Abstract
We investigate the utility of near-infrared spectroscopy (NIRS) as an alternative technique for studying infant speech processing. NIRS is an optical imaging technology that uses relative changes in total hemoglobin concentration and oxygenation as an indicator of neural activation. Procedurally, NIRS has the advantage over more common methods (e.g., fMRI) in that it can be used to study the neural responses of behaviorally active infants. Older infants (aged 6–9 months) were allowed to sit on their caretakers’ laps during stimulus presentation to determine relative differences in focal activity in the temporal region of the brain during speech processing. Results revealed a dissociation of sensory-specific processing in two cortical regions, the left and right temporal lobes. These findings are consistent with those obtained using other neurophysiological methods and point to the utility of NIRS as a means of establishing neural correlates of language development in older (and more active) infants.
The last 30 years have seen important advances in the development and application of behavioral measures that help us better understand how preverbal infants perceive and process speech. This work suggests that infants’ ability to perceive speech actually begins in the womb and progresses dramatically across the first year of life (DeCasper & Fifer, 1980; Werker & Tees, 1984). During the last trimester in-utero (Mehler et al., 1988) and during the first 12 months of life postnatally (Jusczyk, 1997), infants become aware of and adjust to regularities in their native language. By using various cues found in adult speech, infants gradually begin to understand and use their native language.
Findings from two recent experiments using functional magnetic resonance imaging (fMRI) (Dehaene-Lambertz, Dehaene, Hertz-Pannier, 2002; Dehaene-Lambertz et al., 2006) indicate that the neurological substrates necessary for language processing may already be present in very young infants. Dehaene-Lambertz and her colleagues (Dehaene-Lambertz, Dehaene, Hertz-Pannier, 2002) initially tested 3-month-old infants using fMRI and observed increases in left lateralized activity in the region of the planum temporale, in particular, in response to forward playing speech relative to reversed speech. More recently, Dehaene-Lambertz and colleagues (Dehaene-Lambertz et al., 2006) tracked the hierarchical pattern by which speech is processed in young infants (aged 2 to 4 months) and found an adult-like pattern of hemodynamic response delays along the superior temporal regions of the brain. More specifically, they observed a “channeling” of language processing toward the left perisylvian cortical regions.
Findings from these studies have focused attention on the left temporal cortex as a primary area of interest for investigations into the neurobiology of language development. However, and despite the way these studies have advanced our understanding of infant language development and how it might be studied, use of fMRI to examine infant language development has limitations that other methods might address. In particular, use of fMRI makes it necessary for researchers to test infants at a young enough age that swaddling can be achieved to control physical movement.
Swaddling has drawbacks. Chief among these is the possibility that young infants may fall into a restive state. For instance, in the initial study using this approach, Dehaene-Lambertz observed that young infants slept through much of the study (Dehaene-Lambertz et al., 2002). Some concerns about this were addressed in her more recent report (Dehaene-Lambertz et al., 2006), in which only one infant in that investigation was observed to have fallen asleep during observation periods in the magnet. Moreover, the authors reported that, in both studies, infants’ neuro-physiological responses to auditory stimuli were consistent across sleeping and waking states. Nevertheless, given the debate about the degree to which conscious processing of sensory stimuli is possible in various stages of sleep even in adults (e.g., Czisch et al., 2002; Redcay, Kennedy, & Courchesne, 2007), there is a need for procedures that do not promote sleeping states in infants or that require researchers to embrace statistical null effects (often on small samples) in order to establish that sleeping did not exert unwanted influences on the observed data.
There are other reasons to be concerned about the effects of swaddling. Consider older infants and the topics one might want to study with older-infant samples. The second half of the first year of life is a period of time during which infants begin to display a wealth of perceptual abilities that help them learn their native language (Jusczyk, 1997). It is thus important for researchers to study the brain function of older infants who are actively attending to the experimental stimuli. Such investigations will not be possible, however, if researchers rely on imaging technology that places strict limitations on infants’ movement or on their ability to respond in a dynamic fashion to perceptual stimuli. Swaddling not only prevents researchers from pursuing methods that allow infant activity, there also are practical limitations that might prevent researchers from using this method with some samples. Although older infants are less at risk of falling asleep as a result of this procedure, any attempt to control their motion in this way might present considerable challenges to the researchers who attempt it. This type of behavioral restriction could cause a level of distress and resistance among older infants that would prevent investigators from going forward with their study protocols.
NEAR-INFRARED SPECTROSCOPY
One method that is being used increasingly with healthy infants in an experimental setting is near-infrared spectroscopy (NIRS) (Baird et al., 2002; Bortfeld, Wruck, & Boas, 2007; Franchescini et al., 2007; Peña et al., 2003; Taga, Asakawa, Maki, Konishi, & Koizumi, 2003; Wilcox, Bortfeld, Woods, Wruck, & Boas, 2005, 2008). NIRS is a safe, non-invasive optical imaging technique that uses changes in blood volume and hemoglobin oxygenation as an index of neural activation. The rationale for this approach rests on the assumption that neural activation in response to a stimulus results in increased energy demands in the area activated. To accommodate the demand for energy, cerebral blood flow (CBF) to the activated brain areas increases, bringing those areas needed oxygen and glucose. Changes in blood flow lead to an increase in blood volume and can be assessed by measuring local concentrations of oxyhemoglobin (oxygenated blood) and deoxyhemoglobin (deoxygenated blood). Typically during cortical activation, local concentrations of oxyhemoglobin (HbO2) increase, whereas concentrations of deoxyhemoglobin (HbR) decrease; from the summated changes in HbR and HbO2, total hemoglobin (HbT) can be computed. Because the HbR response tends to be much smaller in magnitude and less reliable than the HbO2 response (Bartocci et al., 2000; Chen et al., 2002; Hoshi & Tamura, 1993; Jasdzewski et al., 2003; Kato, Kamei, Takashima, & Ozaki, 1993; Sakatani, Chen, Lichty, Zuo, & Wang, 1999; Strangman, Culver, Thompson, & Boas, 2002; Strangman, Franceschini, & Boas, 2003), researchers typically focus on HbO2 in assessing changes in activation across experimental conditions.
