Abstract
Introduction
Febrile neutropaenia is a frequently occurring and occasionally life-threatening complication of treatment for childhood cancer, yet many children are aggressively over-treated. We aimed to undertake a systematic review and meta-analysis to summarise evidence on the discriminatory ability and predictive accuracy of clinical decision rules (CDR) of risk stratification in febrile neutropaenic episodes.
Methods
The review was conducted in accordance with Centre for Reviews and Dissemination methods, using random effects models to undertake meta-analysis. It was registered with the HTA Registry of systematic reviews, CRD32009100453.
Results
We found 20 studies describing 16 different CDR assessed in 8388 episodes of FNP. No study compared different approaches and only one CDR had been subject to testing across multiple datasets. This review cannot conclude that any system is more effective or reliable than any other.
Conclusion
To maximise the value of the information already collected by these and other cohorts of children with febrile neutropaenia, an individual-patient-data (IPD) meta-analysis is required to develop and test new and existing CDR to improve stratification and optimise therapy.
Keywords: Systematic review, Meta-analysis, Neutropaenic sepsis, Clinical decision rules, Diagnosis
1. Introduction
Children undergoing treatment for malignancy have an excellent chance of survival, with overall rates approaching 75%.1 In most cases, children who die following treatment for cancer do so of their disease, but despite huge improvements in supportive care, around 16% of deaths within 5 years of diagnosis are due to the complications of therapy.2,3 One such life-threatening complication in immunocompromised children remains infection, frequently presenting as the occurrence of fever with neutropaenia.4 A robust risk stratification model which reliably predicted those children at very low risk of having a significant infection could result in reduced intensity and/or duration of hospitalised antibiotic therapy. Those at high risk of complications could be targeted for more aggressive management. At present there are many differing policies for the management of febrile neutropaenia in paediatric practice5,6 with lack of agreement about how and which clinical decision rules (CDR), if any, are used.
A clinical decision rule is a tool designed to be used at the bedside to assist clinical decision making.7 These rules should be validated by assessing them on a separate population; to test both how well the rule differentiates the risk groups (discriminatory ability) and to determine the absolute estimates of risk within these groups (predictive accuracy).
In adult oncology practice the Multinational Association for Supportive Care in Cancer (MASCC) risk index8 provides a CDR to identify patients at low risk of serious medical complications during febrile neutropaenia. The factors identified included ‘young age’ (<60 years) and no chronic obstructive airways disease, among other features specific to the disease type and presentation at each episode, and has been used as the basis for the out-patient management of fever in low-risk neutropaenic adult patients.9 The MASCC rule is of very limited applicability in this group: it did not include children in its derivation, the age criterion is non-discriminatory, and chronic airways disease is extremely rare. Accordingly, studies on children and young people require separate, detailed examination.
This systematic review aimed to identify, critically appraise and synthesise evidence on the discriminatory ability and predictive accuracy of existing CDRs in febrile neutropaenic episodes in children and young people undergoing treatment for malignant disease.
2. Materials and methods
The review was conducted in accordance with ‘Systematic reviews: CRD’s guidance for undertaking reviews in health care10 and registered on the HTA Registry of systematic reviews: CRD32009100453. It sought studies which aimed to derive or validate a CDR in children or young people (aged 0–18 years) presenting with febrile neutropaenia. Both prospective and retrospective cohorts were included, but those using a case–control (‘two-gate’) approach were excluded as these have been previously shown to exaggerate diagnostic accuracy estimates.11 Studies exclusively addressing the prediction of radiologically confirmed pneumonia are subject to a separate review [in submission to Arch Dis Child – Still under consideration].
2.1. Search strategy and selection criteria
An electronic search strategy (see Web Appendix 1) was developed which examined the following databases from their inception to February 2009:
-
•
MEDLINE
-
•
MEDLINE In-Process and Other Non-Indexed Citations
-
•
EMBASE
-
•
CINAHL
-
•
Cochrane Database of Systematic Reviews (CDSR)
-
•
Database of Abstracts of Reviews of Effects (DARE)
-
•
Health Technology Assessment Database (HTA)
-
•
Cochrane Central Register of Controlled Trials (CENTRAL)
-
•
Conference Proceedings Citation Index - Science (CPCI-S)
-
•
Literatura Latinoamericana y del Caribe en Ciencias de la Salud (LILACS)
Reference lists of relevant systematic reviews and included articles were reviewed for further relevant articles. Published and unpublished studies were sought and no language restrictions applied. Non-English language studies were translated. Two reviewers independently screened the title and abstract of studies for inclusion, and then the full text of retrieved articles. Disagreements were resolved by consensus.
