Summary
Mycobacterium tuberculosis has an on-going impact on global public health and new therapeutics to treat tuberculosis are urgently required. The emergence of drug resistant tuberculosis poses a serious threat to the control of this pathogen, and the development of drugs that are active against the resistant strains is vital. A medium-throughput assay using the Alamar Blue reagent was set-up to identify novel inhibitors of M. tuberculosis from a library of known drugs, for which there has already been extensive research investigating their suitability and safety as human therapeutics. Of the 1514 compounds screened, 53 were demonstrated to possess inhibitory properties against M. tuberculosis at a concentration of 5 μM or below. Of these, 17 were novel inhibitors while 36 were known tuberculosis drugs or had been previously described as possessing anti-tuberculosis activity. Five compounds were selected as those which represent the most promising starting points for new anti-tuberculosis agents. It was demonstrated that all five were active against intracellular M. tuberculosis in a macrophage model of infection. The anti-tuberculosis agents identified in this screen represent promising new scaffolds on which future drug development efforts can be focused.
Keywords: Mycobacterium tuberculosis, Alamar blue, Anti-tuberculosis agents, Therapeutics, Johns Hopkins Clinical Compound Library, Novel inhibitors
Introduction
There is an urgent need to develop new anti-tuberculosis drugs: highlighted by the on-going rise in tuberculosis (TB) cases worldwide. One worrying factor in the current TB problem is the prevalence of multi-drug resistant (MDR) strains, which emerged as a threat to TB control over 10 years ago. Yet, despite on-going attempts to control the worldwide problem, the situation appears to have escalated further 1, 2. Mycobacterium tuberculosis strains resistant not only to the front-line drugs isoniazid and rifampicin, but also to an increasing number of second-line drugs, are becoming more common 3, 4. These strains, termed extensively drug resistant (XDR) TB, are virtually untreatable using current therapeutics and, without the strengthening of the current TB control measures combined with a drive to introduce new anti-tuberculosis drugs, the situation is only set to worsen.
The high cost of developing a new drug combined with the past hesitation of pharmaceutical companies to take on a TB drug discovery program, has resulted in few new anti-tuberculosis therapeutics being brought to market during the last decade. However, a new drive towards TB drug development is currently underway, with a large number of academic research groups working towards the identification of suitable TB drug targets, and a range of companies investing in TB programs. This has resulted in several promising new drug candidates making their way through the drug development pipeline, such as moxifloxacin 5 and other fluoroquinolones 6. Fluoroquinolones represent an example of a drug previously originally introduced for the treatment of another infection being found to also be useful as a tuberculosis drug. In fact, since the introduction of rifampicin over 40 years ago, all tuberculosis therapeutics developed have been either old drugs with a new use, or new formulations of existing agents 7.
In the present study, we have used a library of known drugs and pharmacologically active compounds as the basis for a screening approach against Mycobacterium tuberculosis. The extensive evaluation of many of the agents in the Johns Hopkins Clinical Compound Library (JHCCL) for their suitability and safety as human drugs could aid in the rapid identification of a novel anti-tuberculosis agent. In addition, it is hoped that the screening of a large range of compounds will lead to the discovery of novel scaffolds suitable for optimisation and further development as TB drugs.
Material and methods
Strains and growth conditions
M. tuberculosis H37Rv wild-type strain was grown at 37°C in Dubos broth supplemented with 0.05% (vol/vol) Tween 80, 0.2% (vol/vol) glycerol, and 4% (vol/vol) Dubos medium albumin (Becton Dickinson), either in 100 ml volumes in a Bellco roll-in incubator (2 rpm) or in 10 ml volumes in static universals. Stocks of bacteria were prepared for use in the Alamar blue assay by first growing to an optical density of 0.5 to 0.6 at 600 nm. Cells were harvested and washed 3 times in 100 ml and resuspended in a final volume of 10 ml of phosphate-buffered saline. The stocks were frozen at −80°C and the colony forming units per ml determined upon thawing for use in the Alamar blue assay.
Chemicals and media
Kanamycin, streptomycin, isoniazid, ethambutol and rifampicin were obtained from Sigma. Drugs were solubilised in distilled water and filter sterilized (0.22-μm pore size). The Johns Hopkins Clinical Compound Library (JHCCL) contains a collection of 1514 known drugs, of which 1082 are FDA approved drugs and 432 are foreign approved drugs (FAD). The JHCCL was provided by Johns Hopkins University in a 96 well plate format with 25 μL aliquots of 10 mM stocks of drug in either water or dimethylsulfoxide. The compounds were diluted in DMSO to a concentration of 500 μM. The highest concentration used in the assays was 5 μM, corresponding to a 1% final DMSO concentration, well below the 5% determined to be inhibitory against M. tuberculosis (data not shown). The Alamar blue reagent was purchased from Promega (Cell Titre-Blue) and used according to the manufacturer’s recommendations.
Alamar blue susceptibility assay
Black, clear-bottomed, 96-well plates (Corning) to minimize background fluorescence were inoculated with 90 μl of the drug dilution at a single concentration for the initial medium-throughput screening assay, and a 2-fold dilution series performed for the subsequent MIC (Minimal inhibitory concentration) determinations. Drug dilutions were initially made in DMSO or distilled water and diluted into Dubos media plus albumin and glycerol supplements (No Tween 80). Wells containing the drug only were used as controls to detect auto-fluorescence of the compounds, and drug-only wells were also tested to determine that the compounds had no effect on the Alamar blue dye itself. The outer wells were inoculated with sterile water to minimize evaporation from the sample wells. 10 μl H37Rv diluted to give approximately 1 × 105 cells per well was added to each well and the plates incubated at 37°C for 7 days. Prior to the completion of the experiment, 20 μl of the Alamar blue reagent (CellTiter-Blue, Promega) and 12.5 μl 20% Tween 80 was added to each well. Plates were observed after 6 hours and incubated for a further 18 hours if the colour change was not sufficient. Fluorescence was measured with an excitation at 530 nm and emission at 590 nm in a Polarstar Galaxy (BMG Labtechnologies, Germany). Percent inhibition was defined as 1- (test well FU/mean FU triplicate bacteria only well) × 100. The MIC was taken to be the lowest concentration of a drug capable of causing ≥90% inhibition compared to the untreated bacteria only controls.