To measure changes in oxyhemoglobin and deoxyhemoglobin, near-infrared light between approximately 650 and 900 nm is projected through the skull and into the brain. At these wavelengths, light is differentially absorbed by oxygenated and deoxygenated blood (Gratton, Sarno, Maclin, Corballis, & Fabiani, 2000; Villringer & Chance, 1997). Below 800 nm, light is relatively more sensitive to deoxygenated blood; above 800 nm, it is relatively more sensitive to oxygenated blood. Hence, measuring the light intensity modulation during stimulus presentation, and comparing it to the light intensity during a baseline event in which no stimulus is presented, provides important information about the hemodynamic response to brain activation. Levels of intensity can likewise be compared across different experimental conditions. Evidence that there is a linear relationship between hemodynamics and neural activity (Gratton, Goodman-Wood, & Fabiani, 2001) and that NIRS produces results consistent with other imaging techniques (i.e., fMRI and PET) used simultaneously (Kleinschmidt et al., 1996; Strangman et al., 2002; Villringer & Chance, 1997), provides converging evidence that NIRS can provide a reliable measure of brain function.
Although researchers increasingly have been using NIRS to examine auditory-specific processing issues in infants, much of this work has involved testing very young infants (e.g., neonates up to infants 3 or 4 months old (e.g., Zaramella et al., 2001; Saito et al., 2007) or have focused on cortical regions outside the auditory-specific regions (e.g., frontal cortices) (Taga et al., 2003). Relevant to the current study, Zaramella et al. (2001) focused specifically on hemodynamic responses in bilateral temporal regions of the brain to auditory stimuli, demonstrating bilateral activation. However, their subjects were newborn infants and their stimuli consisted entirely of tonal frequency sweeps, thus limiting the degree to which their findings can be extended to issues relevant to speech perception.
More recently, Homae and colleagues (Homae, Watanabe, Nakano, Asakawa, & Taga, 2006) compared 3-month-old infants’ processing of prosodically normal and flattened speech sounds, observing bilateral activation in the frontal, temporal, and temporoparietal regions in response to both types of speech when the infants were sleeping. In subsequent work (Homae, Watanabe, Nakano, & Taga, 2007), the same researchers tested 10-month-old infants using the same stimuli, again finding increases in activity in right temporoparietal regions and bilateral prefrontal regions in response to flattened speech relative to normal speech. The authors interpreted these findings as indicative of the development of prosody-specific sensitivity on the part of these older infants relative to the 3 month olds in the earlier study, an interpretation that is consistent with recent work implicating the right temporoparietal region in pitch perception in adults (Griffiths & Warren, 2002; Zatorre, Belin, & Penhune, 2002). It remains unclear, however, whether similar patterns of neural activation would be observed when the prosodic features of the speech are not made salient through acoustic manipulation of a normal signal. Relevant to more typical speech processing scenarios, it is becoming increasingly clear that distinct subareas of bilateral human auditory cortex have different response properties (e.g., Seifritz et al., 2002). Such region-specific acoustic representation presumably underlies humans’ ability to identify subtle distinctions in both spectral and temporal components of a variety of sounds in both speech and non-speech. Efforts to identify and dissociate these processing regions are ongoing in current research on adult humans (Zatorre, 2003); even less is known about auditory processing characteristics specific to infants.
In previous work (Bortfeld et al., 2007), we observed robust and reliable patterns of neural activation in the left temporal and primary occipital regions of young infants’ brains during their exposure to sensory-specific material, as indicated by NIRS-based measures of cerebral blood flow and volume change. Given this initial methodological step, we can now use NIRS to interrogate regions of the infant brain that are specific to language processing, namely the bilateral temporal cortices. A series of recent studies using hemodynamic-based measures suggest that infants will show focal lateralization of cortical systems that serve language function. For instance, Peña and colleagues (Peña et al., 2003) found evidence of hemispheric lateralization using NIRS. However, these results were obtained from sleeping neonates in which responses to forward playing speech, reversed speech, and silence—in a manner consistent with the research by Dehaene-Lambertz and colleagues (Dehaene-Lambertz et al., 2002; Dehaene-Lambertz et al., 2006). Studies of infants with focal brain injury have also been suggestive. These provide evidence for specific lesion-to-language delay relations consistent with lateralization (Dehaene-Lambertz, Peña, Christophe, & Landrieu, 2004; Hertz-Pannier et al., 2002; Bates, 1999). Although promising, these studies do not determine whether hemodynamic indicators of lateralization of language-specific processing can be found in healthy, awake infants.
PRESENT RESEARCH
The present research was designed to evaluate NIRS as a method for assessing region-specific processing in older infants in response to linguistic stimuli. We focused our attention specifically on older infants who were actively engaged in multimodal perceptual processing. The reason for this is twofold. First, older infants present a challenge to existing technologies that might require swaddling to try to limit the movement typical of this active stage of development (Bortfeld et al., 2007; Wilcox et al., 2005). Our study thus examines a potential way of circumnavigating this limitation. Second, behavioral research indicates that a variety of language-specific processing biases emerge in the second half of the first year of life (Bortfeld, Morgan, Golinkoff, & Rathbun, 2005; Singh, Nestor, & Bortfeld, 2008; see Werker & Curtin, 2005, for a review). This sample thus provided a strong-enough basis of knowledge that measurement validity could be assessed.
