayed a well-developed cirrhosis. In contrast, well-developed cirrhosis was not detected in any of the failed allografts in which rejection was the only cause of graft failure or in any of the allografts removed from a patient having had PBC. Two failed allografts removed from patients transplanted for a condition other than PBC were cirrhotic. The processes separate from or in addition to rejection which were thought to contribute to the development of cirrhosis in these two cases were viral liver disease (type B) and “autoimmune” hepatitis.
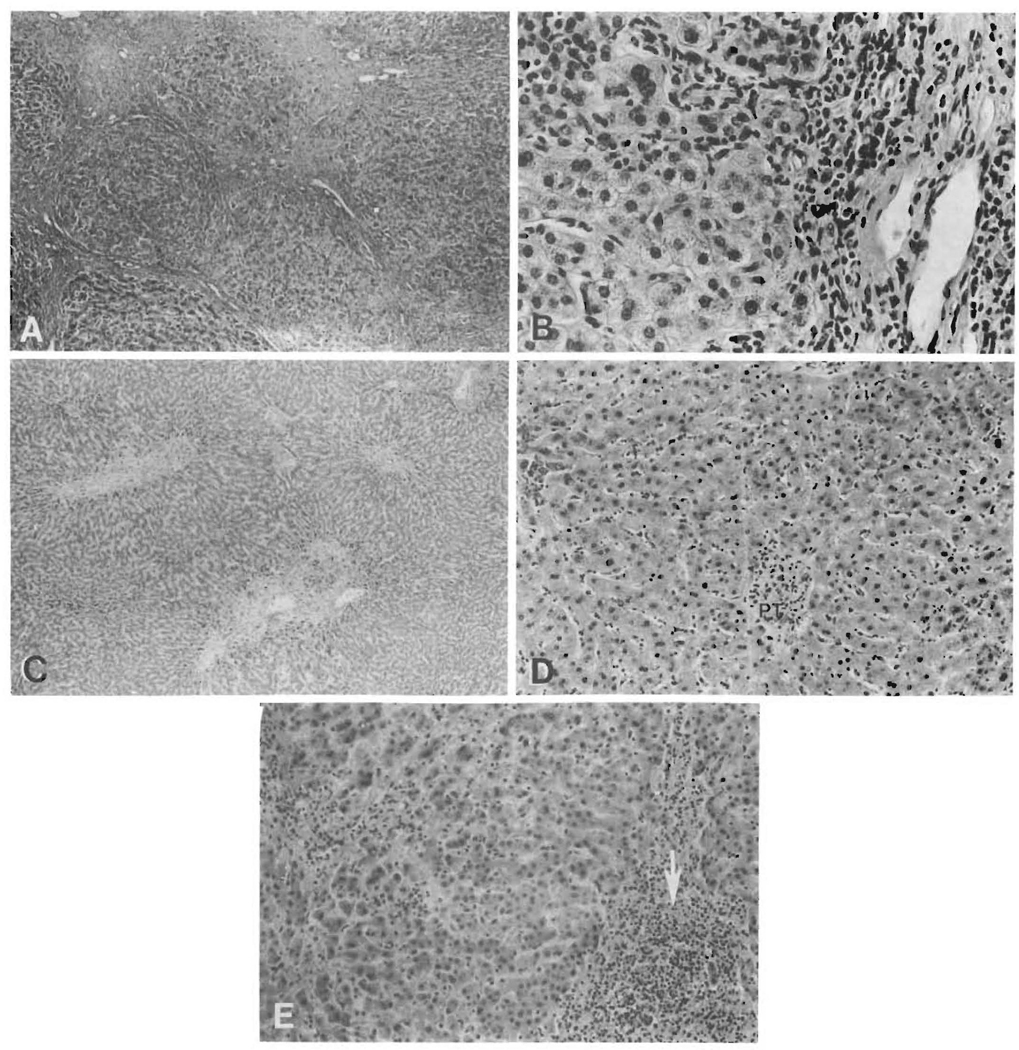
The appearance of the periportal region of chronic rejection and PBC differed importantly (Figure 4). In general, there was an irregular margin between the portal tract/septae and the lobule in PBC, whereas in chronic rejection the limiting plate was either nonbreached or had a “moth-eaten” appearance (19 cases; 87%). The irregular margin in PBC was the result of a typical “piecemeal necrosis,” which included all four types (lymphocytic, biliary, ductular and fibrotic) seen in classic cases of PBC (16, 17). All but six (94%) of the native PBC livers demonstrated a mixture of the biliary and fibrotic types of piecemeal necrosis. The native PBC livers with a predominant lymphocytic and ductular type of piecemeal necrosis were judged to be in Stage 2 to 3 or 3. The periportal hepatocellular damage seen in chronic rejection (no. = 3, 13%) did not particularly fit the typical appearance of any particular type of piecemeal necrosis but most closely resembled the lymphocytic type (Figure 4). Biliary, ductular and fibrotic types were not evident in livers with chronic rejection.