Statistical analysis
Data from this study was analyzed in Excel. Z-prime values were used as a measure of assay quality, using the following formula: Z’ = 1− 3×SSD/R, where SSD is the sum of the Standard Deviation of the negative controls and Standard Deviation of the positive controls, and R is the mean of the maximum signal control minus the mean of the negative signal control 7.
Bone marrow-derived macrophage infections
Bone marrow cells were flushed from the hind legs of 8 week old female BALB/C mice as previously described 8. Cells were differentiated for 7 days in RPMI 1640 medium plus 20% L-cell conditioned supernatant, 10% fetal calf serum, 0.02 mM L-glutamine, 10 mM sodium pyruvate, 0.1 mM HEPES and 0.5 μM β-mercaptoethanol at 37°C and 5% CO2 atmosphere. Macrophages were seeded overnight in 24-well plates at 2 × 105 cells/well in fresh medium containing 5% L-cell conditioned supernatant and then infected at a multiplicity of infection of 0.5:1 (0.5 bacteria per macrophage) for 6 hours. Extracellular bacteria were removed by washing three times with PBS, and fresh pre-warmed medium containing the drug dilutions was added to the wells. After 5 days, intracellular bacteria were enumerated by lysing the macrophages with water plus 0.05% Tween-80 and plating on Middlebrook 7H11 agar plates containing Middlebrook OADC supplement for viable counts. To determine if the drugs were toxic to macrophages, uninfected cells were incubated with the drugs dilutions in 96-well plates for 5 days. 20 μl Alamar blue was then added to the wells, incubated at 37°C for 6 hours and the fluorescence determined as above for the M. tuberculosis Alamar blue assay. Compound toxicity to macrophages was considered acceptable if the fluorescence was within 80% of that observed for the untreated macrophages.
Results
The Alamar Blue oxidation-reduction dye is used as an indicator of cell viability. The blue, non-fluorescent compound, reseazurin, is reduced to the pink, fluorescent resorufin within the cytoplasm of a viable cell, and the fluorescence is directly proportional to cell number 9. A fluorometric microplate-based Alamar blue assay has previously been demonstrated to be a rapid and inexpensive method for the medium-throughput screening of compounds for anti-tuberculosis activity 10. Preliminary experiments (data not shown) demonstrated that this method is accurate in the determination of antibiotic MICs, yielding results representative of those published by Collins and Franzblau 10. Briefly, 2-fold dilutions of the known anti-tuberculosis drugs kanamycin, rifampicin, streptomycin, isoniazid and ethambutol were incubated at 37°C with approximately 1 × 105 cells per well in 96-well microplates for 7 days. Untreated M. tuberculosis and media only controls were included on every plate. The Alamar blue reagent was added on the final day of the incubation, and the fluorescence measured at the completion of the experiment. The visual determination of the antibiotic MICs was seen to agree with the results from the fluorescence readings. The cut-offs between inhibited and non-inhibited wells was observed to be fairly sharp in most cases, with a clear visual and fluorescence difference between the MIC of the drug and the following dilution. On average, the media only control was observed to give fluorescence readings 95% less than those seen for the untreated M. tuberculosis wells. A 90% inhibition of fluorescence compared to the untreated control was chosen as the cut-off for the determination of inhibitory concentrations. It should be noted that in some cases, particularly where the drug was known to possess bacteriostatic rather than bacteriocidal activities, the concentration of drug immediately below the MIC did yield a fluorescent signal greater than the no bacteria controls but less than the 90% cut-off. It is thought that at these concentrations, the drug was incapable of completely sterilizing the well, but was sufficient to significantly impair the bacterial growth over the time period tested.
The initial screen was performed at a single concentration of 5 μM. Z-prime values were determined using the triplicate positive and negative control wells present on every plate, with a median value of 0.85 being obtained; indicating that the quality of the results was generally high. Z-prime is commonly used as a measure of assay quality, with a maximum of 1.0, and values >0.5 being taken to indicate a reliable assay 7.
While a 90% inhibition of fluorescence, relative to the positive control, was chosen as the cut-off for the determination of inhibitory concentrations, compounds which displayed any inhibition greater than 50% in the initial medium-throughput screen were chosen for further evaluation. A lower cut-off was decided upon after observing the relatively small proportion of the library resulting in any inhibition of M. tuberculosis growth at 5 μM. While this did mean that a large number of these potential candidates were rapidly removed during the more rigorous down-stream screening steps, it reduced the likelihood that any inaccuracies in the medium-throughput screening would lead to compounds being wrongly discarded in the early screening stages.
In total, 87 compounds were chosen to be screened in duplicate at a range of concentrations from 5 μM to 0.02 μM to determine the MIC of each (Figure 1). Fifty-five compounds exhibited significant inhibition of M. tuberculosis growth as determined by an inhibition of fluorescence units of greater than 80% at 5 μM or lower. Of these, 43 led to a greater than 90% inhibition in fluorescence units when compared with the untreated positive control at 1 or more drug concentrations. Of the 32 compounds which did not meet the requirements to be defined as possessing inhibitory properties in the 2nd more robust screen, some were observed to lead to a slight inhibition of fluorescence units but were not considered suitable for further investigation. Those compounds observed to be inhibitory in the second screen were re-tested to confirm the MIC values.