To this end, infants aged 6–9 months were tested in two conditions: during exposure to speech coupled with visual stimuli (audiovisual condition) and during exposure to visual stimuli alone (visual only condition). Regions of interest were both the left and right temporal cortices. Two hypotheses were tested: First, that significant changes in neural activation—as measured by an increase in HbO2—would be observed in area T3 (left temporal region) in response to the audiovisual condition relative to the visual condition, and second, that relatively little change in neural activation would be observed in T4 (right temporal region) in response to either condition. We reasoned that only the audiovisual condition included auditory features that would elicit spectral-specific processing in the temporal regions, and that the language-specific nature of those features would lead to greater activation of the left relative to the right temporal area. Furthermore, and in contrast to Homae et al.’s (2007) findings, we did not expect to see significant recruitment of the right temporal region. This prediction was made because, where Homae et al. (2007) highlighted the prosodic aspects of their stimuli by contrasting artificially induced acoustic characteristics, we did not contrast the auditory component of our audiovisual stimuli with any other form of acoustic stimuli.
METHODS
Participants
Participants were 21 infants (13 males; 8 females between the ages of 6 and 9 months). Seven additional infants were tested but eliminated from the sample because of large artifacts in the optical signal throughout the experimental run due to motion or hair obstruction (N = 5), failure to obtain more than one useable block of trials (N = 1), and failure to obtain optical data from more than one channel (N =1). Infants’ names were obtained from birth announcements in the local newspaper and commercially produced lists, and infants and parents were offered a new toy as compensation for their participation. Informed consent was obtained from the parents before testing began.
Apparatus
During the experiment, each infant sat on a parent’s or caretaker’s lap in a testing booth. Infants were positioned facing a 53-cm flat panel computer monitor (Macintosh G4) 76 cm away (28.1° visual angle at infants’ viewing distance based on a 36-cm-wide screen). The monitor was positioned on a shelf, immediately under which audio speakers and a low-light video camera were positioned, oriented toward infants. The monitor was framed by a façade that functioned to conceal the rest of the equipment. The façade was made of three sections. The upper third was a dark black curtain that covered the wall from side to side, and dropped down 84 cm from the ceiling. The middle section, measuring 152 cm (wall to wall horizontally) × 69 cm high, was constructed of plywood and covered with dark black cloth. The plywood had a rectangular hole cut out of its center that coincided with the size of the viewing surface of computer monitor (48 cm diagonal). A dark curtain hung from the bottom edge of the section to the floor. The testing area was separated by a curtain from a control area, where an experimenter controlled the NIRS instrument out of the infant’s view. Fiber optic cables (15 m each) extended from the instrument to the testing booth and into a custom headband on the infant’s head. The cables were bundled into a single strand secured on the wall just over the parent’s right shoulder.
The NIRS instrument consisted of three major components: (1) two fiber optic cables that delivered near-infrared light to the scalp of the participant (i.e., emitter fibers); (2) four fiber optic cables that detected the diffusely reflected light at the scalp and transmitted it to the receiver (i.e., detector fibers); and (3) an electronic control box that served both as the source of the near-infrared light and the receiver of the refracted light. The signals received by the electronic control box were processed and relayed to a DELL Inspiron 7000™ laptop computer. A custom computer program recorded and analyzed the signal.
The imaging device used in these studies produced light at 680 and 830 nm wavelengths with two laser-emitting diodes (Boas, Franceschini, Dunn, & Strangman, 2002). Laser power emitted from the end of the fiber was 4 mW. Light was square wave modulated at audio frequencies of approximately 4 to 12 kHz. Each laser had a unique frequency so that synchronous detection could uniquely identify each laser source from the photodetector signal. Any ambient illumination that occurred during the experiment (e.g., from the visual stimuli) did not interfere with the laser signals because environmental light sources modulate at a significantly different frequency. No detector saturation occurred during the experiment.
The light was delivered via fiber optic cables (i.e., fibers), each 1 mm in diameter and 15 m in length. These originated at the imaging device and terminated in the headband that was placed on the infant’s head. The headband was made of elastic terry-cloth and was fitted with the two light-emitting and four light-detecting fibers. These were grouped into two emitter/detector fiber sets (i.e., optical probes), each containing two detector fibers placed at 2 cm distance on either side from the central emitter fiber. One optical probe was used to deliver near-infrared light to the left temporal region at approximately position T3 according to the International 10–20 system, and the other delivered light to the right temporal region at approximately position T4 according to the International 10–20 system. NIRS data were analyzed first by channel within cortical region (where one paired emitter and detector fiber within each optical probe constituted a channel and each optical probe contained two channels). Data from the two channels within each optical probe then were averaged and responses were compared across probes (that is, across cortical regions).
Stimuli and Design
The stimuli consisted of speech recordings and visual animations. We presented infants with 5 blocks of two stimulus trials and two baseline (rest) periods. Infants first saw a blank (dark) screen for 20 sec before stimulus presentation began. Stimulus presentation proceeded as follows: 20 sec of silence with visual animation, 10 sec of silence with a blank screen, 20 sec of speech with visual animation, 10 sec of silence with a blank screen. Each block thus consisted of 20 sec visual + 10 sec baseline + 20 sec audiovisual + 10 sec baseline, in that order. This sequence repeated five times over the course of the experiment. Upon analysis of the data, we excluded the first (20 sec) stimulus trial to control for the influence of infants’ initial orientation on the hemodynamic data. We also excluded the last (20 sec) stimulus trial to produce a balanced design of 4 complete stimulus blocks. Excluding data from the first (20 sec visual + 10 sec baseline) and last (20 sec audiovisual + 10 sec baseline) stimulus trials created a design with 4 identical blocks of stimuli (20 sec audiovisual + 10 sec baseline + 20 sec visual + 10 sec baseline) for our analyses. We employed this alternating design in order to establish whether NIRS could track dynamic changes in cerebral blood flow across the course of alternating perceptual events (e.g., the 60 sec blocks). The alternating pattern of stimuli also served to keep the infants attentive throughout the experiment. Given the extremely short run-time of the entire experiment (5 min), we were not concerned that infants would develop expectations about the pattern of stimulus trials. Nonetheless, we coded the in-fants’responses for anticipatory orientation toward the speaker and screen prior to trial onset (during the baseline period). No reliable changes in behavior during baseline were observed across the course of the experiment.