FIG. 4.
Differences in the periportal regions. Reflective of their gross appearance, the majority of the native PBC specimens were cirrhotic (Stage 4) (A, H&E, 40×), presumably as a result of the ongoing activity at the edge of the limiting plate (B, H&E, 400×). This in contrast to chronic rejection (C, H&E, 40×) where there was a lack of a well-developed cirrhosis and most often only a mild residual portal infiltrate (D, PT = portal tract, H & E, 150X). Even when the residual portal infiltrate was more intense (E, arrow, H&E, 150×), destruction of periportal hepatocytes was not a prominent feature.
Marginal ductular proliferation, a hallmark of the second stage of PBC (Figure 2), was not a prominent feature of chronic rejection, even in the face of extensive ductular damage and loss.
Other histologic differences between advanced PBC and chronic rejection seen in the periportal region include the presence of a “clearing” between the portal tract/septae and the lobule, hyalin (no. = 21, 20%) and copper-associated protein deposits (no. = 23/25, 93%) in periportal hepatocytes and chole/cholate-stasis (no. = 101,96%), all of which were seen in the PBC specimens but were absent in all of the cases of chronic rejection.
Cholestasis was always seen at the endstage of both disorders, but consistently differed in its location. The cholestasis associated with rejection, whether in the acute or chronic stage, was always predominantly centrilobular whereas the cholestasis seen in PBC was most prominent periportally and spilled over toward the center of regenerative nodules. Copper-associated protein deposition was not a prominent feature of chronic rejection but was consistently seen in advanced PBC.
Lobular alterations present in both disorders included small clusters of sinusoidal foam cells, a mild spotty hepatocellular necrosis and slight Kupffer cell activation.
Hilar structures, including the major bile ducts, were largely unaffected in PBC. In contrast, focal mural necrosis, fibrosis, lymphocytic epithelial infiltration, epithelial sloughing and acute cholangitis (Figure 3) were seen in large hilar bile ducts either as a consequence or in association with rejection (no. = 6, 27%). Some of the large bile duct lesions seen in chronic rejection were clearly the result of coexistent arterial pathology which was present in the cases of rejection but not in PBC.
Hilar Lymph Nodes
Enlarged hilar lymph nodes (up to 3 cm) were commonly seen in acutely rejecting livers and in endstage PBC. The nodes from 52 livers removed for PBC were available for examination. Twelve (23%) of these contained granulomas (nonlipid), two of which were necrotizing (4%). Eosinophilic histiocytic cells in the cortex and paracortex, atrophic “burnt out” germinal centers and sinusoids filled with pigmented macrophages (no. = 51, 94%) were common features in these nodes. None of these features were seen in the hilar nodes from livers that were acutely rejected except for the relative lack of germinal center formation and an occasional poorly formed granuloma associated with infective organisms. The histologic features of nodes from acutely and chronically rejecting allografts consist of changes similar to those described for graft vs. host (11).
A summary of the histologic comparison of acute and chronic rejection to PBC is shown in Table 3.
TABLE 3.
Histopathologic comparison of PBC and liver allograft rejection
| Acute rejection | Early PBC | Chronic rejection | Late PBC | |
|---|---|---|---|---|
| Portal/septal inflammation | ||||
| Intensity | +++ | +++ | + | ++ |
| Naturea | L, but mixed | L, G, P | L, P | L, P, G |
| Bile duct lesion | ||||
| Presence | ++ | ++ | +++ | + |
| Sizeb | S, M, L | M, S | S, M, L | M, S |
| Bile duct loss | 0 | 0 | +++ | +++ |
| Piecemeal necrosis | ± | ± | ± | ++ |
| Cholangiolar proliferation | ± | ++ | − | + |
| Cholestasisc | C | − | C | P, C |
| With copper deposition | None | None | None | +++ |
| Arterial lesionsd | Inflammatory or necrotizing | None | SIFC, T | IS |
| Venous lesionse | SEL | None | SIFC, CVS | PVT |
Nature of inflammation: L = lymphocytic/monocytic; G = granulomatous; P = plasmacytic.