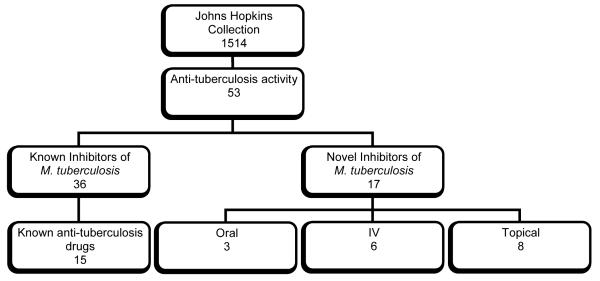
Figure 1.
Distribution of anti-tuberculosis activity in the Johns Hopkins Library of known drugs: 53 compounds exhibited more than 80% growth inhibition at 5 μM. Of these, 17 compounds were novel anti-tuberculosis agents: 3 of these were oral, 6 were intravenous and 8 were topical therapeutics.
Literature searches were conducted to determine the current therapeutic uses of the 53 compounds identified in the 2nd screen, particularly to allow identification of those compounds already shown in the literature to possess anti-tuberculosis activity (Table 1). Thirty-six compounds proved to be previously known anti-tuberculosis agents, providing an excellent validation of the screening assay. It should be noted that the identity of the inhibitors was unknown at the time of the screening: compounds were identified only by well number until positive hits had been identified in the initial screen. Seventeen compounds were identified as having no known anti-tuberculosis application, and to our knowledge, this is the first time these compounds have been investigated.
Table 1.
Compounds with greater than 80% growth inhibition of M. tuberculosis at 5 μM.
| i. WHO recommended drugs | |||
|---|---|---|---|
|
| |||
| Compound | Current Use | Route of delivery |
Anti-tuberculosis MIC (μM) |
| Rifampicin (Rifampin) | Antibacterial (First-line anti- tuberculosis agent) |
Oral | 0.02 |
| 3-formyl Rifamycin | Antibacterial (Anti-tuberculosis agent) |
Oral | 0.08 |
|
Isoniazid (Isonicotinic
acid hydrazide) |
Antibacterial (First-line anti- tuberculosis agent) |
Oral | 0.16 |
| Moxifloxacin HCl | Antibiotic (Fluoroquinolone – anti-tuberculosis agent) |
Oral | 0.16 |
| Amikacin | Antibiotic (Anti-tuberculosis agent) |
Intravenous | 0.31 |
| Ofloxacin | Antibiotic (Fluoroquinolone – anti-tuberculosis agent) |
Oral | 0.63 |
| Clofazimine | Antibacterial (Anti-tuberculosis agent) |
Oral | 1.25 |
| Gatifloxacin | Antibiotic (Fluoroquinolone – anti-tuberculosis agent) |
Oral | 1.25 |
| Levofloxacin HCl | Antibiotic (Fluoroquinolone – anti-tuberculosis agent) |
Oral | 1.25 |
| Ofloxacin | Antibiotic (Fluoroquinolone – anti-tuberculosis agent) |
Oral | 2.5 |
| Protionamide | Antibacterial (Second-line anti-tuberculosis agent) |
Oral | >5uM |
| Rifamycin sv | Antibacterial (First-line anti- tuberculosis agent) |
Oral | >5uM |
|
Thiacetazone
(Amithiozone) |
Antibacterial (Second-line anti-tuberculosis agent) |
Oral | >5uM |
| p-Aminosalicylic acid | Antibacterial (Second-line anti-tuberculosis agent) |
Oral | >5uM |
| Kanamycin B sulfate salt | Antibiotic (Anti-tuberculosis agent) |
Intravenous | >5uM |
| ii. Anti-tuberculosis drugs – compounds with known anti-mycobacterial activity | |||
|---|---|---|---|
|
| |||
| Compound | Current Use | Route of delivery |
Anti-tuberculosis MIC (μM) |
| Thiostrepton | Antibiotic | Topical | 0.08 |
| Rifaximin | Antibiotic | Oral (Non- systemic) |
0.08 |
| Sparfloxacin32, 33 | Antibiotic (Fluoroquinolone) | Oral | 0.08 |
| Doxycycline hyclate | Antibiotic (Anti-mycobacterial agent) |
Oral | 0.16 |
| Clinafloxacin HCl 34 | Antibiotic (Fluoroquinolone) | Oral | 0.31 |
|
Minocycline
hydrochloride salt 35 |
Antibiotic (Anti-mycobacterial agent) |
Oral | 0.31 |
| Aconiazide 36 | Antibacterial (Anti-tuberculosis agent) |
Oral | 0.63 |
| Doxycycline | Antibiotic (Anti-mycobacterial agent) |
Oral | 0.63 |
|
Vancomycin HCl
hydrate 35 |
Antibiotic (Agent) | Oral | 0.63 |
| Enrofloxacin | Antibiotic (Fluoroquinolone) | Oral | 1.25 |
| Tetracycline 37 | Antibiotic | Oral | 1.25 |
| Fleroxacin 38 | Antibiotic (Fluoroquinolone) | Oral | 2.5 |
| Tosufloxacin | Antibiotic (Fluoroquinolone) | Oral | 2.5 |
| Lomefloxacin33, 39 | Antibiotic (Fluoroquinolone) | Oral | 5 |
| Nialamide21, 22 | Antidepressant | Oral | 5 |
| Pazufloxacin 40 | Antibiotic (Fluoroquinolone) | Oral | 5 |
| Sarafloxacin HCl 41 | Antibiotic (Fluoroquinolone) | Oral | 5 |
|
Chlorhexidine
gluconate 42 |
Antiseptic | Topical | 5 |
| Methylene blue hydrate 43 | Antimethemoglobinemic | Intravenous | >5uM |
| Miconazole 44 | Antifungal | Topical | >5uM |
| Sulfathiazole | Antibiotic (Anti-mycobacterial agent) |
Oral | >5uM |
| iii. Novel anti-tuberculosis drugs | |||
|---|---|---|---|
|
| |||
| Compound | Current Use | Route of delivery |
Anti-tuberculosis MIC (μM) |
| Pyrvinium pamoate | Antihelmintic (Agent) | Oral | 0.31 |
| Bismuth subnitrate | Antacid | Oral | 2.5 |
|
Cefmenoxime
hydrochloride |
Antibiotic | Intravenous | 2.5 |
|
Pyrithione zinc (1-
Hydroxypyridine-2-thione Zinc Salt) |
Antibacterial | Topical | 2.5 |
|
Cetalkonium chloride
(Benzyldimethylhexadecyl ammonium chloride) |