The voice recordings were made by a female speaker relaying segments from children’s stories using highly animated, infant-directed speech. The content of each speech segment was different, but all contained speech from the same speaker with the same animated intonational variation and positive affective tone. Transcriptions of the story segments are included in the Appendix. Recordings were made using a Sony digital camcorder, and then converted to .wav sound files using Sound Forge 6.0™ audio editing software. The visual stimuli were distinct across trials, but each consisted of animations that were similar in color contrast and motion parameters. They consisted of simple, 3-dimensional objects (e.g., spirals, circles, and rectangles) that rotated and moved slowly in front of a high-contrast, colored background. The animations were produced using 3-D Studio Max™ computer graphics software. The auditory and animated digital files were combined using Adobe Premier 6.5™ video editing software, which produced .avi movie files that were then recorded onto a blank DVD. The recorded DVD was then played through the computer monitor and speakers. The hidden speakers were 82 cm from infants, facing them and producing audio stimuli at 75 dB SPL when measured from the approximate location of the infant.
Procedure
After the parent and infant were seated, a head circumference measurement was taken from the infant using a standard cloth tape measure. Each parent was instructed to refrain from talking and interacting with the infant during the course of the experiment, and to hold the infant up so that he or she was able to comfortably view the screen. Parents were also asked to guide infants’hands down and away from the headband if they began to reach up during the experiment. The experimenter then placed the NIRS headband on the infant. Following the 10–20 system, the two optical probes were adjusted over the left and right temporal areas, centered over the T3 position (on the left) and T4 position (on the right). The experimenter moved to the control area. The room lights in both the experimental and control areas were turned off, leaving only a low-intensity light to sufficiently illuminate the experimental area, and light from the computer monitor to light the control area. The source lights of the imaging device were turned on, and optical recordings began. At this time, stimulus presentation began as well. Infants were video recorded for the duration of the experimental session for offline coding of looking times.
RESULTS
Looking-Time Data
The length of time infants spent toward the audiovisual and visual trials was coded by two observers. Looking times were calculated for each 20-sec trial, and a grand average was computed for visual and audiovisual conditions. Looking times during baseline periods were not calculated. The average looking time during the visual only condition was 16.5 sec (SD = 1.7). The average looking time during the audiovisual condition was 17.3 (SD = 2.0). Interobserver agreement was measured for all infants and averaged 95% per test trial per infant. Data were examined to determine if, in any blocks, infant (a) cumulated less than 10 sec total looking time per trial or (b) looked away from the display for more than 5 consecutive sec. No infants who completed the entire experiment met these behavioral criteria, and therefore no blocks were excluded for any of the infants based on looking times.
Optical Data
The two detector fibers within each optical probe recorded raw optical signals for subsequent digitization at 200 Hz for each of the four channels. NIRS data collected for each of the two stimulus conditions were analyzed the same way. The NIRS apparatus converted the signals to optical density units, which were digitally low-pass filtered at 10.0 Hz for noise reduction, and decimated to 20 samples per sec. The control computer then converted the data to relative concentrations of oxygenated (HbO2) and deoxygenated (HbR) hemoglobin using the modified Beer-Lambert law (Strangman, Boas, & Sutton, 2002), which calculates the relationship between light absorbance and concentration of particles within a medium. Based on this conversion, HbO2 and HbR concentration changes across the four 60 sec experimental blocks (each composed of the entire 20 sec audiovisual trial, the 10 sec baseline period, the entire 20 sec visual only trial, and the 10 sec baseline period) were first averaged and plotted by channel. Artifacts originating in infant physiology and movement were spatially filtered using a principal components analysis (PCA) of the signals across the four channels (Zhang, Brooks, Franceschini, & Boas, 2005). This approach is based on the assumption that systemic components of interference are spatially global and have higher energy than the signal changes evoked by the perceptual stimuli themselves (Zhang et al., 2005). Further filtering was conducted to eliminate large motion artifacts, objectively defined as a signal change greater than 5% in a tenth of second. This filtering step resulted in the removal of a total of 13 blocks (of 84 possible) for an average of 3.38 useable blocks per infant, with all infants providing at least two. At this stage, because there was no indication that one channel was preferentially activated over another in either cortical region, and because there remains some uncertainty about the relationship between the 10–20 coordinates and underlying brain coordinates, data were averaged across the two channels per region.
Hemodynamic Response Functions and Analyses
Focusing on the 71 useable blocks, average changes in HbO2 concentration were established for each cortical region during each of the two stimulus-specific trial types relative to that trial type’s own baseline. We limited our focus to HbO2 as this chromophore provided the most robust contrast-to-noise ratio across infants. Because changes in concentration began manifesting 2–3 sec after stimulus onset and showed signs of abating near the stimulus offset, concentration changes were calculated by measuring the average relative HbO2 concentration during the 10–20 sec period for each stimulus trial and comparing it the relative HbO2 concentration at time −2 to 0 sec (baseline) prior to the trial. Figure 1 illustrates these relative changes (in micromolars).
FIGURE 1.