2.2. Validity assessment and data extraction
The validity of each study was assessed using 11 of the 14 questions from the QUADAS assessment tool for diagnostic accuracy studies.12 The QUADAS tool was adapted specifically for the review,13 omitting questions on ‘time between index and reference test’, ‘intermediate results’ and ‘explanation of withdrawals’ (see Web Appendix 2). The CDR and reference tests are necessarily related, and the design of a CDR means that ‘intermediate’ results are included in any analysis. The issue of incomplete data was addressed in the analysis of the method of derivation or validation, and as such was not included as a quality criterion.
Data were extracted by one reviewer and checked by the other. The data extracted included age and sex distribution of the included participants, geographical location of the study and participant inclusion/exclusion criteria. The performance of the CDR as a 2 * k table (where k refers to the number of strata described) as well as the methods used to derive the CDR (where applicable), the variables considered, methods of statistical analysis and approach to multiple episodes in individual patients and missing data were also extracted.
2.3. Methods of analysis/synthesis
Quantitative synthesis was undertaken for studies which tested the same CDR and, where appropriate, was investigated for sources for heterogeneity.
For dichotomous test data, analyses were attempted with a bivariate model (using ‘metandi’ in STATA1014). For tests with very small numbers of studies to pool (n ⩽ 4) fitting a bivariate model is problematic as the procedure frequently fails to converge. In these cases, a univariate approach was used (pooling sensitivity and specificity separately).15
For tests where three-level (low, medium and high risk) results were produced, an approach based on a previous meta-analysis of three-level CDR results was used.16 This random-effects meta-analysis was undertaken using WinBUGS 1.4.317 to estimate the proportions of individuals classified as low, medium or high risk in the bacteraemic and non-bacteraemic groups. As an extension to this method, bivariate random effects were applied to the calculation of each proportion. Data from studies which used a similar rule but provided only two of the risk categories (i.e. low versus medium–high) were also included in this analysis.18 These proportions were used to calculate likelihood ratios (LR) for each risk category and the corresponding 95% credible intervals (CrI).
Heterogeneity between study results was explored through consideration of study populations, study design, predictor variables assessed and outcomes chosen, although the small number of studies in each category limited this approach. Sensitivity analysis was undertaken by comparing results when the original (derivation) dataset was included and excluded.
For those areas where a quantitative synthesis was not possible, a narrative approach was used.
3. Results
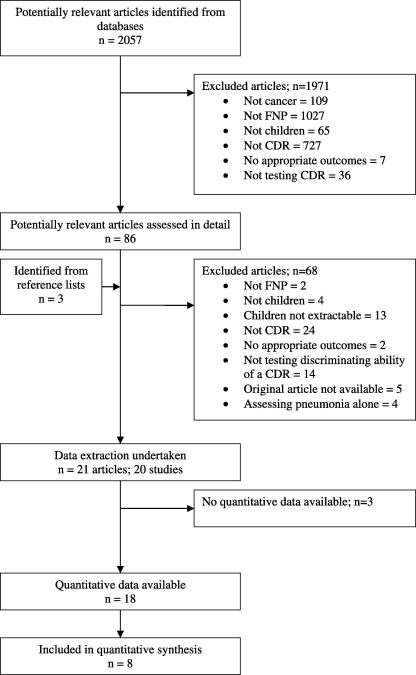
Twenty-one articles reporting on 20 studies19–38 were eligible for inclusion in the review (see Fig. 1). The studies included patients from 1 month to 23 years old, with a wide range of malignancies, and a total of 7840 episodes of FNP describing 11 outcomes, summarised in 5 clusters: death, critical care requirement, serious medical complication, significant bacterial infection and bacteraemia (see Table 1). Eight of these studies were prospective,25,26,29,30,32,33,36,37 11 were retrospective19–23,27,28,31,34,35,38 and 1 was a retrospective analysis of prospectively collected data.24
Fig. 1.
Flow diagram of study selection process.
Table 1.
Studies of clinical decision rules.
