Abstract
Background
We have demonstrated that donor cell chimerism is associated with a lower incidence of obliterative bronchiolitis (OB) in lung recipients, and that donor chimerism is augmented by the infusion of donor bone marrow (BM). We herein report the intermediate results of a trial combining the infusion of donor BM and lung transplantation.
Methods
Clinical and in vitro data of 26 lung recipients receiving concurrent infusion of donor bone marrow (3.0 to 6.0 × 108 cells/kg) were compared with those of 13 patients receiving lung transplant alone.
Results
Patient survival and freedom from acute rejection were similar between groups. Of the patients whose graft survived greater than 4 months, 5% (1 of 22) of BM and 33% (4 of 12) of control patients, developed histologic evidence of OB (p = 0.04). A higher proportion (but not statistically significant) of BM recipients (7 of 10, 70%) exhibited donor-specific hyporeactivity by mixed lymphocyte reaction assays as compared with the controls (2 of 7, 28%).
Conclusions
Infusion of donor BM at the time of lung transplantation is safe, and is associated with recipients’ immune modulation and a lower rate of obliterative bronchiolitis.
We and others have reported that a low level of bone-marrow derived cells was detectable in the peripheral blood and tissues of long-term survivors of liver [1], kidney [2], heart [3], lung, and heart-lung [4] allografts. This phenomenon of donor cell chimerism, which occurs by seeding of the host’s tissues with cells from the graft [5], was associated with a lower incidence of chronic rejection in lung recipients [4]. To augment donor cell chimerism, in order to modulate the response of the recipient to the allograft, we initiated a prospective trial combining the infusion of donor bone marrow and lung transplantation. Reported herein is the intermediate outcome of this clinical trial.
Patients and Methods
This study was approved by the Institutional Review Board of the University of Pittsburgh on July 14,1993, and informed consent was obtained from all patients. Between September 1993 and July 1998, 26 adult patients received combined infusion of donor bone marrow and lung transplant. In addition, unavailability of consent to retrieve donor bone marrow has resulted in the accrual of 13 contemporaneous control patients, who received lung transplant alone. These patients, all undergoing primary lung transplantation, were not conditioned by myeloablative or myeloreductive regimen before transplantation.
Bone Marrow Preparation and Infusion
Donor bone marrow cells were isolated from thoracolumbar vertebrae as described [6]. Unmodified bone marrow cells, at a dose of 3.0 to 6.0 × 106 cells/kg of recipient’s body weight, were resuspended in 200 mL of the suspension medium, and infused into the patient within 2 hours after preparation, and between 6 to 10 hours after revascularization of the heart.
Immunosuppression
Immunosuppression consisted of tacrolimus (FK506, Prograf; Fujisawa USA, Deerfield, IL) and steroids, as previously described. During the first postoperative month, the dose of tacrolimus was targeted to maintain whole blood trough levels of 15 to 20 ng/mL. Depending on the side effects and history of rejection, tacrolimus dose was gradually reduced to achieve levels of 10 to 15 ng/mL. Methylprednisolone (500 mg; Upjohn Pharmaceuticals, Kalamazoo, MI) was given intraoperatively before revascularization of the lung graft. Subsequently, a short course of steroid cycle was initiated on postoperative day (POD) 0, starting with 200 mg of methylprednisolone per day administered intravenously in 4 divided doses. The dose of methylprednisolone was tapered by a daily decrement of 40 mg/day and converted to oral prednisone, (20 mg/day) on POD 5. Systematic reduction of prednisone dose (by 2.5 to 5 mg decrements) was initiated in all patients 3 months after transplantation, if there was no significant rejection by transbronchial biopsy. Azathioprine (2 mg/kg/day; Imuran; Burroughs Wellcome, Research Triangle Park, NC) of mycophenolate mofetil (2 g/day; Cell-Cept; Roche Laboratories, Basel, Switzerland) was added if there was recurrent rejection, or when renal dysfunction (serum creatinine > 2.0 mg/dL) necessitated a reduction of tacrolimus dose.