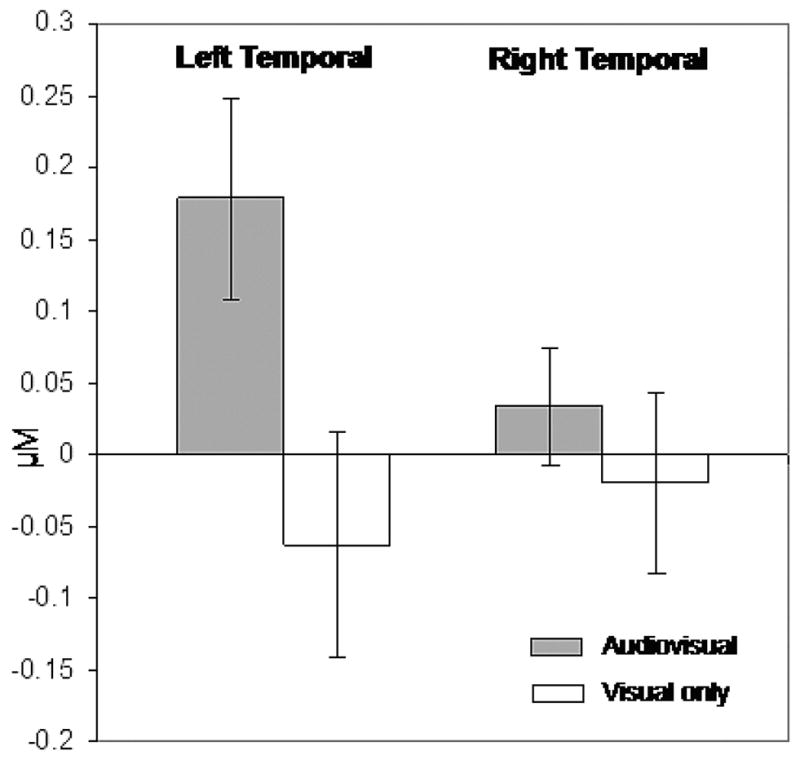
Bar graph illustrates average change (and standard error) in HbO2 concentration (micromolar) from time −2 to 0 seconds (baseline) to 10–20 seconds (second half of trial) for each stimulus condition and cortical region.
We then conducted statistical comparisons of the average relative response to each stimulus condition (audiovisual and visual) in each cortical region (left and right temporal cortex). Planned comparisons revealed mean concentration change was significantly greater in the left-cortical, audiovisual presentation (M = 0.18, SD = 0.32) than in the other three conditions, F (1, 20) = 6.17, p < .02. Moreover, mean concentration change did not differ (ps > .30) in the left-cortical, visual-only presentation (M = −.06, SD = 0.36), the right-cortical audiovisual presentation (M = .04, SD = .19) or the right-cortical visual-only condition (M = −.02, SD = .29). Although not central to our predictions, a traditional 2 (cortical region: left versus right temporal) × 2 (stimulus condition: visual versus audiovisual) within-subjects ANOVA was also conducted. This revealed only a significant difference in the concentration of HbO2 by cortical region, F(1, 20) = 5.48, p < .03. Together, these results demonstrate a dissociation of homologous regions of the infant brain during exposure to speech stimuli.
DISCUSSION
Findings from the current study highlight the utility of the NIRS method for studying the neurobiological basis for early perceptual processing. We found reliable hemodynamic changes in the left temporal cortex of infants in the second half of the first year of life in response to audiovisual stimuli relative to visual-only stimuli, as well as relative to changes across stimulus conditions in homologous regions of the right cortex in the same infants. These results are consistent with a long history of behavioral and neurophysiological research with adult humans demonstrating that the left temporal cortex is a primary area for language processing due to its structural and functional characteristics (see Hutsler & Galuske, 2003, for a review). The results of the current study, along with those of Bortfeld et al. (2007), are also consistent with recent reports that very young infants demonstrate significant hemodynamic changes in the left temporal cortex in response to speech-specific auditory stimuli (Dehaene-Lambertz et al., 2002; Peña et al., 2003). These current results thus suggest that NIRS can be used to assess infants’ neurophysiological responses in a variety of processing tasks.
Research will need to build on and expand the current findings. For example, it will be important to investigate the extent to which these results replicate in infants of the same age when no visual stimuli accompany the auditory stimuli, a process that is ongoing in our lab currently. Specific to language development, additional research will need to investigate the specific areas within the bilateral temporal cortex, as well as other cortical regions, that respond selectively to specific features of the auditory signal. Finally, it will be important to determine how different experimental manipulations influence infants’ processing biases. For example, contrasts in classes of structured auditory stimuli (e.g., native vs. nonnative speech; speech vs. music) may reveal important changes across the first year of life attributable to environmental experience. Indeed, because the hemispheric lateralization for speech processing observed in infants is not as strong as it is in adults (Holland et al., 2001), it would seem that consolidation occurs during the course of learning language and that researchers should see changes across the first year, as sensitivity to language-specific features increases. Such findings would bolster our understanding of the neural basis for the variety of behavioral findings from infants, many of which demonstrate increasingly sophisticated perceptual capacities that are quickly modified by the linguistic environment (e.g., Jusczyk, Luce, & Charles-Luce, 1994; Jusczyk, 1997). Likewise, and consistent with recent findings using NIRS (Homae et al., 2007), it is possible that infants’ right temporal cortex, a region that is important to prosodic perception in adults (Baum & Pell, 1999; Ross, Thompson, & Yenkosky, 1997; Friederici & Alter, 2004; Grimshaw, Kwasny, Covell, & Johnson, 2003), would respond more robustly to particular auditory stimuli than others given a paradigm that highlights that aspect of the signal. Given that infants rely differentially on cues unique to their native language to segment the incoming auditory stream into linguistic units, this type of approach would allow researchers to map the functional development of hemispheric specialization and determine the role it plays in tuning auditory processing during the first year of life.
Another direction for future research will be to combine multiple techniques, both behavioral and neurophysiological, to determine if known developmental sequences reflect biological changes in the neurophysiology underlying perceptual processing. In the current study, looking times were tracked only to verify that infants were attending to the stimuli being presented during any particular trial. The utility of looking-time differences has been made clear by behavioral researchers, who operationalize them as indicative of differential sensitivity (whether characterized as preference or recognition; e.g., Bortfeld et al., 2005; Singh, Nestor, & Bortfeld, 2008). Therefore, the development of experimental paradigms that combine measures of differential looking behavior with measures of neurophysiological response will do much to bridge the gap between findings in the behavioral and the neurobiological literatures on human infant development.