| Citation | Derivation or validation study | Study location (years) | Inclusion criteria | Exclusion criteria | Total number of patients | Total number of episodes | Age of patients | Clinical prediction rulea | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Adcock (1999) | Derivation of CDR | North Carolina, USA (1995–1996) | ANC <1000 cells/mm3, temperature ⩾38 °C, HIV −ve | 33 | 88 | Median 5 y (range 1–18 y) | High risk = hypotension/septic shock, inflamed central line site, recent high dose Ara-C | Gram-positive bacteraemia | |
| Alexander (2002) | Derivation of CDR | Boston, USA (1994–1995) | ANC ⩽500/mm3, temperature >38.5 °C. Outpatient status | Post stem cell transplant | 104 | 188 | Mean 8.9 y (SD 5.7 y) | Low risk = not AML/Burkitts/Induction ALL/Progressive-relapsed with marrow involvement (‘Anticipated neutropaenia <7 d’) and no significant comorbidity (defined as hypotension, tachypnea/hypoxia <94%, new CXR changes, altered mental status, severe mucositis, vomiting or abdominal pain, focal infection, other clinical reason for in-patient treatment) | Bacteraemia |
| Serious medical complication Death | |||||||||
| High risk = hypotension/septic shock, inflamed central line site, recent high dose Ara-C | |||||||||
| Ammann (2003) | Derivation of CDR | Berne, Switzerland (1993–2001) | ANC ⩽500 cells/mm3 or ⩽1000 cells/mm3 and falling, axilliary temperature ⩾38.5 °C for ⩾2 h, or once ⩾39 °C | Patients with FN due to malignant bone marrow suppression, or following myeloablative therapy | 132 | 364 | Not reported | Low risk = not AML/Burkitts/Induction ALL/Progressive-relapsed with marrow involvement (‘Anticipated neutropaenia <7 d’) and no significant comorbidity (defined as hypotension, tachypnea/hypoxia <94%, new CXR changes, altered mental status, severe mucositis, vomiting or abdominal pain, focal infection, other clinical reason for in-patient treatment) | Severe bacterial infection, (death from bacterial infection, a positive culture of normally sterile body fluids, radiologically proven pneumonia, clinically unequivocal diagnosis of a bacterial infection, or CRP > 150 mg/L) |
| (regression models) | Low risk ⩽ 3 factors (model #2) or ⩽4 factors (model #3). Risk factors = bone marrow involvement, absence of clinical signs of viral infection, high serum CRP level, low leucocyte count, presence of a central venous catheter, high haemoglobin level, and Pre-B-cell leukaemia | Severe bacterial infection (death from bacterial infection, a positive culture of normally sterile body fluids, radiologically proven pneumonia, clinically unequivocal diagnosis of a bacterial infection, or CRP > 150 mg/L) | |||||||
| Ammann (2004) (same population as Ammann (2003)) | Derivation of CDR | Additionally, patients with established severe bacterial infection | 111 | 285 | Median 6.3 y (interquartile range 3.2–12.1 y) | Low risk = all of: maximum temp ⩽ 39.7 °C, no comorbidity requiring hospitalisation, leucocyte count > 0.5 × 109/L, and in partial or complete remission | Bacteraemia | ||
| Gala-Peralta (2005) | Validation of CDR | Barcelona, Spain (2002) | ANC ⩽ 500/mm3, ‘fever’ (temperature not defined) | 30 | 62 | Mean 8.7 y (range 1.2–14.7 y) | Low risk ⩽ 2 of: <1 y, poor bone marrow response (plt < 75, ANC < 100),uncontrolled solid tumour or relapsed leukaemia, chemotherapy <10 d earlier, rapid neutropaenia, cardiac and renal dysfunction | Positive blood culture | |
| Hann (1997) | Derivation of CDR | Multiple centres across Western Europe (1986–1994) | ANC ⩽1000 cells/mm3, temperature ⩾ 38.0 °C twice in <12 h, or once ⩾ 38.5 °C, in an EORTC trial | 759 | 759 | Median 8 y | No rule described | Bacteraemia | |
| Individual features = disease type, IV line, shock, duration of granulocytopaenia and admission temperature | |||||||||
| Jones (1996) | Derivation of CDR | North Carolina, USA (1987–1993) | ANC <500 cells/mm3, oral temperature ⩾ 38.0 °C ⩾12 h, or once >38.5 °C | None reported, but ‘none of the children were undergoing BMT’ | 127 | 276 | Mean 8 y (range 2 m to 21 y) | Low risk = ANC ⩾ 200, outpatient at onset, in remission | Bacteraemia Clinical infection |
| Lucas (1996) | Derivation of CDR | New York, USA (1990–1992) | ANC <500 cells/mm3 or <1000 cells/mm3 and falling, temperature ⩾ 38.0 °C ⩾2 occasions in ⩾12 h, or once ⩾38.5 °C. Outpatient status | Received blood product transfusions within 6 h or cytosine arabinoside within 2 d of presentation | 161 | 509 | Mean 9.2 y (range 1–18 y) | Low risk = no chills, hypotension, or a requirement for fluid resuscitation at admission | Positive blood culture ICU admission Septic death |