Monitoring and Treatment of Rejection
Surveillance bronchoscopy and transbronchial biopsy was performed in all patients between POD 14 and 21, unless clinical criteria warranted earlier intervention. Subsequently, surveillance biopsies were obtained every 3 months in the first year, and every fourth month in the second year. Thereafter, the biopsy schedule was dictated by clinical symptoms, and by results of pulmonary function tests. Follow-up biopsies are generally performed 3 to 4 weeks following treatment of rejection, or after cytomegalovirus pneumonia. Acute rejection was defined by histologic criteria [7], with grade II or higher considered significant, and required treatment. Previously described criteria were used for the histological diagnosis and clinical staging of obliterative bronchiolitis (OB) [8, 9]. The histological diagnosis for OB was made in a blinded fashioned. Acute rejection was treated with pulses of methylprednisolone (1 g/day for 3 consecutive days). Cytolytic therapy was used when the rejection was refractory to 2 to 3 courses of pulse steroids.
Detection of Chimerism
After transplantation, recipients were typed for donor chimerism in peripheral blood leukocytes by flow cytometry, as previously reported [6]. Blood samples (20 mL) from the patients were obtained on day 0 (time of transplantation), day 15, day 30, day 60, and then every other month during the first 2 years after transplantation, for the detection of donor cells. After staining with the appropriate antibody against donor MHC class I antigens, single-color fluorescence-activated cell sorting (FACS) analysis was performed to identify donor cells, using an EPICS Elite Flow Cytometer (Coulter Corp, Hialeah, FL). Fifty thousand events were collected per sample for analyses. Values of circulating donor cells of less than 0.5% were considered not quantifiable.
Immune Monitoring
Pretransplant and serial posttransplant (every other month) monitoring of recipients’ immune status was carried out by evaluating the proliferative responses of their peripheral blood leukocytes to mitogens (concanavalin A, phytohemagglutinin), and mixed leukocvte reactions (MLR), as previously described [10]. Recipients’ donor-specific MLR responses (D) at various times post-transplantation were compared to the recipients’ pre-transplant donor-specific responses, and to responses to cells from third party controls (TP). Donor-specific reactivity was classified according to the previously described criteria [10]. Briefly, donor-specific hyporeactivity (category I) was defined as at least a 70% decrease in posttransplant versus pretransplant donor-specific MLR responses, while maintaining reactivity to both third party stimulators (D/TP ratio < 40%) and to mitogens (> 50% of pretransplant responses). Donor-specific intermediate reactivity (category II) was designated when there was a 40% to 70% inhibition of antidonor activity with retention of third party responsiveness, whereas reactive (category III) meant that there was minimal or no decline in donor-specific nonreactivity. Suppression (category IV) connoted a nonspecific diminished proliferative response to mitogens as well as to alloantigens.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD), and compared using t-test or Mann-Whitney test when appropriate. Differences in proportions were compared using the χ2 or Fisher exact test. Survival and freedom from acute rejection were estimated by Kaplan-Meier method, and compared using the log-rank test. A p value less than 0.05 was considered statistically significant. A software package (CSS Statistica, Release 4.5; Statsoft, Tulsa, OK) was used for statistical analyses.
Results
Clinical Course and Patient Survival
The infusion of donor bone marrow was well tolerated. None of the 26 BM recipients developed graft-versus-host disease or had complications related to the infusion of donor bone marrow. The 1 and 3-year actuarial patient survival rates were 77%, 77% for the controls, and 81% and 77% for the BM group, respectively (p = 0.9). Seven patients in the BM group died from bacterial infection (n = 2), OB (n = 1), fungal infection (n = 1), renal failure (n = 1), cerebral bleeding (n = 1), and cardiac arrest (n = 1). In the control group there were 5 deaths, and the causes include bacterial infection (n = 1), OB (n = 1), respiratory failure (n = 1), pancreatitis (n = 1), and multisystem organ failure (n = 1).