Limitations of the current study can be discussed in terms of the particular experimental design employed and the physiological basis for the NIRS technique. The infant population requires certain concessions and creative adaptations of experimental design in order to accommodate infants’ general inattention and their likelihood of moving during experimental trials. In the current study, we used stimulus alternating blocked trials. This design was effective at holding infants’ attention throughout the experiment, while NIRS was able to characterize the corresponding dynamic perceptual processing. Nonetheless, this design necessarily limited our ability to draw conclusions based entirely on the auditory component of the stimuli. A paradigm that would allow us to test infants with auditory stimuli alone would employ probabilistically determined event-related trials (Friston et al., 1998; Friston, Zarahn, Josephs, Henson, & Dale, 1999), in which responses to individual stimuli are modeled as brief bursts of neural activity that are convolved with a hemodynamic response function. This design approach likewise would address the second limitation in this experiment, the relatively sluggish temporal resolution of hemodynamic-based measures in general. Techniques such as evoked response potentials (ERP) and electroencephalogram (EEG) can be used to map electrical signals based on action potentials occurring perpendicular to the surface of the scalp and are extremely accurate temporally, but their spatial acuity is quite limited relative to NIRS. The use of an event-related design targeting specific cortical regions using NIRS would provide a relatively sensitive measure of the temporal nature of cortical responses in those relatively specific cortical regions.
Finally, the temporal specificity of processing provided by ERP complements the localization specificity provided by NIRS. Notable advances have been made in recent years in combining a variety of infant behavioral measures (e.g., bimodal preference paradigms; combinations of preferential listening and eye-tracking). Likewise, simultaneous tracking of multiple neurophysiological measures stands to substantially advance our understanding of the processing that underlies infants’ perceptual experience. For example, much progress has been made in the application of ERP to help predict a range of language outcome measures (e.g., Espy, Molfese, Molfese, & Modglin, 2004; Mills et al., 2004; Molfese et al., 2006). Future research that succeeds in combining these two approaches (NIRS and ERP) in the assessment of processing abilities in infants and young children will maximize the data obtainable from these populations. Developmental researchers will gain much from the current push to combine these and other technologies in the measurement of adult neurophysiology (e.g., Roche-Labarbe, Wallois, Ponchel, Kongolo, & Grebe, 2007).
The findings reported here continue our extension of NIRS to use with older infants. In particular, they highlight the utility of this technology as a tool for tracking hemodynamic activity in infants, even when they are actively processing relatively complex material. A variety of questions remain, however, about the region specific acoustic representation that underlies humans’ ability to identify subtle distinctions in both spectral and temporal components of a variety of sounds (in both speech and non-speech). It is becoming increasingly clear that distinct subareas of bilateral human auditory cortex have different response properties (e.g., Seifritz et al., 2002) and that, although ongoing research on adult humans (e.g., Zatorre, 2003) is expanding our understanding of the nature of these response properties, relatively little is known about auditory processing characteristics specific to infants. Our results are an initial demonstration that NIRS is sufficiently sensitive to assess the neural basis of perceptual processing in awake and behaving infants. Although much additional research is needed to examine a variety of important issues underlying this result, the current study demonstrates the wealth of information available through thoughtful application of this technology to the study of infant development.
Acknowledgments
This research was supported by grants from the James S. McDonnell Foundation 21st Century Research Award, Bridging Brain, Mind, and Behavior and from HD046533 to Heather Bortfeld and P41-RR14075 to David Boas.
The authors thank the undergraduate assistants in the Lil’ Aggies’ Language Learning Laboratory at Texas A&M University for their help with data collection and the parents who so graciously agreed to have their infants participate in the research.
APPENDIX
The hippo was very hot. He sat on the riverbank and gazed at the little fishes swimming in the water. If only I could be in the water he thought, how wonderful life would be. Then he jumped in and made a mighty splash.
Robert was out riding his bike. He saw his friend Will by the old fence. Did you lose something, he asked. I thought I saw a frog, Will said. I used to have a pet frog named Greeney he’d wait for me by the pond where I lived. He must miss me a lot.
While mama hangs the wash out and pappa rakes the leaves, Oliver chases a big yellow leaf down the hill. He follows it under a twisty tree and all the way to the edge of the woods. From far away, Oliver hears mama calling him. Oliver runs all the way home.
Sometimes the chick and other young penguins dig their beaks into the ice to help them walk up the slippery hill. They toboggon down fast on their fluffy bellies. The chick grows and grows, in a short while, he’ll be a junior penguin.
One very hot afternoon, the farmer and his animals were dozing in the barn. A warm breeze blew through the open doors. The only sound was the buzz buzz of a lazy fly. Suddenly, the buzzing stopped.
Contributor Information
Heather Bortfeld, Department of Psychology, Texas A&M University, College Station, Texas.
Eswen Fava, Department of Psychology, Texas A&M University, College Station, Texas.