| Petrelli (1991) | Validation of CDR | Camargo, Brazil (1988–1989) | ANC ⩽ 500 cells/mm3, temperature ⩾ 37.5 °C ⩾3 occasions in ⩾24 h, or once ⩾38.0 °C. Outpatient status | Fever associated with blood product transfusions or drugs | 146 | 240 | Mean 7.3 y | Low risk: patients with solid tumours and lymphomas stages I and II. High risk: patients with leukaemias and lymphomas stages III and IV | Positive blood culture |
| Riikonen (1993) | Derivation of CDR | Helsinki, Finland (1989–1990) | ANC < 200 cells/mm3, temperature >38.0 °C ⩾2 occasions in ⩾4 h, or once >39.0 °C | Antibiotics (excluding Septrin) in the preceding 3 weeks | 46 | 91 | Range 1–16 y | No rule described. No variables emerged as significant | Bacteraemia Focal infection Suspected sepsis/fever of unknown origin |
| Rojo (2008) | Derivation of CDR | Santiago, Chile (2003 –2006) | Episode of febrile neutropaenia which was ‘low risk’ according to the PINDA criteria | 33 | 47 | Median 5.8 y (1.1–15.7 y) | No rule described. No variables emerged as significant | ‘Unfavourable outcome’ – Compound of: haemodynamic instability, new focus if bacterial infection, 72 h persistent fever, unresponsive CRP, or continuing +ve blood cultures 72 h after treatment | |
| Rondinelli (2006) | Derivation of CDR | San Paulo, Brazil (2000– 2003) | ANC < 500 cells/mm3 or ⩽1000 cells/mm3 and falling, temperature ⩾37.8 °C ⩾3 occasions in ⩾24 h, or once >38.0 °C. First episode per patient (new or relapsed disease) | Second or subsequent episode. Episodes in progressive disease (<6 m from between completing therapy and relapse). History of BMT | 283 | 283 | Mean 5.2 y | Low risk = 2.5–5 points: Intermediate risk = 5.5– 9 points: High risk = Greater than 9 points. 4.5 points for: clinical site of infection; 2.5 points for: no URTI; 2 points for: CVC; 1 point for: aged ⩽ 5 y, fever > 38.5 °C, Hb ⩽7 g/dL | ‘Serious infectious complication’ – sepsis, shock, +ve blood cultures, infection-related death |
| Tezcan (2006) | Derivation of CDR | Antalya, Turkey (1996–2004) | ANC < 500 cells/mm3 or <1000 cells/mm3 and falling, axilliary temperature ⩾38.0 °C ⩾2 occasions at 4 h intervals, or once >38.3 °C | Fever that occurred following transfusion of blood and blood products or administration of G-CSF | 240 | 621 | Median 6 y (range 1 m to 17 y) | No rule described. Significant association between hypotension, uncontrolled cancer and mortality. Duration of fever only independent risk factor for microbiologically documented infection | Death Clinically suspected infection Microbiologically documented infection |
| West (2004) (internally validated using bootstrap) | Derivation of CDR | California, USA (1994–1998) | ANC < 500 cells/mm3 or <1000 cells/mm3 and falling, axilliary temperature ⩾38.0 °C ⩾3 occasions in 24 h, or once ⩾38.5 °C, within 21 d of chemotherapy | Induction, relapse and refractory disease. Collapse within 1 h of admission | 143 | 303 | Mean 7.6 y (SD 4.6 y) | Very high risk = temp > 39.5 °C and CRT > 3 s; High risk = temp > 39.5 °C or CRT > 3s; Low risk = neither | Requirement for critical care within 24 hs of presentation (fluid boluses ⩾60 ml/kg, inotropes or ventilation) |
| Paganini rule | |||||||||
| Paganini (2007) | Derivation | Multiple centres across Argentina (derived 1 institution, validated in 7 further ones) (2000–2004) | ANC < 500 cells/mm3 or <1000 cells/mm3 and falling, temperature ⩾ 38.0 °C ⩾2 occasions in 24 h, or once ⩾38.5 °C | History of BMT | 458 | 714 | Mean 7 y (range 1 m to 17.9 y: derivation set) | Low risk < 4. Mid risk = 4. High risk ⩾ 4. Advanced stage of disease = 3 points, Comorbidity = 2 points, Bacteraemia = 1 point | Death |
| and validation of ‘Paganini rule’ | 523 | 806 | Mean 7.1 y (range 1 m to 17.5 y: validation set) | ||||||
| Rackoff rule | |||||||||
| Rackoff (1996) | Derivation of ‘Rackoff rule’ | Indianapolis, USA (1994–1995) | ANC < 500 cells/mm3, temperature >38.0 °C ⩾3 occasions in ⩾ 24 h, or once >38.5 °C. Outpatient status | 72 | 115 | Range 9 ms to 18 y: derivation set | Low risk = AMC > 100; Mid risk = AMC < 100, and temp < 39; High risk = AMC < 100, but temp ⩾ 39 | Bacteraemia Clinical reason for admission |
|
| Rackoff (1996) | Revision of ‘Rackoff rule’ | (1993) | 57 | Validation set not reported | Low risk = AMC > 100 | ||||