Acute Rejection and Obliterative Bronchiolitis
The linearized rejection rates (episode per patient) during the first 6 months after transplantation were 2.6 ± 0.3 and 2.0 ± 0.2 in the BM and control groups, respectively (p = 0.5). Only patients who survived for at least 6 months after transplantation were included in the calculation of the linearized rejection rate. Freedom from acute rejection at 100 days after transplantation (by Kaplan-Meier method) was 25% in the bone marrow group and 8% in the control (p = 0.9).
Of the patients whose graft survival time was greater than 4 months, 5% (1 of 22) bone marrow patients and 33% (4 of 12) control patients developed histological evidence of OB on transbronchial biopsy (p = 0.04). Two patients in the bone marrow group (2 of 22) and 2 in the control group (2 of 12) have clinical bronchiolitis obliterans syndrome (BOS), and one in each group had both OB and BOS. In other words, the proportion of patients with either OB or BOS in the bone marrow and control groups was 9% (2 of 22) and 42% (5 of 12), respectively (p = 0.07; Table 1).
Table 1.
Prevalence of Pathological Obliterative Bronchiolitis and Clinical Bronchiolitis Obliterans Syndrome
| Patient Group | Bone Marrow | Control | p Valuea |
|---|---|---|---|
| Surviving >4 months | 22 | 12 | |
| With OB | 1 (5%) | 4 (33%) | 0.04 |
| With BOS | 2 (9%) | 2 (17%) | 0.60 |
| With either OB or BOS | 2 (9%) | 5 (42%) | 0.07 |
Two-tailed, Fisher exact test.
BOS = bronchiolitis obliterans syndrome; OB = obliterative bronchiolitis.
Immunosuppression Profiles
At last follow-up, or at the time of death, the dose and level of tacrolimus, the dose of prednisone, and the number of patients on prednisone were similar between the two groups (Table 2), However, the number of patients requiring a third drug (either azathioprine or mycophenolate mofetil), because of persistent rejection or renal dysfunction (defined as having a serum creatine > 2 mg/dL), is higher in the control group (77% [10 of 13] versus 17% [4 of 23], P = 0.0009).
Table 2.
Immunosuppression Profilesa of Controls and Lung Recipients Receiving Bone Marrow Infusion
| Immunosuppression | Bone Marrow | Control |
|---|---|---|
| FK dose (mg) | 10.8 ± 7.1 | 8.2 ± 3.6 |
| FK level (ng/mL) | 13.3 ± 5.3 | 12.0 ± 3.9 |
| Prednisone dose (mg) | 8.3 ± 4.3 | 6.8 ± 2.3 |
| Patients on prednisone | 18/23 (78%) | 12/13 (92%) |
| Patients on third drug | 4/23 (17%) | 10/13 (77%)b |
Only in patients surviving more than 1 month after transplantation.
p = 0.0009, two-tailed, Fisher exact test.
FK = tacrolimus.
Donor Chimerism
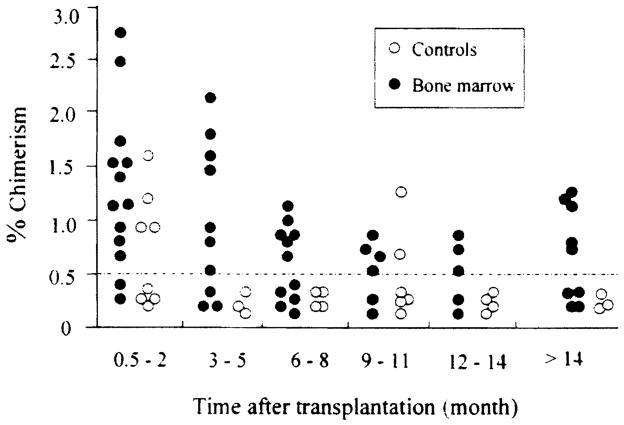
Detection of donor cells was feasible (when appropriate antibody to donor HLA antigens were available) in 13 BM patients and 8 controls. The numbers are too small for meaningful statistical analyses, however there is trend toward higher level of donor chimerism in the peripheral blood of BM patients, as compared with the controls (Fig 1, 2). In both groups the level of donor chimerism is higher in the early postoperative period, and decreases with time. However, the chimerism persists much longer in the BM group than in the control. None of the control group had detectable level of donor chimerism at 1 year, whereas 63% (5 of 8) of BM recipients still had detectable level of chimerism (by flow cytometry) more than 1 year after transplantation.