David A. Boas, Anthinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Harvard Medical School, Charlestown, Massachusetts
References
- Baird AA, Kagan J, Gaudette T, Walz KA, Hershlag N, Boas DA. Frontal lobe activation during object permanence: Data from near-infrared spectroscopy. NeuroImage. 2002;16:1120–1126. doi: 10.1006/nimg.2002.1170. [DOI] [PubMed] [Google Scholar]
- Bartocci M, Winberg J, Ruggiero C, Bergqvist LL, Serra G, Lagercrantz H. Activation of olfactory cortex in newborn infants after odor stimulation: A functional near-infrared spectroscopy study. Pediatric Research. 2000;48:18–23. doi: 10.1203/00006450-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Bates E. Plasticity, localization and language development. In: Fletcher S, editor. The changing nervous system: Neurobehavioral consequences of early brain disorders. New York: Oxford University Press; 1999. pp. 214–253. [Google Scholar]
- Baum S, Pell M. The neural bases of prosody: Insights from lesion studies and neuroimaging. Aphasiology. 1999;13:581–608. [Google Scholar]
- Boas DA, Franceschini MA, Dunn AK, Strangman G. Noninvasive imaging of cerebral activation with diffuse optical tomography. In: Frostig RD, editor. In vivo optical imaging of brain function. Boca Raton: CRC Press; 2002. pp. 193–221. [PubMed] [Google Scholar]
- Bortfeld H, Morgan J, Golinkoff R, Rathbun K. Mommy and me: Familiar names help launch babies into speech stream segmentation. Psychological Science. 2005;16:298–304. doi: 10.1111/j.0956-7976.2005.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortfeld H, Wruck E, Boas DA. Assessing infants’cortical response to speech suing near-infrared spectroscopy. Neuroimage. 2007;34:407–415. doi: 10.1016/j.neuroimage.2006.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Sakatani K, Lichty W, Ning P, Zhao S, Zuo H. Auditory-evoked cerebral oxygenation changes in hypoxic-ischemic encephalopathy of newborn infants monitored by near-infrared spectroscopy. Early Human Development. 2002;67:113–121. doi: 10.1016/s0378-3782(02)00004-x. [DOI] [PubMed] [Google Scholar]
- Czisch M, Wetter T, Kaufmann C, Pollmächer Holsboer F, Auer D. Altered processing of acoustic stimuli during sleep: Reduced auditory activation and visual deactivation detected by a combined fMRI/EEG study. Neuro-image. 2002;16:251–258. doi: 10.1006/nimg.2002.1071. [DOI] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: Newborns prefer their mothers’voices. Nature. 1980;208:1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Peña M, Christophe A, Landrieu P. Phoneme perception in a neonate with a left sylvian infarct. Brain and Language. 2004;88:26–38. doi: 10.1016/s0093-934x(03)00284-0. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proceedings of the National Academy of Sciences. 2006;103:14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espy KA, Molfese D, Molfese V, Modglin A. Development of auditory event-related potentials in young children and relations to word-level reading abilities at age 8 years. Annals of Dyslexia. 2004;54:9–38. doi: 10.1007/s11881-004-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini MA, Thaker S, Themelis G, Krishnamoorthy K, Bortfeld H, Diamond S, Boas DA, Arvin K, Grant E. Assessment of infant brain development with frequency-domain near-infrared spectroscopy. Pediatric Research. 2007;61:546–551. doi: 10.1203/pdr.0b013e318045be99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions—The dynamic dual pathway model. Brain and Language. 2004;89:267–276. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, OAP, Rugg MD, Turner R. Event-related fMRI: Characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, ORNA, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–619. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Gratton G, Goodman-Wood MR, Fabiani M. Comparison of neuronal and hemodynamic measures of the brain response to visual stimulation: An optical imaging study. Human Brain Mapping. 2001;13:13–25. doi: 10.1002/hbm.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Sarno A, Maclin E, Corballis PM, Fabiani M. Toward noninvasive 3-D imaging of the time course of cortical activity: Investigation of the depth of the event-related optical signal. Neuroimage. 2000;11:491–504. doi: 10.1006/nimg.2000.0565. [DOI] [PubMed] [Google Scholar]
- Griffiths T, Warren J. The planum temporale as a computational hub. Trends in Neuroscience. 2002;25:348–353. doi: 10.1016/s0166-2236(02)02191-4. [DOI] [PubMed] [Google Scholar]
- Grimshaw GM, Kwasny KM, Covell E, Johnson RA. The dynamic nature of language lateralization: Effects of lexical and prosodic factors. Neuropsychologia. 2003;41:1008–1019. doi: 10.1016/s0028-3932(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Hertz-Pannier L, Chiron C, Jambaqué I, Renaux-Kieffer V, Van de Moortele P, Delalande O, Fohlen M, Brunelle F, Le Bihan D. Late plasticity for language in a child’s non-dominant hemisphere: A pre- and post-surgery fMRI study. Brain. 2002;125:361–372. doi: 10.1093/brain/awf020. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Asakawa K, Taga G. The right hemisphere of sleeping infant perceives sentential prosody. Neuroscience Research. 2006;54:276–280. doi: 10.1016/j.neures.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic porcessing in the developing brain. Neuroscience Research. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Tamura M. Dynamic multichannel near-infrared optical imaging ofhuman brain activity. Journal of Applied Physiology. 1993;75:1842–1846. doi: 10.1152/jappl.1993.75.4.1842. [DOI] [PubMed] [Google Scholar]
- Hutsler J, Galuske RAW. Hemispheric asymmetries in cerebral corticalnetworks. Trends in Neurosciences. 2003;26:429–435. doi: 10.1016/S0166-2236(03)00198-X. [DOI] [PubMed] [Google Scholar]
- Jasdzewski G, Strangman G, Wagner J, Kwong KK, Poldrack RA, Boas DA. Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuro-image. 2003;20:479–488. doi: 10.1016/s1053-8119(03)00311-2. [DOI] [PubMed] [Google Scholar]
- Jusczyk PW. The discovery of spoken language. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Jusczyk PW, Luce PA, Charles-Luce J. Infants’ sensitivity to phonotactic patterns in the native language. Journal of Memory and Language. 1994;33:630–645. [Google Scholar]