| Baorto (2001) | Validation/recalibration of ‘revised Rackoff rule’ | St. Louis, Dallas and Houston, USA (1990–1996) | ANC < 500 cells/mm3, temperature ⩾ 38 °C, 12 m or older | History of BMT | 558 | 1171 | Mean 8.0 y (range 1–23 y) | Low risk = AMC > 100 | Bacteraemia ICU/Death related to bacteraemia within 72 h of admission for FN |
| Klaassen (2000) | Derivation | Toronto, Canada (1996–1998) | ANC < 500 cells/mm3 or ⩽1000 cells/mm3 and falling. Temperature > 38.0 °C ⩾2 occasions in ⩾ 12 h, or once >38.5 °C, or localised infection | New malignant diagnosis; bone marrow or stem-cell transplantation in preceding 6 m. Another medical condition that independently required inpatient observation. Interstitial infiltrate or lobar consolidation on chest X-ray | 140 | 227 | Median 6.8 y (range 6 m to 17 y: derivation set) | Low risk = AMC > 100; Mid risk = AMC < 100, and temp ⩽ 39; High risk = AMC < 100, but temp > 39 | Bacteraemia Significant bacterial infection (defined as any blood or urine culture positive for bacteria, interstitial or lobar consolidation on CXR, or unexpected death from infection before ANC recovery (>0.5 × 109/L)) |
| and validation of CDR (‘Rackoff rule’) | Unclear | Unclear | 136 | Median 7.6 y (range 1–18 y: validation set) | |||||
| Madsen (2002) | Validation/recalibration of ‘Rackoff rule’ | Indianapolis, USA (1997) | New admissions ‘coded’ as ‘fever of unknown origin’ and ANC < 500 cells/mm3 | History of BMT. AML. In-patient status | 76 | 157 | Mean 8 y (range 2 m to 18 y) | Low risk = AMC > 100; Mid risk = AMC < 100, and temp < 39; High risk = AMC < 100, but temp ⩾ 39 | Positive blood culture |
| Tezcan (2006) | Validation of ‘Rackoff rule’ | Antalya, Turkey (1996–2004) | ANC ⩽ 500 cells/mm3 or ⩽1000 cells/mm3 and falling, axilliary temperature ⩾38.0 °C ⩾2 occasions at 4 h intervals, or once >38.3 °C | Fever that occurred following transfusion of blood and blood products or administration of G-CSF | 240 | 621 | Median 6 y (range 2 m to 17 y) | Low risk = AMC > 100 | Death Clinically suspected infection Microbiologically documented infection |
| Santolaya rule | |||||||||
| Santolaya (2001) | Derivation of ‘Santolaya rule’ | 5 centres in Santiago, Chile (1996–1997) | ANC ⩽ 500 cells/mm3, axilliary temperature ⩾38.0 °C ⩾ 2 occasions 1 h apart, or once ⩾38.5 °C | Not reported | 257 | 447 | Mean 7 y (range 6 m to 18 y) | Low risk = 0 factors or isolated low plts or <7 d from chemotherapy. High risk = >1 risk factor, or isolated high CRP, hypotension or relapsed leukaemia. Risk factors: CRP ⩾ 90, hypotension, relapsed leukaemia, plts ⩽50, chemotherapy within 7 d | Invasive bacterial infection (positive blood culture – 2 for CoNS, positive bacterial culture from usually sterile site, or sepsis syndrome and/or focal organ involvement and haemodynamic instability and severe malaise) Death |
| Santolaya (2002) | Validation of ‘Santolaya rule’ | 6 centres in Santiago, Chile (1999–2000) | ANC ⩽ 500 cells/mm3, axilliary temperature ⩾38.0 °C ⩾ 2 occasions 1 h apart, or once ⩾38.5 °C | Not reported | 170 | 263 | Mean 7 y (range 7 m to 17 y) | Low risk = 0 factors or isolated low plts or <7 d from chemotherapy. High risk = >1 risk factor, or isolated high CRP, hypotension or relapsed leukaemia. Risk factors: CRP ⩾90, hypotension, relapsed leukaemia, plts ⩽ 50, chemotherapy within 7 d | Invasive bacterial infection (positive blood culture – 2 for CoNS, positive bacterial culture from usually sterile site, or sepsis syndrome and/or focal organ involvement and haemodynamic instability and severe malaise) |
Age: y, years; m, months. ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; AMC, absolute monocyte count; ANC, absolute neutrophil count; BMT, bone marrow transplant; CoNS, coagulase-negative Staphylococcus; CRP, C-reactive protein; CRT, capillary refill time; CXR, chest X-ray; Hb, haemoglobin; HIV, human immunodeficiency virus; Plt, platelets.
Unless stated, the rule dichotomises into low and high risk groups.
3.1. Quality assessment
The studies varied in quality. Potential biases due to threats to independent outcome assessment were present in some studies (see Web Appendix 3). The applicability of the studies to specific populations also varied (see Table 1). Thirteen definitions of febrile neutropaenia were used, with 12 definitions of fever and 4 of neutropaenia. However, all definitions are clinically similar, with any variation at the ‘lowest risk’ part of the spectrum of classification.