Fig 1.
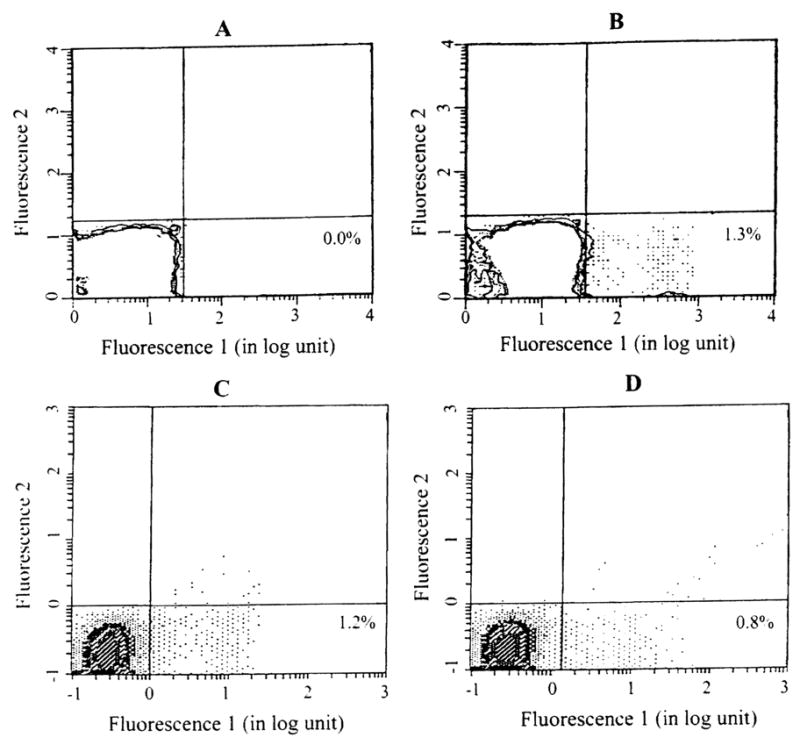
Donor chimerism in the peripheral blood leukocytes of a lung recipients who received bone marrow infusion. Recipient cells were stained with a primary mouse antibody against human MHC class I, then counterstained with fluorescein isothiocyanate (FITC) conjugated goat anti-mouse secondary antibody, and analyzed using an EPICS Elite Flow Cytometer (Coulter Corp, Hialeah, FL). (A) Before transplantation; (B) 1 month, (C) 6 months, and (D) and 9 months after transplantation. The number in the right lower quadrant indicates the percentage of donor cells. The level of chimerism was higher after transplantation, but dwindled with time.
Fig 2.
Donor cell chimerism detected by flow cytometry in lung recipients. Each circle represents 1 patient. Level below 0.5% is considered not quantifiable.
In Vitro Immune Testing
In vitro immune testing was possible only when donor splenocytes were available. Recipients’ donor-specific, mixed leukocyte responses to donor antigens at various times posttransplantation were compared with the recipient’s pretransplant responses. Using previously described criteria [10] we found that, a higher proportion (but not statistically Significant, p = 0.15) of BM recipients (7 of 10; 70%) exhibit donor-specific hyporeactivity as compared with the control group (2 of 7; 28%). Figure 3 depicts the profile of a mixed leukocyte response of a BM-lung recipient who demonstrated donor-specific hyporesponsiveness.
Fig 3.