- Kato T, Kamei A, Takashima S, Ozaki T. Human visual cortical function during photic stimulation monitoring by means of near-infrared spectroscopy. Journal of Cerebral Blood Flow and Metabolism. 1993;13:516–520. doi: 10.1038/jcbfm.1993.66. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A, Obrig H, Requardt M, Merboldt KD, Dirnagl U, Villringer A, Frahm J. Simultaneous recording of cerebral blood oxygenation changes during human brain activation by magnetic resonance imaging and near-infrared spectroscopy. Journal of Cerebral Blood Flow and Metabolism. 1996;16:817–826. doi: 10.1097/00004647-199609000-00006. [DOI] [PubMed] [Google Scholar]
- Mehler J, Jusczyk PW, Lambertz G, Halsted G, Bertoncini J, Amiel-Tison C. A precursor of language acquisition in young infants. Cognition. 1988;29:143–178. doi: 10.1016/0010-0277(88)90035-2. [DOI] [PubMed] [Google Scholar]
- Mills D, Prat C, Stager C, Zangl R, Neville H, Werker J. Language experience and the organization of brain activity to phonetically similar words: ERP evidence from 14- and 20-month olds. Journal of Cognitive Neurosci-ence. 2004;16:1452–1464. doi: 10.1162/0898929042304697. [DOI] [PubMed] [Google Scholar]
- Molfese D, Key AF, Spencer K, Cunningham N, Terrell S, Ferguson M, Molfese V, Bonebright T. Below-average, average, and above-average readers engage different and similar brain regions while reading. Journal of Learning Disabilities. 2006;39:352–363. doi: 10.1177/00222194060390040801. [DOI] [PubMed] [Google Scholar]
- Peña M, Maki A, Kovaciæ D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J. Sounds and silence: An optical topography study of language recognition at birth. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11702–11705. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Kennedy D, Courchesne E. fMRI during natural sleep as a method to study brain function during early childhood. Neuroimage. 2007;38:696–707. doi: 10.1016/j.neuroimage.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Roche-Labarbe N, Wallois F, Ponchel E, Kongolo G, Grebe R. Coupled oxygenation oscillation measured by NIRS and intermittent cerebral activation on EEG in premature infants. Neuroimage. 2007;36:718–727. doi: 10.1016/j.neuroimage.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Ross ED, Thompson RD, Yenkosky J. Lateralization of affective prosody in brain and the callosal integration of hemispheric language functions. Brain and Language. 1997;56:27–54. doi: 10.1006/brln.1997.1731. [DOI] [PubMed] [Google Scholar]
- Saito Y, Kondo T, Aoyama S, Fukumoto R, Konishi N, Nakamura K, Kobayashi M, Toshima T. The function of the frontal lobe in neonates for response to a prosodic voice. Early Human Development. 2007;83:225–230. doi: 10.1016/j.earlhumdev.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Sakatani K, Chen S, Lichty W, Zuo H, Wang Y. Cerebral blood oxygenation changes induced by auditory stimulation in newborn infants measured by near infrared spectroscopy. Early Human Development. 1999;55:229–236. doi: 10.1016/s0378-3782(99)00019-5. [DOI] [PubMed] [Google Scholar]
- Seifritz E, Esposito F, Hennel F, Mustovic H, Neuhoff J, Bilecen D, Tedeschi G, Scheffler K, Di Salle F. Spatiotemporal pattern of neural processing in the human auditory cortex. Science. 2002;297:1706–1708. doi: 10.1126/science.1074355. [DOI] [PubMed] [Google Scholar]
- Singh L, Nestor S, Bortfeld H. Overcoming the effects of variation in infant speech segmentation: Influences of word familiarity. Infancy. 2008;13:57–74. doi: 10.1080/15250000701779386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biological Psychiatry. 2002;52:679–693. doi: 10.1016/s0006-3223(02)01550-0. [DOI] [PubMed] [Google Scholar]
- Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during function brain activation. Neuroimage. 2002;17:719–731. [PubMed] [Google Scholar]
- Strangman G, Franceschini MA, Boas DA. Factors affecting the accuracy of near-infrared spectroscopy concentration calculations for focal changes in oxygenation parameters. Neuroimage. 2003;18:865–879. doi: 10.1016/s1053-8119(03)00021-1. [DOI] [PubMed] [Google Scholar]
- Taga G, Asakawa K, Maki A, Konishi Y, Koizumi H. Brain imaging in awake infants by near-infrared optical topography. Proceedings of the National Academy of Sciences. 2003;100:10722–10727. doi: 10.1073/pnas.1932552100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villringer A, Chance B. Non-invasive optical spectroscopy and imaging of human brain function. Trends in Neuroscience. 1997;20:435–442. doi: 10.1016/s0166-2236(97)01132-6. [DOI] [PubMed] [Google Scholar]
- Werker J, Curtin S. PRIMIR: A developmental framework of infant speech processing. Language Learning and Development. 2005;12:197–234. [Google Scholar]
- Werker J, Tees RC. Cross-language speech perception: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development. 1984;7:49–63. [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Using near-infrared spectroscopy to assess neural activation during object processing in infants. Journal of Biomedical Optics. 2005;10:011010, 1–9. doi: 10.1117/1.1852551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox T, Bortfeld H, Woods R, Wruck E, Boas DA. Hemodynamic response to featural changes in the occipital and inferior temporal cortex in infants: A preliminary methodological exploration. Developmental Science. 2008;11:361–370. doi: 10.1111/j.1467-7687.2008.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaramella P, Freato F, Amigoni A, Salvadori S, Marangoni P, Suppjei A, Schiavo B, Chiandetti L. Brain auditory activation measured by near-infrared spectroscopy (NIRS) in neonates. Pediatric Research. 2001;49:213–219. doi: 10.1203/00006450-200102000-00014. [DOI] [PubMed] [Google Scholar]
- Zatorre R. Sound analysis in the auditory cortex. Trends in Neuroscience. 2003;26:229–230. doi: 10.1016/S0166-2236(03)00074-2. [DOI] [PubMed] [Google Scholar]
- Zatorre R, Belin P, Penhune V. Structure and function of auditory cortex: Music and speech. Trends in Cognitive Science. 2002;6:37–46. doi: 10.1016/s1364-6613(00)01816-7. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brooks DH, Franceschini MA, Boas DA. Eigenvector-based spatial filtering for reduction of physiological interference in diffuse optical imaging. Journal of Biomedical Optics. 2005;10:011014, 1–11. doi: 10.1117/1.1852552. [DOI] [PubMed] [Google Scholar]