3.2. Techniques of CDR derivation
The 16 reports of attempts to derive a CDR varied in the populations included the predictor variables and adverse outcomes they reported. The model-building technique, the reporting and handling of missing data and multiple-episode data and the use and categorisation of continuous and categorical variables were also assessed. Details are available in Web Appendices 4 and 5.
3.3. CDR performance
The CDR had diverse test performance (see Table 2 for detail). This heterogeneity has largely been explored using a narrative structure, as pooling across all the studies was not possible due to the varied rules, outcomes and populations studied. It was examined by analysis of the tabulated CDR performance data and graphically with plots of sensitivity and specificity (Web Fig. 1 for unpooled studies and Figs. 2 and 3 for pooled studies).
Table 2.
Predictive performance of clinical decision rules.
| Citation | Clinical prediction rule | Number in study | Outcome | Number with outcome | Predictive accuracy |
||
|---|---|---|---|---|---|---|---|
| % Low | LR Low | LR High | |||||
| Models with one supporting dataset | |||||||
| Adcock (1999) | High risk = hypotension/septic shock, inflamed central line site, recent high dose Ara-C | 33 | Gram-positive bacteraemia | 6 | Data not given | ||
| Alexander (2002) | Low risk = Nnt AML/Burkitts/Induction ALL/Progressive-relapsed with marrow involvement and no significant comorbidity | 104 | Bacteraemia | 13 | 58%∗ | 0.24 | 2.39 |
| Ammann (2003) | |||||||
| (model #1: bootstrapped) | Final decision tree model: four covariates were used to classify low risk; bone marrow involvement, leucocyte count > 0.5 × 109/L, with clinical signs of a viral infection, and aged up to 6 years at presentation. For those with a leucocyte count ⩽ 0.5 × 109/L, they were further classified according to CRP level (⩽ or >50 mg/L) | 111 | Severe bacterial infection, (death from bacterial infection, a positive culture of normally sterile body fluids, radiologically proven pneumonia, clinically unequivocal diagnosis of a bacterial infection, or CRP > 150 mg/L) | 90 | 10% | 0 | 1.18 |
| (model #2) | Low risk ⩽ 3 factors. Risk factors = bone marrow involvement, absence of clinical signs of viral infection, high serum CRP level, low leucocyte count, presence of a central venous catheter, high haemoglobin level, and Pre-B-cell leukaemia | (111) | As above | (90) | 14% | 0 | 1.29 |
| (model #3) | Low risk ⩽ 4 factors. Risk factors = bone marrow involvement, absence of clinical signs of viral infection, high serum CRP level, low leucocyte count, presence of a central venous catheter, high haemoglobin level, and Pre-B-cell leukaemia | (111) | As above | (90) | 20% | 0.07 | 1.39 |
| Ammann (2004) | Low risk = all of: maximum temp ⩽ 39.7 °C, no comorbidity requiring hospitalisation, leucocyte count > 0.5 × 109/L, and in partial or complete remission | 132 | Bacteraemia | 85 | 26% | 0.15 | 1.40 |
| Gala-Peralta (2005) | Low risk ⩽ 2 of: <1 year, poor bone marrow response (plt < 75, ANC < 100),uncontrolled solid tumour or relapsed leukaemia, chemotherapy <10 d earlier, rapid neutropaenia, cardiac and renal dysfunction | 30 | Positive blood culture | 16 | 27% | 0.18 | 1.44 |
| Jones (1996) | Low risk = ANC ⩾ 200, outpatient at onset, in remission | 127 | Bacteraemia | 68 | 17% | 0.71 | 1.07 |
| Lucas (1996) | Low risk = no chills, hypotension, or a requirement for fluid resuscitation at admission | 509 | Positive blood culture | 82 | 87% | 0.72 | 4.05 |