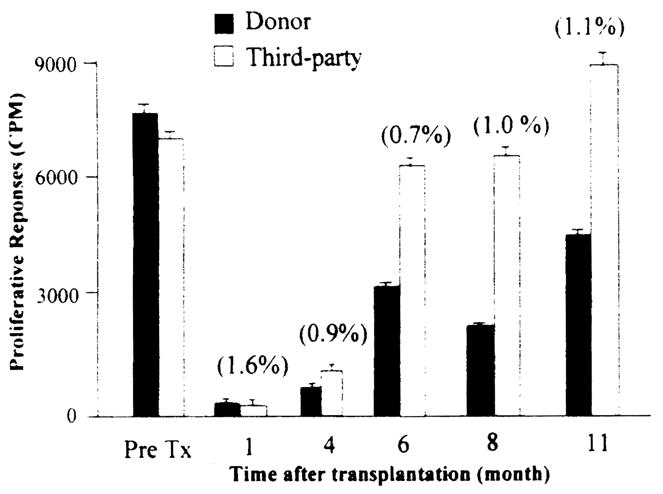
Mixed lymphocyte response demonstrating donor-specific hyporesponsiveness, lung recipient who received donor bone marrow infusion. Freshly isolated recipient peripheral blood leukocytes were used as responders, while γ-irradiated donor splenocytes and third-party cells were used as stimulators. The response to donor stimulators was lowered than that to third-party stimulators. Numbers in parentheses indicate the level of chimerism (by flow cytometry).
Comment
The use of bone marrow-derived cells (splenocytes) to achieve donor-specific transplantation tolerance in neonatal mice was first reported by Billingham, Brent, and Medawar [11]. Subsequently, chimerism and donor-specific transplantation tolerance was achieved in adult animals by preconditioning the host with different regimens which include, among others, total body irradiation [12], total lymphoid irradiation [13], and the use antilymphocyte globulin [14]. The clinical use of donor bone marrow to prolong the survival of organ allograft was first attempted in kidney transplant recipients. Monaco and colleagues [15] were the first to report the use of antilymphocyte globulin induction and delayed (25 days after organ transplantation) donor bone marrow infusion in a kidney transplant recipient. Subsequently, Barber and associates adopted the approach of Monaco, and preconditioned a series of renal transplant recipients with antilymphocyte globulin, cyclosporine, prednisone, and azathioprine before infusion of donor bone marrow, and demonstrated better graft survival and less acute rejection in the bone marrow group [16]. Low levels of donor chimerism were detected (by PCR) in approximately half of the bone marrow group [17]. In another study, Kahn and colleagues used high dose total lymphoid irradiation (5,400 to 6,000 cGy) to precondition a series of heart recipients before heart transplantation and intraperitoneal injection of donor bone marrow [18], and reported high perioperative death from infection. Of note is the fact that in these trials antilymphocytes globulin, and radiation had been used to precondition the recipients before bone marrow infusion.
The scientific rationale for the current study, which did not involve preconditioning of the recipient before bone marrow infusion, was based on the discovery by Starzl and associates, that donor cells of bone marrow origin persisted at low level in peripheral blood, lymphoid organs, and skin of long-surviving liver and kidney recipients [1, 2]. Based on these observations, we posited that donor cell chimerism was perhaps essential for the long-term allograft acceptance. Therefore, we hypothesized that augmenting this spontaneously occurring event with perioperative donor bone marrow infusion may further enhance the acceptance of the graft, especially of those organs which are not endowed with a large quantity of passenger leukocytes. To test this hypothesis, in December 1992, we initiated a trial combining donor bone marrow infusion with solid organ transplantation, without preconditioning of the host [6]. Our aim was to augment this de novo phenomenon (chimerism) with the hope to reduce the incidence of rejection.
The preliminary results in lung recipients reported herein, indicate that the infusion of unmodified donor bone marrow concurrently with lung transplantation is safe, and is associated with a trend towards higher level of donor cell chimerism, and less donor alloreactivity (by MLR assay). Patients in the bone marrow group had less requirement for immunosuppression (17% of the BM patients required a third drug beside tacrolimus versus 77% of the controls), lower incidence of obliterative bronchiolitis, at least by histologic criteria, than the control. One of the limitations of the current study is its small sample size and its short follow-up.