Antibacterial | Topical | 5 |
| Hexadimethrine bromide | Antidote | Intravenous | 5 |
|
Methylbenzethonium
chloride |
Antiseptic | Topical | 5 |
| Primaquine | Antimalarial | Oral | 5 |
|
Protamine chloride, grade
V |
Antidote | Intravenous | 5 |
| Thonzonium bromide | Antiseptic | Topical | 5 |
|
Triple dye (Brilliant green
FW 482.64, Gentian violate FW 408, Proflavine hemisulfate FW 258.29) |
Antiseptic | Topical | 5 |
| Pentamide | Antiprotozoal | Intravenous | >5uM |
| Aurothioglucose | Antirheumatic | Intravenous | >5uM |
| Brilliant Blue | Antiseptic | Topical | >5uM |
| Demecarium bromide | Cholinergic | Topical (ophthalmic) |
>5uM |
| Gold sodium thiomalate | Antirheumatic | Intravenous | >5uM |
| Oxiconazole nitrate | Antifungal | Topical | >5uM |
| Sodium aurothiomalate | Antirheumatic | Intravenous | >5uM |
Inhibitors with known anti-tuberculosis activity
Thirty-six of the 55 hit compounds were found to possess known anti-tuberculosis activity and a number were current anti-tuberculosis drugs. The current treatment for new TB cases, as recommended by the World Health Organization, consists of a standardized regime involving a combination of front-line drugs 11. Isoniazid, rifampicin, pyrazinamide, and ethambutol are the most common, taken daily for two months, followed by four to six months of just two drugs daily (usually rifampicin and isoniazid). Drug resistance requires the use of the reserve or second-line TB drugs, of which there are 6 classes: aminoglycosides (amikacin, kanamycin), polypeptides (capreomycin, viomycin, enviomycin), fluoroquinolones (ciprofloxacin, moxifloxacin), cycloserine and p-aminosalicylic acid. Other TB drugs are available, such as rifabutin, clarithromycin, thioacetazone and linexolid, although these are not on the list of WHO recommended drugs. Of these known anti-tuberculosis drugs, a number were present in the JHCCL. Isoniazid and rifampicin were among the most inhibitory drugs in the screen. Both were also included in the initial validation of the assay using a number of known TB drugs; ethambutol was also used in the validation, but was not in the JHCCL. A number of the second-line tuberculosis drugs were also included in the medium-throughput screen, including amikacin, kanamycin, a number of fluoroquinolones, cycloserine, thiacetazone and p-aminosalicylic acid, all of which were seen to inhibit the growth of M. tuberculosis. It is important to mention that, of all the known anti-tuberculosis drugs present in the JHCCL, all except one were observed to possess inhibitory activity against M. tuberculosis in the current screen. The one exception was seen with cycloserine, a compound which possesses a published MIC against M. tuberculosis of greater than 5 μM.
The fluoroquinolones require a specific mention as they were very highly represented in the hits obtained from the initial screen. Fluoroquinolones, which are fluorine-containing nalidixic acid derivatives, were introduced in the 1980’s and are rapidly emerging as important drugs for the treatment of tuberculosis 5, 6. Currently, they are recommended as second-line treatments 12, in combination with other drugs to minimize the emergence of drug resistance, a common problem with these agents. However, their strong in vitro and in vivo activity against M. tuberculosis has led to them currently being evaluated as first-line drugs.
Potential anti-tuberculosis drug scaffolds
Several of the anti-tuberculosis therapeutics identified in this study are topical agents, with antibacterial, antiseptic, and antifungal applications. Despite their activity against M. tuberculosis, such compounds are not suitable for use directly as tuberculosis therapeutics as they cannot be administered internally. This most highly inhibitory, non-tuberculosis drug identified in this screen was Thiostrepton, a topical agent. This drug is a complex bacterial natural product that inhibits protein synthesis and was initially used as a topical veterinary antibiotic. The last decade has seen a number of publications investigating the effect of thiostrepton on the malaria parasite, Plasmodium falciparum 13. Despite originally being used as a topical antibiotic, Thiostrepton has been used intravenously to treat malaria infected mice, leading to clearance of the parasite 14. An equal amount of interest has been shown in the ability of thiostrepton to specifically target certain human cancer cells 15, with minimal toxicity against non-cancer cells 16. However, there is little available research concerning the potential of thiostrepton to inhibit M. tuberculosis 17, and we believe this could be an interesting area of future investigation.