S = small (<50 µm); M = medium (≥75 µm); L = hilar excretory ducts. Listed in order of prevalence.
Location of cholestasis: C = central; P = peripheral/paraseptal.
SIFC = subintimal foam cells; IS = intimal sclerosis; T = thrombosis.
SEL = subendothelial lymphocytes; CVS = central vein sclerosis; PVT = portal vein thrombi.
DISCUSSION
We were unable to find a difference in the incidence or pathologic appearance of histologically documented episodes of acute cellular rejection between patients transplanted for PBC and those grafted for other indications. Nor was patient or graft survival affected by the presence of PBC prior to transplantation. We did, however, note a trend toward an increased incidence of allograft loss because of chronic rejection in PBC patients as compared to those who did not have this disorder. In addition, we were unable to diagnose recurrent PBC after transplantation.
The argument could be made that our observation of a higher incidence of graft failure because of chronic rejection and lack of confirmation of recurrent PBC was the result of a mistaken histopathologic interpretation. This argument becomes even more appealing if one conjectures that recurrent disease in an allograft might be different histopathologically since the patients were receiving potent immunosuppressive therapy. Although we have been unable to recognize recurrent disease in the allograft, the data presented do not offer definitive proof that PBC cannot recur after transplantation. We can only state that we were unable to diagnose the disease using the criteria offered by Neuberger et al. (10). The reasons we were unable to diagnose recurrent disease with certainty included the different histopathologic appearance of PBC and chronic rejection, the lack of correlation of AMA levels with liver dysfunction and/or hepatic pathology and our ignorance of the effect of cyclosporin therapy on the histopathology of PBC.
One of the most striking histopathologic differences between PBC and chronic rejection is the nature and extent of the vascular pathology. Immune mechanisms probably account for the arterial lesions of rejection, which were not prominent in PBC. However, altered hemodynamics, infections and possibly altered lipid metabolism may also play a role. Altered lipid metabolism and hemodynamics may account for the mild arteriosclerotic changes observed in PBC livers. Nevertheless, it must be stated clearly that the arterial changes seen in PBC are quite mild when compared to those seen in rejection.
The architectural consequences of the putative Immune damage, particularly in the periportal regions, and the size of damaged bile ducts (see “Results”) also differs between these two disorders. This probably accounts for the frequent development of cirrhosis in PBC and its relative infrequency in chronic rejection. The reason for this difference is unclear at present. It may be due to an intrinsic difference in antigenic targets, alterations in the host immune system after transplantation because of obligatory immunosuppressive therapy or surgical interventions prior to the development of cirrhosis.
The differences in the histopathologic appearance of chronic rejection and PBC are probably related to the putative targets of immunologic attack in each disorder.
The targets of the lymphocytic attack occurring as part of the rejection reaction in any solid organ allograft are thought to be the major histocompatibility complex antigens which are expressed on structures targeted by the rejection reaction (8,9). Functional analysis of graft-infiltrating lymphocytes cultured from liver specimens during a rejection reaction have confirmed proliferative and cytotoxic activity directed at these antigens (18).
The precise antigenic target(s) in PBC has yet to be precisely defined, but several reports have implicated biliary glycoproteins (19–22). However, the specificity and role of these antigens or even the effector mechanisms involved in the liver damage in PBC are poorly understood.
The serologic data presented indicate that there is either recurrence or failure to lose AMA, following transplantation in the vast majority of PBC patients. The presence of elevated postoperative AMA titers was not associated with any evidence of hepatic dysfunction which could be detected by utilizing standard liver injury parameters to monitor hepatic function in either this series (13) or that of Haagsma et al. (23). Similarly, no apparent correlation between the AMA titers and presence of chronic rejection was seen in this study. Therefore, the precise role of these antibodies in the pathogenesis of PBC is quite unclear.
The postoperative clinical course appears to be a clinical criterion for distinguishing between the recurrent PBC and allograft rejection in that rejection tends to pursue an accelerated course while recurrent PBC, If It occurs at all, appears to progress indolently (10). Based on this the livers removed from the patients in our series who were originally transplanted for PBC most likely experienced rejection rather than recurrent PBC as all of the allografts failed within 3 years of the date of the transplant procedure (Table 2). The validity of this particular criterion must be questioned, however, because patients whose original disease was not PBC can experience progressive dysfunction and graft failure with bile duct loss over a period of time which is reported to be more consistent with recurrent PBC (5 to 6 years).