| Petrelli (1991) | Low risk: patients with solid tumours and lymphomas stages I and II. High risk: patients with leukaemias and lymphomas stages III and IV, | 146 | Positive blood culture | 35 | 45% | 0.58 | 1.42 |
| Rondinelli (2006) | Low risk = 2.5–5 points: Intermediate risk = 5.5–9 points: High risk = greater than 9 points 4.5 points for: clinical site of infection; 2.5 points for: no URTI; 2 points for: CVC; 1 point for: aged ⩽5 years, fever >38.5 °C, Hb ⩽7 g/dL | 283 | ‘Serious infectious complication’ – sepsis, shock, +ve blood cultures, infection-related death | 93 | Odds ratio only: | Low 1.0 | |
| Mid 13 | |||||||
| High 50 | |||||||
| West (2004) (bootstrapped) | High risk = temp > 39.5 °C and CRT > 3 s; Mid risk = temp > 39.5 °C or CRT > 3s; Low risk = neither | 143 | Requirement for critical care within 24 h of presentation (fluid boluses ⩾ 60 ml/kg, inotropes or ventilation) | 36 | Low 89% | 0.73 | Infinite |
| Mid 10% | 2.70 | ||||||
| Models with >1 supporting dataset | |||||||
| Santolaya rule | |||||||
| Clinical prediction rule | Number in study | Outcome | Number with Outcome | % Low | LR Low | LR High | |
| Santolaya (2001) | Low risk = 0 factors or isolated low plts or <7 d from chemotherapy. High risk = >1 risk factor, or isolated high CRP, hypotension or relapsed leukaemia. Risk factors: CRP ⩾ 90, hypotension, relapsed leukaemia, plts ⩽ 50, chemotherapy within 7 d | 407 | Invasive bacterial infection = positive blood culture (2 for Coagulase-negative Staphylococcus spp), positive bacterial culture from usually sterile site, or sepsis syndrome and/or focal organ involvement and haemodynamic instability and severe malaise | 178 | 42% | 0.22 | 2.41 |
| Santolaya (2002) | As above | 263 | As above | 140 | 40% | 0.11 | 3.91 |
| Rackoff dichotomous rule | |||||||
| Rackoff (1996) | |||||||
| (proposed from validation set) | Low risk = AMC > 100; High risk = AMC < 100 | 57 | Bacteraemia | 10 | 23% | 0 | 1.45 |
| Baorto (2001) | As above | 1171 | Bacteraemia | 189 | 21% | 0.45 | 1.45 |
| Tezcan (2006) | As above | 671 | Microbiological documented infection | 225 | 58% | 0.70 | 1.60 |
| Clinical prediction rule | Number in study | Outcome | Number with Outcome | % Low | % Mid | LR Low | LR Mid | LR High | |
|---|---|---|---|---|---|---|---|---|---|
| Paganini rule | |||||||||
| Paganini (2007) | Low risk < 4. Mid risk = 4. High risk = >4. Advanced stage of disease = 3 points, Comorbidity = 2 points, Bacteraemia = 1 point | Death | 18 | 82% | 10% | 0 | 2.38 | 12.0 | |
| (validation set) | As above | 806 | Death | 19 | 82% | 12% | 0.12 | 2.76 | 9.86 |
| Rackoff three-category rule | |||||||||
| Rackoff (1996) | |||||||||
| (derivation set) | Low risk = AMC > 100; Mid risk = AMC < 100, and temp < 39; High risk = AMC < 100, but temp ⩾ 39 | 115 | Bacteraemia | 24 | 17% | 65% | 0 | 0.87 | 3.44 |
| (validation set) | As above | 57 | Bacteraemia | 10 | 23% | 40% | 0 | 0.21 | 3.52 |
| Klaassen (2000) | As above | 226 | Bacteraemia | 28 | 37% | 37% | 0.35 | 0.75 | 2.57 |
| Significant bacterial infection | 43 | 0.39 | 0.94 | 2.29 | |||||
| (validation set) | As above | 136 | Bacteraemia | 28 | 42% | 33% | 0.21 | 4.34 | 3.09 |
| Significant bacterial infection | 26 | 0.59 | 1.28 | 1.41 | |||||
| Madsen (2002) | As above | 157 | Bacteraemia | 12 | 25% | 64% | 0.31 | 0.91 | 3.72 |
ALL, acute lymphoblastic leukaemia; AML, acute myeloid leukaemia; AMC, absolute monocyte count; CoNS, coagulase-negative Staphylococcus; CRP, C-reactive protein; CRT, capillary refill time; CXR, chest X-ray; Hb, haemoglobin. Plt, platelet.
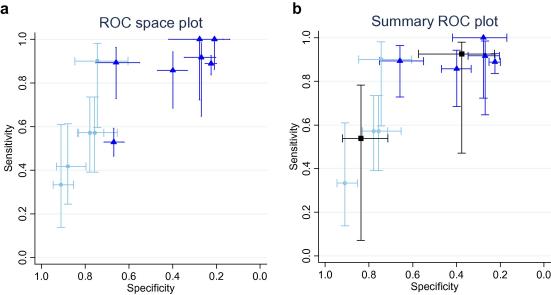
Fig. 2.