Intravenous drugs are also not ideal for the treatment of uncomplicated tuberculosis, which is currently achieved over a period of up to 9 months using a combination of orally administered drugs. However, the novel therapeutics identified in this study may represent interesting starting points for further development as they have already undergone clinical testing. Hexadimethrine bromide (heparin antidote), protamine choloride, grade V (nerve gas antidote), gold sodium thiomalate (antirheumatic), methylene blue hydrate (antimethemoglobinemic), pentamide (antiprotozoal), aurothioglucose (antirheumatic), and sodium aurothiomalate (antirheumatic) were identified as injectable drugs with inhibitory effects against M. tuberculosis. Of these compounds, pentamide is the only drug currently used in the treatment of an infectious disease. We were therefore interested in further investigating the possibility that it may represent a starting point for a new M. tuberculosis therapeutic. The mechanism of action of pentamide is currently unknown, although it is thought that the drug interferes with nuclear metabolism producing inhibition of the synthesis of DNA, RNA, phospholipids, and proteins. It is used in prophylaxis against Leishmaniasis and sleeping sickness, as well as for the treatment of HIV-associated pneumonia due to Pneumocystis carinii. It has also been suggested as a potential anti-cancer drug 18. It is interesting to note that, in the treatment of P. carinii, aerosolised pentamide is used over extremely long periods and is well-tolerated 19.
Inhibitors currently administered as oral therapeutics represent ideal starting points for tuberculosis drug development. Such compounds have been clinically tested and formulated to allow delivery orally. A number of the agents in the JHCCL fall into this category. Pyrvinium pamoate (antihelmintic), bismuth subnitrate (antacid), nialamide (antidepressant), and primaquine (antimalarial), were identified as orally administered drugs capable of inhibiting M. tuberculosis growth. Bismuth subnitrate, whilst being an oral agent, is poorly absorbed gastrointestinally, with less than 0.005% estimated to be taken up systemically: this limits the compound from having any useful anti-tuberculosis activity.
On further investigation, nialamide was found to be a derivative of the front-line tuberculosis drug, Isoniazid. A few publications from the 1960s mention nialamides possessing anti-tuberculosis activity 20-22, and one investigated the cross-resistance of isoniazid resistant isolates to nialamide 23. Isoniazid itself is a pro-drug which is metabolically activated within the bacterial cell. There is limited evidence that pro-drugs of isoniazid can have improved anti-tuberculosis activity due to better mycobacterial cell wall permeability 24. It is thought that nialamide may prove to be a good starting point for further work in this area, potentially yielding new isoniazid derivatives with improved anti-tubercular activity.
Pyrvinium pamoate possessed strong anti-tuberculosis activity in this study. The biochemical mechanisms underlying the anti-helmintic action of this drug are not fully understood, but it is considered to exert its killing effects through the inhibition of glucose and glycogen utilization 25. It may be interesting in terms of an anti-tuberculosis agent in that there is also some evidence to suggest that it inhibits fumarate reductase activity in worms 26. M. tuberculosis fumarate reductase plays a central role in the tricarboxylic acid pathway during hypoxia. Genes suspected of having an involvement in survival under low oxygen conditions represent interesting drug targets because of their likely importance in a latent tuberculosis infection, although it remains to be determined whether the anti-tuberculosis activity of pyrvinium pamoate is related to its inhibition of fumarate reductase. The long period of treatment required to cure an M. tuberculosis infection does raise the issue of toxicity when considering pyrvinium pamoate as an anti-tuberculosis drug: for the treatment of helminth infections it is administered as a single dose repeated after 14 days. However, this compound appears to be structurally different from all other known anti-tuberculosis agents, and we propose that it represents a promising new starting point for future drug development. This drug has also recently been demonstrated to have interesting anti-cancer properties 26.
The anti-tuberculosis activity of primaquine appears to be a novel finding. In addition, this anti-malarial agent possesses a structure not seen among known tuberculosis drugs, raising the possibility that it could represent a new drug template with possibly a novel mechanism of action against M. tuberculosis. Primaquine’s anti-malarial mechanism of action is not well understood, and may involve the generation of reactive oxygen species or interference with electron transport. While toxicity issues associated with this compound must be addressed when considering primaquine’s suitability as a new tuberculosis therapeutic, it is hoped that it may represent a novel scaffold from which a new therapeutic can be developed.
Intracellular Activity
Five of the drugs with anti-tuberculosis activity were chosen for further investigation as we believe they represent the strongest starting points for a new tuberculosis therapeutic. Thiostrepton, primaquine, pentamide, nialamide and pyrvinium pamoate were tested for their ability to inhibit growth of M. tuberculosis in an intracellular macrophage model of infection. The majority of the drugs with novel anti-tuberculosis activity were ruled out for further investigation due to their toxicity, and these compounds would require chemical optimisation before they could be tested in an intracellular model of infection. The shortlisted drugs were applied to M. tuberculosis infected bone marrow-derived macrophages at a range of concentrations. The infected monolayers were lysed after 5 days and the viable bacteria enumerated by colony counts. In untreated control wells, viable bacteria increased in numbers by more than 10-fold (colony count increasing from 8.2×103 at T=0 to 9.8×104, representative of three repeats). Isoniazid, included as a control example of a bacteriostatic anti-tuberculosis agent, prevented intracellular growth of M. tuberculosis at concentrations up to 1 μg/ml.