There are numerous possible explanations for the increased incidence of chronic rejection in PBC patients, including patient age, sex, individual variations of immunosuppressive therapy, etc. These factors were not taken into consideration. A highly speculative conjecture for this perceived difference may be that a PBC patient’s immune system may be specifically (i.e. against biliary antigens) or nonspecifically “sensitized.” In this sense, the unique immunologic milieu of patients with PBC may be a contributory factor in the postoperative course. Perhaps mechanisms other than, or in addition to, an immune response directed at incompatible major histocompatibility complex antigens is occurring in patients transplanted for PBC.
Despite the apparent pathologic differences between chronic rejection and PBC, a clear separation of these two disease processes may be made difficult as a result of the cyclosporin therapy. The difficulty of using pathologic observations for the diagnosis of PBC after transplantation is underscored by the experience at Cambridge (10). Since their original report, the Cambridge group has identified patients who developed a PBC-like syndrome (clinically and pathologically) after transplantation but did not have the disease originally, nor did they convert to AMA positivity (24). Until the genesis of PBC (including the role of AMA) and the effect of cyclosporin therapy on PBC histology are better understood, we may be forced to wait for more definitive disease markers to determine if PBC recurs or not.
Although this series is rather large in comparison to others, it should be remembered that the number of posttransplant biopsies taken later than 3 years posttransplant in our study is limited. In addition, because our patients have been maintained on cyclosporin whereas those reported by the Cambridge group having recurrent disease received azathioprine and not cyclosporin, it may be impossible to reconcile the pathologic findings of the two groups. Finally, no number of negative cases can prove that PBC or any other disorder cannot recur; only one classic case needs to be documented to prove otherwise (10). Differences in the postoperative course of patients transplanted for PBC have been seen compared to those transplanted for other disorders. However, these differences have not affected the early (<4 years) graft or patient survival in the majority of patients transplanted for PBC. This is opposed to disorders such as viral hepatitis B (25) or certain hepatic malignancies (26) in which recurrent disease is more easily proven and affects both graft and patient survival.
REFERENCES
- 1.Epstein O. The pathogenesis of primary biliary cirrhosis. Mol Aspects Med. 1985;8:293–305. doi: 10.1016/0098-2997(85)90011-1. [DOI] [PubMed] [Google Scholar]
- 2.Epstein O, Thomas HC, Sherlock S. Primary biliary cirrhosis is a dry gland syndrome with features of chronic graft versus host disease. Lancet. 1980;1:1166–1168. doi: 10.1016/s0140-6736(80)91621-9. [DOI] [PubMed] [Google Scholar]
- 3.James SP, Hoofnagle JH, Stober W, et al. NIH conference: primary biliary cirrhosis: a model autoimmune disease. Ann Intern Med. 1983;99:500–512. doi: 10.7326/0003-4819-99-4-500. [DOI] [PubMed] [Google Scholar]
- 4.MacSween RNM, Burt AD. The cellular pathology of primary biliary cirrhosis. Mol Aspects Med. 1985;8:269–291. doi: 10.1016/0098-2997(85)90010-x. [DOI] [PubMed] [Google Scholar]
- 5.Fennell R. Ductular damage in liver transplant rejection. Its similarity to that of primary biliary cirrhosis and graft versus host disease. Pathol Annu. 1981;2:289–294. [PubMed] [Google Scholar]
- 6.Portmann B, O’Grady J, Williams R. Disease recurrence following orthotopic liver transplantation. Transplant Proc. 1986;18 Suppl. 4:136–143. [Google Scholar]
- 7.Bernuau D, Feldmann G, Deott C, et al. Ultrastructural lesions of bile ducts in primary biliary cirrhosis: a comparison with the lesions observed in graft versus host disease. Hum Pathol. 1981;12:782–793. doi: 10.1016/s0046-8177(81)80081-0. [DOI] [PubMed] [Google Scholar]
