Abstract
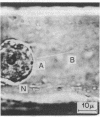
The ability of infrared laser traps to apply controlled forces inside of living cells is utilized in a study of the mechanical properties of the cytoplasm of plant cells. It was discovered that infrared traps are capable of plucking out long filaments of cytoplasm inside cells. These filaments exhibit the viscoelastic properties of plastic flow, necking, stress relaxation, and set, thus providing a unique way to probe the local rheological properties of essentially unperturbed living cells. A form of internal cell surgery was devised that is capable of making gross changes in location of such relatively large organelles as chloroplasts and nuclei. The utility of this technique for the study of cytoplasmic streaming, internal cell membranes, and organelle attachment was demonstrated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. S., Allen R. D. Cytoplasmic streaming in green plants. Annu Rev Biophys Bioeng. 1978;7:497–526. doi: 10.1146/annurev.bb.07.060178.002433. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M. Optical trapping and manipulation of viruses and bacteria. Science. 1987 Mar 20;235(4795):1517–1520. doi: 10.1126/science.3547653. [DOI] [PubMed] [Google Scholar]
- Ashkin A., Dziedzic J. M., Yamane T. Optical trapping and manipulation of single cells using infrared laser beams. Nature. 1987 Dec 24;330(6150):769–771. doi: 10.1038/330769a0. [DOI] [PubMed] [Google Scholar]
- Berns M. W. Directed chromosome loss by laser microirradiation. Science. 1974 Nov 22;186(4165):700–705. doi: 10.1126/science.186.4165.700. [DOI] [PubMed] [Google Scholar]
- Berns M. W. Partial cell irradiation with a tunable organic dye laser. Nature. 1972 Dec 22;240(5382):483–485. doi: 10.1038/240483a0. [DOI] [PubMed] [Google Scholar]
- Block S. M., Blair D. F., Berg H. C. Compliance of bacterial flagella measured with optical tweezers. Nature. 1989 Apr 6;338(6215):514–518. doi: 10.1038/338514a0. [DOI] [PubMed] [Google Scholar]
- Brotschi E. A., Hartwig J. H., Stossel T. P. The gelation of actin by actin-binding protein. J Biol Chem. 1978 Dec 25;253(24):8988–8993. [PubMed] [Google Scholar]
- Cecchi G., Griffiths P. J., Taylor S. Stiffness and force in activated frog skeletal muscle fibers. Biophys J. 1986 Feb;49(2):437–451. doi: 10.1016/S0006-3495(86)83653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskell R. C., Carlson F. D. Quasi-elastic light-scattering studies of single skeletal muscle fibers. Biophys J. 1981 Jan;33(1):39–62. doi: 10.1016/S0006-3495(81)84871-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Koonce M. P., Euteneuer U., McDonald K. L., Menzel D., Schliwa M. Cytoskeletal architecture and motility in a giant freshwater amoeba, Reticulomyxa. Cell Motil Cytoskeleton. 1986;6(5):521–533. doi: 10.1002/cm.970060511. [DOI] [PubMed] [Google Scholar]
- Petersen N. O., McConnaughey W. B., Elson E. L. Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5327–5331. doi: 10.1073/pnas.79.17.5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Schwarz W. H., Pollard T. D. Dependence of the mechanical properties of actin/alpha-actinin gels on deformation rate. 1987 Feb 26-Mar 4Nature. 325(6107):828–830. doi: 10.1038/325828a0. [DOI] [PubMed] [Google Scholar]
- Sato M., Wong T. Z., Allen R. D. Rheological properties of living cytoplasm: endoplasm of Physarum plasmodium. J Cell Biol. 1983 Oct;97(4):1089–1097. doi: 10.1083/jcb.97.4.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P. Contractile proteins in cell structure and function. Annu Rev Med. 1978;29:427–457. doi: 10.1146/annurev.me.29.020178.002235. [DOI] [PubMed] [Google Scholar]
- Taylor D. L., Condeelis J. S. Cytoplasmic structure and contractility in amoeboid cells. Int Rev Cytol. 1979;56:57–144. doi: 10.1016/s0074-7696(08)61821-5. [DOI] [PubMed] [Google Scholar]
- YAGI K. The mechanical and colloidal properties of Amoeba protoplasm and their relations to the mechanism of amoeboid movement. Comp Biochem Physiol. 1961 Aug;3:73–91. doi: 10.1016/0010-406x(61)90134-7. [DOI] [PubMed] [Google Scholar]
- Zaner K. S., Hartwig J. H. The effect of filament shortening on the mechanical properties of gel-filtered actin. J Biol Chem. 1988 Apr 5;263(10):4532–4536. [PubMed] [Google Scholar]