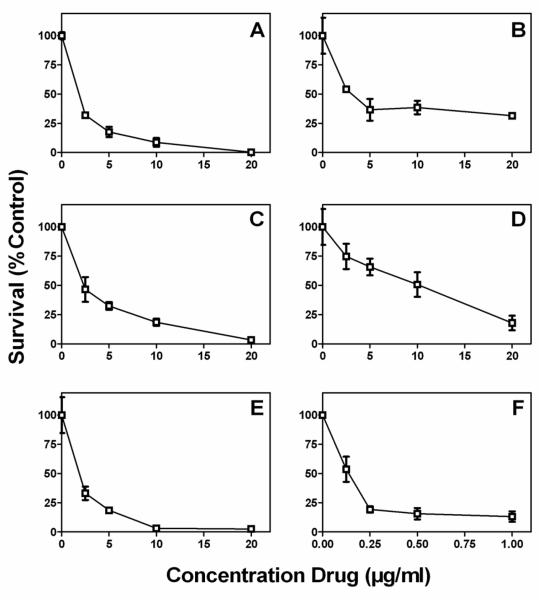
All of the compounds tested were inhibitory to growth of intracellular M. tuberculosis (Figure 2). Not all drugs were capable of completely preventing growth of the bacteria, and only thiostrepton appeared to have a bacteriocidal effect. Pyrvinium pamoate and pentamide did not result in complete inhibition of growth compared to the untreated control at concentrations up to 20 μM, although growth of intracellular bacteria was inhibited to some degree at concentrations above 2.5 μM. Nialamide inhibted growth of intracellular bacteria at 10 μM, while Primaquine and Thiostrepton were inhibitory at 5 μM. Thiostrepton also had some bactericidal activity at 10 μM, reducing the initial colony count by 4 fold.
Figure 2.
Intracellular activity of Johns Hopkins drugs in a bone marrow derived macrophage model of infection. Primaquine (A), Pentamide (B), Nialamide (C), Pyrvinium pamoate (D), Thiostrepton (E), and Isoniazid (F) were incubated with macrophages for 5 days and the surviving bacilli enumerated by colony counts. Inhibition of growth is shown as a percentage of the untreated control.
None of the compounds were observed to have any effect on viability of the macrophages at the concentrations inhibitory in the intracellular assay, as tested with the Alamar blue reagent (results not shown), indicating that the reduction in colony count was a result of the bactericidal effect of the drugs, rather than a non-specific lethal effect on the macrophages.
Discussion
Screening for novel anti-tuberculosis agents is important to the drug discovery effort. The growth inhibition assay described here has been demonstrated to be appropriate for the screening of potential inhibitory compounds against M. tuberculosis. In this study, we have used the Alamar blue-based assay to screen a library of 1,514 known drugs and pharmacologically active compounds, identifying several novel inhibitors of M. tuberculosis. The large number of known M. tuberculosis drugs highlighted during the screen serves to confirm that the assay is suitable for the detection of compounds with anti-tuberculosis activity. In addition to the known tuberculosis drugs identified in this experiment, a large number of anti-bacterials with previously described anti-tuberculosis activity were also highlighted. Although these have been demonstrated to inhibit M. tuberculosis, not all have been used in the treatment of TB. While many are not sufficiently active against M. tuberculosis to present viable drug options in their present state, they could perhaps represent some interesting starting points for further development.
In the past, efforts to identify new tuberculosis therapeutics have considered the possibility of re-working existing antibacterial agents to target tuberculosis. It was such an approach that has recently demonstrated the usefulness of fluoroquinolones and their newer derivatives as anti-tubercular agents 12. By revisiting some of the early antibiotic structures, it may be possible to find new scaffolds on which improved anti-tuberculosis drugs can be based. It should be remembered that a key problem for any M. tuberculosis therapeutic is that it must be capable of gaining entry to the cell by crossing the highly hydrophobic cell wall. Therefore, those drugs capable of entering the cell and inhibiting growth, even only at higher concentrations, should not be immediately discounted before attempts at improving their inhibitory action have been made.
A number of oral agents with anti-tubercular activity were identified in this study; primaquine, nialamide and pyrvinium pamoate represent the most promising potential inhibitors. Both nialamide and primaquine were inhibitory in an intracellular model of infection. While not suitable for the treatment of tuberculosis in their current state, it is hoped that they will yield interesting starting points for drug development. In addition to these oral agents, the intravenous and topical inhibitors identified should not be discounted. Pentamide and Nialamide were both observed to possess anti-mycobacterial activity, both in vitro and against intracellular bacteria. While the current treatment for uncomplicated tuberculosis relies on a long regimen of oral therapeutics, injectable agents are recommended for drug resistant cases 27. One possibility is that injectable agents identified in this study, or compounds derived from them, could be used as a final mode of attack to combat infections in the developed world that have failed to respond to all other therapeutics, such as in HIV sufferers or cases of extreme drug resistance.
Topical agents may also prove to have useful activities against mycobacterial infections and it is hoped that some topical agents identified in this study may possess structures that can easily be altered to provide an agent capable of being administered orally. Thiostrepton is one such agent, which has already been demonstrated to have the potential for intravenous use in the treatment of other infectious diseases and cancer 15. Another intriguing possibility is that topical agents may be suitable for the treatment of certain mycobacterial skin infections in their current formulation. Mycobacterium ulcerans is a slow-growing organism, which causes a little understood tropical disease known as Buruli ulcer 28. Infection leads to skin and soft tissue destruction, which can leave patients with long-term disability if not treated early. The limited knowledge of this disease poses a problem for its prevention, diagnosis and treatment, particularly because it mainly affects poor rural communities with little access to adequate healthcare. Currently, treatment for this condition requires a combination of the tuberculosis drugs rifampicin and streptomycin or amikacin, taken for 8 weeks 29. In addition, surgery is often required to remove the infected portions of tissue. The use of a topical agent to treat this condition, combined with better diagnosis to detect cases early in infection, may enable patients to be treated at home, minimizing their loss of earnings during long stays in hospital. The potential use of topical agents against M. ulcerans has been reported 30, and a small study demonstrated that topical nitrogen oxides are efficacious against Buruli ulcer 31. However, there is yet to be any topical agent introduced as a treatment for this neglected disease.