- 8.Ballardini G, Mirakian R, Bianchi FB, et al. Aberrant expression of HLA-DR antigens on bile duct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984;2:1009–1013. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- 9.Demetris AJ, Lasky S, Van Thiel DH, et al. Induction of DR/IA antigens in human liver allografts. An immuno-cytochemical and clinicopathologic analysis of twenty failed grafts. Transplantation. 1985;40:504–509. doi: 10.1097/00007890-198511000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neuberger J, Portmann B, MacDougall BRD, et al. Recurrence of primary biliary cirrhosis after liver transplantation. N Engl J Med. 1982;306:1–4. doi: 10.1056/NEJM198201073060101. [DOI] [PubMed] [Google Scholar]
- 11.Demetris AJ, Jaffe R, Starzl TE. A review of adult and pediatric post transplantation liver pathology. Pathol Annu. 1987;22(Part II):347–386. [PubMed] [Google Scholar]
- 12.Detre KM. The NIDDK liver transplantation database. In: Terasaki PJ, editor. Clinical Transplants. Vol. 29. Los Angeles: University of California; 1986. [PubMed] [Google Scholar]
- 13.Esquivel CO, Bernardos A, Demetris AJ, et al. Liver transplantation for primary biliary cirrhosis in 76 patients during the cyclosporine era. Gastroenterology. 1988;94:1207–1216. doi: 10.1016/0016-5085(88)90014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg PA, Reinhild K. Clinical and prognostic relevance of different mitochondrial antibody profiles in primary biliary cirrhosis (PBC) Mol Aspects Med. 1985;8:235–247. doi: 10.1016/0098-2997(85)90008-1. [DOI] [PubMed] [Google Scholar]
- 15.Demetris AJ, Markus BH, Burnham J, et al. Antibody deposition in liver allografts with chronic rejection. Transplant Proc. 1987;19:121–125. [PubMed] [Google Scholar]
- 16.Portmann B, Popper H, Neuberger J, et al. Sequential and diagnostic features in primary biliary cirrhosis based on serial histologic study in 209 patients. Gastroenterology. 1985;88:1777–1790. doi: 10.1016/0016-5085(85)90001-0. [DOI] [PubMed] [Google Scholar]
- 17.Popper H. The problem of histological evaluation of primary biliary cirrhosis. Pathol Anat Histol. 1978;379:99–102. doi: 10.1007/BF00432478. [DOI] [PubMed] [Google Scholar]
- 18.Fung JJ, Zeevi A, Starzl TE, et al. Functional characterization of infiltrating T-lymphocytes in human hepatic allografts. Hum Immunol. 1986;16:182–199. doi: 10.1016/0198-8859(86)90047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarlane IG. Autoreactivity against biliary tract antigens in primary biliary cirrhosis. Mol Aspects Med. 1985;8:249–267. doi: 10.1016/0098-2997(85)90009-3. [DOI] [PubMed] [Google Scholar]
- 20.McFarlane IG, Wojcicka BM, Tsantoulas DC, et al. Leukocyte migration inhibition in response to biliary antigens in primary biliary cirrhosis, sclerosing cholangitis and other chronic liver diseases. Gastroenterology. 1979;76:1333–1340. [PubMed] [Google Scholar]
- 21.Vento S, O’Brien CJ, McFarlane BM, et al. T-lymphocytes sensitization to hepatocyte antigens in autoimmune chronic active hepatitis and primary biliary cirrhosis: evidence for different underlying mechanisms and different antigenic determinants as targets. Gastroenterology. 1986;91:810–817. doi: 10.1016/0016-5085(86)90680-3. [DOI] [PubMed] [Google Scholar]
- 22.Baum H, Palmer P. The PBC-specific antigen. Mol Aspects Med. 1985;8:201–234. doi: 10.1016/0098-2997(85)90007-x. [DOI] [PubMed] [Google Scholar]
- 23.Haagsma EB, Manns M, Klein R, et al. Subtypes of antimitochondrial antibodies in primary biliary cirrhosis before and after orthotopic liver transplantation. Hepatology. 1987;7:129–133. doi: 10.1002/hep.1840070125. [DOI] [PubMed] [Google Scholar]
- 24.Portmann B, Wight DGD. Pathology of liver transplantation (excluding rejection) In: Calne R, editor. Liver transplantation. Ed. 2. Orlando, Florida: Grune and Stratton; 1987. pp. 437–479. [Google Scholar]
- 25.Demetris AJ, Jaffee R, Sheahan DG, et al. Recurrent hepatitis B in liver allograft recipients. Differentiation between viral hepatitis B and rejection. Am J Pathol. 1986;125:161–172. [PMC free article] [PubMed] [Google Scholar]
- 26.Iwatsuki S, Gordon RD, Shaw B, et al. Role of liver transplantation in cancer therapy. Ann Surg. 1985;202:401–407. doi: 10.1097/00000658-198510000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]