Following our initial report [6], the transplant group at the University of Miami initiated a series of studies using single and multiple infusions of donor bone marrow in kidney and liver recipients. Our results in lung recipients reported herein are in agreement with the data on kidney recipients from this group. Garcia-Morales and colleagues [19] studied 40 kidney recipients who received unmodified donor bone marrow infusion, and 100 controls who received kidney alone. Their immunosuppression protocol included OKT3 induction therapy, tacrolimus, and steroid maintenance therapy, and in some patients, mycophenolate mofetil. The authors used a newly developed PCR-flow assay (a combination of PCR and flow cytometric techniques that detect donor versus recipient histocompatibility genes as well as cell surface CD epitope markers) to measure donor cell chimerism in the recipient’s peripheral blood lymphocytes (PBL) and bone marrow. The bone marrow recipients have higher level of donor chimerism than the controls. Notably, the level of donor chimerism (especially CD3+ and CD34+ cells) was 10-fold higher in the bone marrow compartment than in the peripheral blood leukocytes. Immunologically, the bone marrow patients displayed more depressed humoral and cellular immune responses than the controls. Recipients who were HLA-DR identical with their donors had a high level of chimerism, and no acute rejection. In their most recent update [20], these investigators analyzed the results of 63 cadaveric kidney recipients who received either one (n = 21), or two (n = 42) infusions of donor bone marrow and 220 cadaveric kidney recipients who did not receive bone marrow (controls). Although there was no difference in the rates of acute rejection, the incidence of chronic rejection in the bone marrow group was lower than the control (p < 0.02). The dose of bone marrow cells and the timing of their infusion appear to influence the immune modulation of the hosts. Ricordi and colleagues administered donor bone marrow to liver transplant recipients at varying schedules after transplantation [21]. Patients receiving multiple infusion of bone marrow cells had significantly longer graft survival than those receiving a single infusion.
Collectively, the results of the present study, along with other clinical trials in which donor bone marrow cells were infused into recipients of solid organs, suggest that donor bone marrow cells may have a modulatory effect on the recipient’s immune systems, resulting in a salutary impact on chronic allograft rejection. While the long-term effect of the donor bone marrow infusion in lung recipients remains speculative, because of the small sample size and relatively short follow-up duration, it is conceivable that presence of donor chimerism will enhance the acceptance of the graft and reduce the incidence of chronic rejection. Future study with larger sample size and longer follow-up will clarify this issue.
Table 3.
Demographics of Lung Recipients
| Variables | Bone Marrow | Control |
|---|---|---|
| n | 26 | 13 |
| Recipient age (y) | 48.1 ± 11.0 | 50.23 ± 8.0 |
| Donor age (y) | 25.3 ± 10.9 | 30.9 ± 10.1 |
| Sex (M:F) | 18:8 | 3:10a |
| Type of transplant | ||
| Single lung | 19.0 | 9.0 |
| Double lung | 7.0 | 3.0 |
| Heart lung | 2.0 | 1.0 |
| Donor ischemic time (min) | 235.1 ± 76.2 | 279.6 ± 51.0 |
| MHC mismatch | 4.8 ± 1.0 | 4.9 ± 1.0 |
| Follow-up duration (days) | 729 ± 475 | 841 ± 445 |
p < 0.05, Fisher’s exact test.
MHC = major histocompatibility complex.
Acknowledgments
Support in part by the American College of Surgeons Faculty Fellowship, The Thoracic Surgery Research Foundation, and the American Lung Association (Si M. Pham), and NIH Grant No. 1R01 A140329-01 (to john J. Fung and Abdul S. Rao).
Footnotes
Presented at the Thirty-fifth Annual Meeting of The Society of Thoracic Surgeons, San Antonio, TX, Jan 25-27, 1999.