Following the successful identification of a number of inhibitory agents which will be further investigated to determine their usefulness as scaffolds for future anti-tuberculosis drug development efforts, the screening of a number of additional libraries is also being planned. It is hoped that, through the screening of less well characterized and diverse compounds, it will be possible to identify additional scaffolds for future drug development.
Acknowledgments
We thank David Sullivan and Jun Liu from the Johns Hopkins University for providing the Johns Hopkins Clinical Compound Library used in this research.
Funding: This research was supported by MRC Technology and the Medical Research Council. KL is a Career Development Fellow supported by a MRC Technology Development Gap Fund grant.
Footnotes
Competing interests: None declared
Ethical approval: Not required
References
- 1.Espinal MA. The global situation of MDR-TB. Tuberculosis (Edinb) 2003;83:44–51. doi: 10.1016/s1472-9792(02)00058-6. [DOI] [PubMed] [Google Scholar]
- 2.Global Alliance for TB Drug Development Tuberculosis. Scientific blueprint for tuberculosis drug development. Tuberculosis (Edinb) 2001;81(Suppl 1):1–52. doi: 10.1054/tube.2001.0288. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs--worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 4.Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martin-Casabona N, Drobniewski F, Gilpin C, Havelkova M, Lepe R, Lumb R, Metchock B, Portaels F, Rodrigues MF, Rusch-Gerdes S, Van Deun A, Vincent V, Laserson K, Wells C, Cegielski JP. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazaki E, Miyazaki M, Chen JM, Chaisson RE, Bishai WR. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–9. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvirez-Freites EJ, Carter JL, Cynamon MH. In vitro and in vivo activities of gatifloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2002;46:1022–5. doi: 10.1128/AAC.46.4.1022-1025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 8.Tascon RE, Soares CS, Ragno S, Stavropoulos E, Hirst EM, Colston MJ. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473–80. doi: 10.1046/j.1365-2567.2000.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed SA, Gogal RM, Jr., Walsh JE. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods. 1994;170:211–24. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 10.Collins L, Franzblau SG. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother. 1997;41:1004–9. doi: 10.1128/aac.41.5.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organisation Treatment of Tuberculosis: guidelines for national programmes. 2003. http://wwwwhoint/en/ WHO/CDS/TB/2003.313.
- 12.Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs. 2007;67:2077–99. doi: 10.2165/00003495-200767140-00007. [DOI] [PubMed] [Google Scholar]
- 13.McConkey GA, Rogers MJ, McCutchan TF. Inhibition of Plasmodium falciparum protein synthesis. Targeting the plastid-like organelle with thiostrepton. J Biol Chem. 1997;272:2046–9. doi: 10.1074/jbc.272.4.2046. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan M, Li J, Kumar S, Rogers MJ, McCutchan TF. Effects of interruption of apicoplast function on malaria infection, development, and transmission. Mol Biochem Parasitol. 2000;109:17–23. doi: 10.1016/s0166-6851(00)00226-7. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou KC, Zak M, Rahimipour S, Estrada AA, Lee SH, O’Brate A, Giannakakou P, Ghadiri MR. Discovery of a biologically active thiostrepton fragment. J Am Chem Soc. 2005;127:15042–4. doi: 10.1021/ja0552803. [DOI] [PubMed] [Google Scholar]
- 16.Kwok JM, Myatt SS, Marson CM, Coombes RC, Constantinidou D, Lam EW. Thiostrepton selectively targets breast cancer cells through inhibition of forkhead box M1 expression. Mol Cancer Ther. 2008;7:2022–32. doi: 10.1158/1535-7163.MCT-08-0188. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen MW, Wu J. Use of thiostrepton as an anti-mycobacterial agent. United States. 2004 [Google Scholar]
- 18.Lee MS, Johansen L, Zhang Y, Wilson A, Keegan M, Avery W, Elliott P, Borisy AA, Keith CT. The novel combination of chlorpromazine and pentamidine exerts synergistic antiproliferative effects through dual mitotic action. Cancer Res. 2007;67:11359–67. doi: 10.1158/0008-5472.CAN-07-2235. [DOI] [PubMed] [Google Scholar]
- 19.Ewig S, Schafer H, Rockstroh JK, Pickenhain A, Luderitz B. Effect of long-term primary aerosolized pentamidine prophylaxis on breakthrough Pneumocystis carinii pneumonia. Eur Respir J. 1996;9:1006–12. doi: 10.1183/09031936.96.09051006. [DOI] [PubMed] [Google Scholar]
- 20.Erlach A. Klinische Untersuchungen uber Cochleotoxidose durch Viomycin and Kanamycin wahrend tuberkulostatischer Therapie (Versuch einer Prophylaxe) Monatsschr Ohrenheilkd Laryngorhinol. 1968;102:624–30. [PubMed] [Google Scholar]
- 21.Dupasquier P, Keita S. A propos de l’activit’e antituberculeuse “in vitro” du n-isonicotinylhydrazide. Rev Tuberc Pneumol (Paris) 1965;29:234–5. [PubMed] [Google Scholar]
- 22.Coletsos PJ. Identit’e de comportement in vitro ’a l”egard de mycobacterium tuberculosis de l’inh et du niamide, thymo-analeptique i.m.a.o. (d’eductions d’ordre biologique et th’erapeutique) Rev Tuberc Pneumol (Paris) 1965;29:165–72. [PubMed] [Google Scholar]
- 23.Klugh GA, Pratt PC, Atwell RJ. Antimycobacterial activity of N-isonicotinyl hydrazide and cross resistance to isoniazid. Am Rev Respir Dis. 1960;82:251–2. doi: 10.1164/arrd.1960.82.2.251. [DOI] [PubMed] [Google Scholar]
- 24.Aboul-Fadl T, Hassanin K. Tetrahydro-2H-1,3,5-thiadiazine-5-(4-pyridylcarboxamide)-2-thione derivatives as prodrugs for isoniazid; synthesis, investigations and in vitro antituberculous activity. Pharmazie. 1999;54:244–7. [PubMed] [Google Scholar]
- 25.Sheth UK. Mechanisms of anthelmintic action. Prog Drug Res. 1975;19:147–57. doi: 10.1007/978-3-0348-7090-0_19. [DOI] [PubMed] [Google Scholar]
- 26.Esumi H, Lu J, Kurashima Y, Hanaoka T. Antitumor activity of pyrvinium pamoate, 6-(dimethylamino)-2-[2-(2,5-dimethyl-1-phenyl-1H-pyrrol-3-yl)ethenyl]-1-me thyl-quinolinium pamoate salt, showing preferential cytotoxicity during glucose starvation. Cancer Sci. 2004;95:685–90. doi: 10.1111/j.1349-7006.2004.tb03330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation Guidelines for the programmatic management of drug-resistant tuberculosis. 2006. http://wwwwhoint/en/ WHO/HTM/TB/2006.361.