(a) Results of the individual ‘Rackoff’ CDR studies. The ROC space plots show each study estimates of sensitivity and specificity as a marker at the point estimate, with 95% confidence intervals demonstrated by lines. In reading such graphs, tests with a better discriminatory ability fall in the top left corner of the plot, and non-discriminatory tests fall on a 45° line between the bottom left and top right. The dashed lines (light blue)/circles represent the dichotomy of low and medium versus high risk groups (5 datasets), the solid lines (darker blue)/triangles between low versus medium and high (7 datasets). The outlier (32) is towards the centre of the graph. (b) Pooled results of the ‘Rackoff’ CDR meta-analysis. The dashed lines (light blue)/circles represent the dichotomy of low and medium versus high risk groups (4 datasets), the solid lines (darker blue)/triangles between low versus medium and high (5 datasets). The meta-analytic summary estimates are shown in heavy lines (black)/squares. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
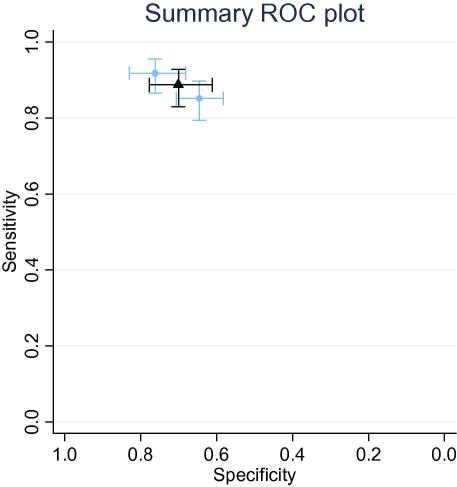
Fig. 3.
Pooled and individual results of the ‘Santolaya’ CDR studies. The dashed lines (light blue)/circles represent the individual studies. The meta-analytic summary estimates are shown in heavy lines (black)/squares. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Meta-analysis of studies which used identical CDR was undertaken in two cases: the ‘Rackoff rule’36 to examine bacteraemia,22,26,28,34,36 and the ‘Santolaya rule’ for serious infectious complications.32,33
The ‘Rackoff rule’ discriminates between three groups of individuals at low, moderate and high risk of bacteraemia. A study which reported ‘microbiologically documented infection’ rather than the narrower ‘bacteraemia’ appeared as a significant outlier (see Fig. 2a).34 Exclusion of this study led to a more Normal distribution of the posterior probability plots. Undertaking a sensitivity analysis by exclusion of the initial rule derivation study demonstrated poorer discriminatory ability (see Fig. 2b for a ‘best estimate’ summary) LR [low] = 0.22 (95% CrI 0.03–1.85), LR [medium] = 0.79 (95% CrI 0.12–2.06) and LR [high] = 3.41 (95% CrI 0.24–18.7). The probability of bacteraemia in each of these groups will vary with the baseline chance of bacteraemia. If we use a 22% overall prevalence of bacteraemia (the average proportion over the included studies which report these data) the predictive values are; Low risk = 6% (95% CrI 1–34%), Mid risk = 18% (CrI 3–37%) and High risk = 49% (95% CrI 6–84%).
The ‘Santolaya rule’ showed a moderate ability to differentiate between low- and high risk groups considering the outcome of ‘invasive bacterial infection’. The derivation sample performed marginally less effectively than the validation set. The pooled estimate of test accuracy is LR [low] = 0.17 (95% CI 0.12–0.23) and LR [high] = 2.87 (95% CI 2.43–3.38), see Fig. 3. Using the average ‘invasive bacterial infection’ rate of 47%, this leads to the probability of ‘invasive bacterial infection’ in the low group as 13% (95% CI 9–13%) and 72% (95% CI 68–75%) in the high group. The two studies examining this rule are from the same research group (although in a multi-centre study environment) and the rule has not been subject to further validation.
Assessments of potential sources of heterogeneity showed that derivation studies generally had better accuracy than validation studies. The outcome studied also appeared to alter rule performance but the heterogeneity of rules and populations make this difficult to examine clearly. Those CDRs developed in a population where the highest risk patients are excluded (e.g. bone marrow transplant recipients) did not seem to differ from rules developed without these exclusions. All these analyses are confounded by the correlation of location, population, outcome and rule. For example: the Santolaya studies took place in Chile, excluded BMT, looked at a broad definition of infectious complications and developed a 5-item rule, the Rackoff model was developed in the United States, did not clearly exclude any patient group, primarily examined bacteraemia and produced a rule based on a single haematological parameter and temperature.
Examination of the detailed content of all the proposed rules shows they address four major domains (Web Appendix 6). The first can be considered stable patient-related factors, including age and the underlying disease. The second group reflects treatment; the presence of a central venous catheter and the type or duration since last chemotherapy. The third group reflects episode-specific clinical features, such as maximum temperature, the patient’s blood pressure or clinical features of infection. The final group contains episode-specific laboratory test values. These are various markers of bone marrow function where, excepting,23 each rule uses a single item which reflects one of the three major cellular components: haemoglobin, platelets, leucocytes (or a subset); and serum inflammatory markers (C-reactive protein). An exploratory analysis of the individual features common across predictive studies shows that age, malignant disease state, clinical assessments of circulatory and respiratory compromise, higher temperatures and bone marrow suppression all have some explanatory power.
4. