Abstract
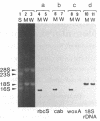
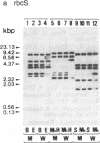
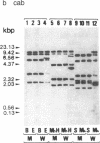
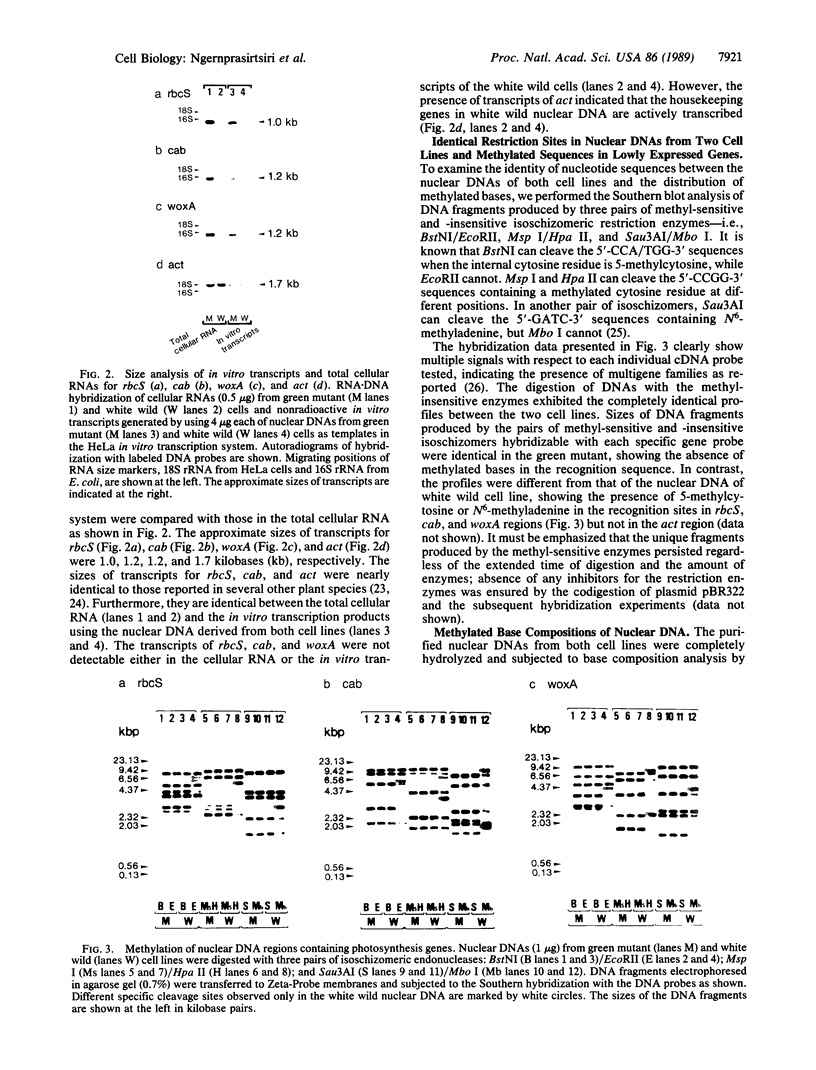
The transcripts of nuclear genes for the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase (rbcS), chlorophyll a/b-binding protein (cab), and extrinsic 33-kDa protein involved in photosystem II water oxidation (woxA) were not detectable in the white wild cultured cells of sycamore (Acer pseudoplatanus), in contrast to their high levels in the sibling green mutant cells and the constitutive expression of actin genes (act) in both cell types. We have examined the template activities of nuclear DNAs using the HeLa cell in vitro transcription system. All of the three photosynthesis genes from the green cell line and act from both cell types were well transcribed in vitro, but these photosynthesis genes from the white cell line were not, indicating that the transcriptional regulation is ascribable to DNA templates. Digestion of nuclear DNA with methyl-sensitive and -insensitive isoschizomeric endonucleases and the subsequent Southern hybridization showed that each gene has the identical recognition sites of restriction enzymes in the green and white cell lines, but some of the sites were methylated only in the photosynthesis genes in the white cells. There was observed a clear inverse relationship between the level of expressed transcripts and the extent of DNA methylation. Thus, it is inferred that the selective methylation of DNA is a likely mechanism for suppressing transcription of nuclear genes for photosynthesis in the nonphotosynthetic plant cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry J. O., Nikolau B. J., Carr J. P., Klessig D. F. Transcriptional and post-transcriptional regulation of ribulose 1,5-bisphosphate carboxylase gene expression in light- and dark-grown amaranth cotyledons. Mol Cell Biol. 1985 Sep;5(9):2238–2246. doi: 10.1128/mcb.5.9.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P. DNA methylation--how important in gene control? Nature. 1984 Feb 9;307(5951):503–504. doi: 10.1038/307503a0. [DOI] [PubMed] [Google Scholar]
- Bligny R. Growth of Suspension-cultured Acer pseudoplatanus L. Cells in Automatic Culture Units of Large Volume. Plant Physiol. 1977 Mar;59(3):502–505. doi: 10.1104/pp.59.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie R., Bellemare G., Bartlett S. G., Chua N. H., Cashmore A. R. Cloned DNA sequences complementary to mRNAs encoding precursors to the small subunit of ribulose-1,5-bisphosphate carboxylase and a chlorophyll a/b binding polypeptide. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7304–7308. doi: 10.1073/pnas.78.12.7304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Feix G., Frendewey D. Accurate in vitro splicing of two pre-mRNA plant introns in a HeLa cell nuclear extract. EMBO J. 1986 Nov;5(11):2749–2758. doi: 10.1002/j.1460-2075.1986.tb04563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Coruzzi G., Broglie R., Cashmore A., Chua N. H. Nucleotide sequences of two pea cDNA clones encoding the small subunit of ribulose 1,5-bisphosphate carboxylase and the major chlorophyll a/b-binding thylakoid polypeptide. J Biol Chem. 1983 Feb 10;258(3):1399–1402. [PubMed] [Google Scholar]
- Doerfler W. DNA methylation and gene activity. Annu Rev Biochem. 1983;52:93–124. doi: 10.1146/annurev.bi.52.070183.000521. [DOI] [PubMed] [Google Scholar]
- Fradin A., Manley J. L., Prives C. L. Methylation of simian virus 40 Hpa II site affects late, but not early, viral gene expression. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5142–5146. doi: 10.1073/pnas.79.17.5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenbaum Y., Naveh-Many T., Cedar H., Razin A. Sequence specificity of methylation in higher plant DNA. Nature. 1981 Aug 27;292(5826):860–862. doi: 10.1038/292860a0. [DOI] [PubMed] [Google Scholar]
- Gruissem W., Barkan A., Deng X. W., Stern D. Transcriptional and post-transcriptional control of plastid mRNA levels in higher plants. Trends Genet. 1988 Sep;4(9):258–263. doi: 10.1016/0168-9525(88)90033-9. [DOI] [PubMed] [Google Scholar]
- Handa H., Kaufman R. J., Manley J., Gefter M., Sharp P. A. Transcription of Simian virus 40 DNA in a HeLa whole cell extract. J Biol Chem. 1981 Jan 10;256(1):478–482. [PubMed] [Google Scholar]
- Hepburn A. G., Clarke L. E., Pearson L., White J. The role of cytosine methylation in the control of nopaline synthase gene expression in a plant tumor. J Mol Appl Genet. 1983;2(3):315–329. [PubMed] [Google Scholar]
- Hightower R. C., Meagher R. B. Divergence and differential expression of soybean actin genes. EMBO J. 1985 Jan;4(1):1–8. doi: 10.1002/j.1460-2075.1985.tb02309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine G., Nash B., Weissbach H., Brot N. Light regulation of the synthesis of the large subunit of ribulose-1,5-bisphosphate carboxylase in peas: Evidence for translational control. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5690–5694. doi: 10.1073/pnas.82.17.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Marmillot P., Job C., Jovin T. M. Transcription of left-handed Z-DNA templates: increased rate of single-step addition reactions catalyzed by wheat germ RNA polymerase II. Biochemistry. 1988 Aug 23;27(17):6371–6378. doi: 10.1021/bi00417a027. [DOI] [PubMed] [Google Scholar]
- Jove R., Sperber D. E., Manley J. L. Transcription of methylated eukaryotic viral genes in a soluble in vitro system. Nucleic Acids Res. 1984 Jun 11;12(11):4715–4730. doi: 10.1093/nar/12.11.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshet I., Lieman-Hurwitz J., Cedar H. DNA methylation affects the formation of active chromatin. Cell. 1986 Feb 28;44(4):535–543. doi: 10.1016/0092-8674(86)90263-1. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Bogorad L., Miles C. D. Nuclear gene-regulated expression of chloroplast genes for coupling factor one in maize. Plant Physiol. 1987 Nov;85(3):757–767. doi: 10.1104/pp.85.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Nelson M. The effect of site specific methylation on restriction endonuclease digestion. Nucleic Acids Res. 1985;13 (Suppl):r201–r207. doi: 10.1093/nar/13.suppl.r201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R. Transcription and RNA stability are important determinants of higher plant chloroplast RNA levels. EMBO J. 1987 Jun;6(6):1571–1579. doi: 10.1002/j.1460-2075.1987.tb02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nairn C. J., Winesett L., Ferl R. J. Nucleotide sequence of an actin gene from Arabidopsis thaliana. Gene. 1988 May 30;65(2):247–257. doi: 