- 28.Thangaraj HS, Evans MR, Wansbrough-Jones MH. Mycobacterium ulcerans disease; Buruli ulcer. Trans R Soc Trop Med Hyg. 1999;93:337–40. doi: 10.1016/s0035-9203(99)90104-9. [DOI] [PubMed] [Google Scholar]
- 29.Chauty A, Ardant MF, Adeye A, Euverte H, Guedenon A, Johnson C, Aubry J, Nuermberger E, Grosset J. Promising clinical efficacy of the combination streptomycin - rifampin for the treatment of Buruli ulcer (Mycobacterium ulcerans disease) Antimicrob Agents Chemother. 2007 doi: 10.1128/AAC.00175-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adjei O, Evans MR, Asiedu A. Phenytoin in the treatment of Buruli ulcer. Trans R Soc Trop Med Hyg. 1998;92:108–9. doi: 10.1016/s0035-9203(98)90977-4. [DOI] [PubMed] [Google Scholar]
- 31.Phillips R, Adjei O, Lucas S, Benjamin N, Wansbrough-Jones M. Pilot randomized double-blind trial of treatment of Mycobacterium ulcerans disease (Buruli ulcer) with topical nitrogen oxides. Antimicrob Agents Chemother. 2004;48:2866–70. doi: 10.1128/AAC.48.8.2866-2870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duman N, Cevikbas A, Johansson C. The effects of rifampicin and fluoroquinolones on tubercle bacilli within human macrophages. Int J Antimicrob Agents. 2004;23:84–7. doi: 10.1016/j.ijantimicag.2003.05.017. [DOI] [PubMed] [Google Scholar]
- 33.Sulochana S, Rahman F, Paramasivan CN. In vitro activity of fluoroquinolones against Mycobacterium tuberculosis. J Chemother. 2005;17:169–73. doi: 10.1179/joc.2005.17.2.169. [DOI] [PubMed] [Google Scholar]
- 34.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–9. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins CH, Uttley AH. In-vitro activity of seventeen antimicrobial compounds against seven species of mycobacteria. J Antimicrob Chemother. 1988;22:857–61. doi: 10.1093/jac/22.6.857. [DOI] [PubMed] [Google Scholar]
- 36.Peloquin CA, James GT, Craig LD, Kim M, McCarthy EA, Ikle D, Iseman MD. Pharmacokinetic evaluation of aconiazide, a potentially less toxic isoniazid prodrug. Pharmacotherapy. 1994;14:415–23. [PubMed] [Google Scholar]
- 37.Hobby GL, Lenert TF. Antituberculous activity of tetracycline and related compounds. Am Rev Tuberc. 1955;72:367–72. doi: 10.1164/artpd.1955.72.3.367. [DOI] [PubMed] [Google Scholar]
- 38.Salfinger M, Hohl P, Kafader FM. Comparative in-vitro activity of fleroxacin and other 6-fluoroquinolones against mycobacteria. J Antimicrob Chemother. 1988;22(Suppl D):55–63. doi: 10.1093/jac/22.supplement_d.55. [DOI] [PubMed] [Google Scholar]
- 39.Piersimoni C, Morbiducci V, Bornigia S, De Sio G, Scalise G. In vitro activity of the new quinolone lomefloxacin against Mycobacterium tuberculosis. Am Rev Respir Dis. 1992;146:1445–7. doi: 10.1164/ajrccm/146.6.1445. [DOI] [PubMed] [Google Scholar]
- 40.Tomioka H, Sato K, Saito H. In vitro antimycobacterial activity of a new quinolone, T-3761. Kekkaku. 1995;70:97–101. [PubMed] [Google Scholar]
- 41.Berlin OG, Young LS, Bruckner DA. In-vitro activity of six fluorinated quinolones against Mycobacterium tuberculosis. J Antimicrob Chemother. 1987;19:611–5. doi: 10.1093/jac/19.5.611. [DOI] [PubMed] [Google Scholar]
- 42.Best M, Sattar SA, Springthorpe VS, Kennedy ME. Efficacies of selected disinfectants against Mycobacterium tuberculosis. J Clin Microbiol. 1990;28:2234–9. doi: 10.1128/jcm.28.10.2234-2239.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristiansen JE, Amaral L. The potential management of resistant infections with non-antibiotics. J Antimicrob Chemother. 1997;40:319–27. doi: 10.1093/jac/40.3.319. [DOI] [PubMed] [Google Scholar]
- 44.Sun Z, Zhang Y. Antituberculosis activity of certain antifungal and antihelmintic drugs. Tuber Lung Dis. 1999;79:319–20. doi: 10.1054/tuld.1999.0212. [DOI] [PubMed] [Google Scholar]