References
- 1.Starzl TE, Demetris A, Trucco M, et al. Systemic chimerism in human female recipients of male livers. Lancet. 1992;340:876–7. doi: 10.1016/0140-6736(92)93286-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Demetris AJ, Trucco M, et al. Chimerism and donor specific nonreactivity 27 to 29 years after kidney allotransplantation. Transplantation. 1993;55:1272–7. doi: 10.1097/00007890-199306000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlitt HJ, Hundrieser J, Hisanaga M, et al. Patterns of donor-type microchimerism after heart transplantation. Lancet. 1994;343:1469–71. doi: 10.1016/s0140-6736(94)92584-4. [DOI] [PubMed] [Google Scholar]
- 4.Keenan RJ, Zeevi A, Banas R, et al. Microchimerism is associated with a lower incidence of chronic rejection after lung transplantation. J Heart Lung Transplant. 1994;13:532. [Google Scholar]
- 5.Starzl TE, Demetris AJ, Murase N, et al. Cell migration, chimerism, and graft acceptance. Lancet. 1992;339:1579–82. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fontes P, Rao AS, Demetris AJ, et al. Bone marrow augmentation of donor-cell chimerism in kidney, liver, heart, and pancreas islet transplantation. Lancet. 1994;344:151–5. doi: 10.1016/s0140-6736(94)92756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 8.Yousem SA, Berry GJJ, Brunt EM, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1990;9:593–601. [PubMed] [Google Scholar]
- 9.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. J Heart Lung Transplant. 1993;12:713–6. [PubMed] [Google Scholar]
- 10.Zeevi A, Pavlick S, Lombardozzi R, et al. Immune status of recipients following bone marrow-augmented solid organ transplantation. Transplantation. 1995;59:616–20. doi: 10.1097/00007890-199502270-00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–6. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 12.Ildstad ST, Sachs DT. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 13.Slavin S, Strober S, Fukes Z, et al. Induction of specific tissue transplantation tolerance using fractionated total lymphoid irradiation in adult mice: long-term survival of allogeneic bone marrow and skin grafts. J Exp Med. 1977;146:34–48. doi: 10.1084/jem.146.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Monaco AP. Use of antilymphocyte serum in the induction of immunological tolerance to tissue allografts. Fed Proc. 1970;29:153–5. [PubMed] [Google Scholar]
- 15.Monaco AP, Clark AW, Wood ML, et al. Possible active enhancement of a human cadaver renal allograft with antilymphocyte serum (ALS) and donor bone marrow: case report of an initial attempt. Surgery. 1976;79:384–92. [PubMed] [Google Scholar]
- 16.Barber WH, Mankin JA, Laskow DA, et al. Long-term results of a controlled prospective study with transfusion of donor specific bone marrow in 57 cadaveric renal allograft recipients. Transplantation. 1991;51:70–5. doi: 10.1097/00007890-199101000-00011. [DOI] [PubMed] [Google Scholar]
- 17.McDaniel DO, Naftilan J, Hulvey K, et al. Peripheral blood chimerism in renal allograft-recipients with donor bone marrow. Transplantation. 1994;57:852–6. doi: 10.1097/00007890-199403270-00014. [DOI] [PubMed] [Google Scholar]
- 18.Kahn DR, Hong R, Greenberg AJ, et al. Total lymphatic irradiation and bone marrow in human heart transplantation. Ann Thorac Surg. 1984;38:169–75. doi: 10.1016/s0003-4975(10)62227-8. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Morales R, Carreno M, Mathew J, et al. The effects of chimeric cells following donor bone marrow infusions as detected by PCR-flow assays in kidney transplant recipients. J Clin Invest. 1997;99:1118–29. doi: 10.1172/JCI119240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J, Mathew J, Garcia-Morales R, et al. The human bone marrow as an immunoregulatory organ. Transplantation. 1999;68:1079–90. doi: 10.1097/00007890-199910270-00001. [DOI] [PubMed] [Google Scholar]
- 21.Ricordi C, Karatzas T, Nary J, et al. High-dose donor marrow infusions to enhance allograft survival: the effect of timing. Transplantation. 1997;63:7–11. doi: 10.1097/00007890-199701150-00003. [DOI] [PubMed] [Google Scholar]