10.1016/0378-1119(88)90461-1. [DOI] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Chollet R., Kobayashi H., Sugiyama T., Akazawa T. DNA methylation and the differential expression of C4 photosynthesis genes in mesophyll and bundle sheath cells of greening maize leaves. J Biol Chem. 1989 May 15;264(14):8241–8248. [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Kobayashi H., Akazawa T. DNA Methylation Occurred around Lowly Expressed Genes of Plastid DNA during Tomato Fruit Development. Plant Physiol. 1988 Sep;88(1):16–20. doi: 10.1104/pp.88.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Kobayashi H., Akazawa T. DNA methylation as a mechanism of transcriptional regulation in nonphotosynthetic plastids in plant cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4750–4754. doi: 10.1073/pnas.85.13.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngernprasirtsiri J., Macherel D., Kobayashi H., Akazawa T. Expression of Amyloplast and Chloroplast DNA in Suspension-Cultured Cells of Sycamore (Acer pseudoplatanus L.). Plant Physiol. 1988 Jan;86(1):137–142. doi: 10.1104/pp.86.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K., Pelcher L. E., Horn D. A rapid, simple method for nuclei isolation from plant protoplasts. Plant Physiol. 1977 Aug;60(2):179–181. doi: 10.1104/pp.60.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichersky E., Bernatzky R., Tanksley S. D., Cashmore A. R. Evidence for selection as a mechanism in the concerted evolution of Lycopersicon esculentum (tomato) genes encoding the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3880–3884. doi: 10.1073/pnas.83.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation in eukaryotic cells. Int Rev Cytol. 1984;92:159–185. doi: 10.1016/s0074-7696(08)61327-3. [DOI] [PubMed] [Google Scholar]
- Resnekov O., Aloni Y. RNA polymerase II is capable of pausing and prematurely terminating transcription at a precise location in vivo and in vitro. Proc Natl Acad Sci U S A. 1989 Jan;86(1):12–16. doi: 10.1073/pnas.86.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D. M., Hightower R. C., Meagher R. B. Genes encoding actin in higher plants: intron positions are highly conserved but the coding sequences are not. J Mol Appl Genet. 1983;2(1):111–126. [PubMed] [Google Scholar]
- Strzalka K., Ngernprasirtsiri J., Watanabe A., Akazawa T. Sycamore amyloplasts can import and process precursors of nuclear encoded chloroplast proteins. Biochem Biophys Res Commun. 1987 Dec 16;149(2):799–806. doi: 10.1016/0006-291x(87)90438-4. [DOI] [PubMed] [Google Scholar]
- Vardimon L., Kressmann A., Cedar H., Maechler M., Doerfler W. Expression of a cloned adenovirus gene is inhibited by in vitro methylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1073–1077. doi: 10.1073/pnas.79.4.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. C., Kaufman L. S., Thompson W. F. Developmental regulation of cytosine methylation in the nuclear ribosomal RNA genes of Pisum sativum. J Mol Biol. 1987 Jan 5;193(1):15–26. doi: 10.1016/0022-2836(87)90622-x. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Imamoto F. Selective and accurate initiation of transcription at the T-DNA promoter in a soluble chromatin extract from wheat germ. Mol Gen Genet. 1987 Oct;209(3):445–452. doi: 10.1007/BF00331148. [DOI] [PubMed] [Google Scholar]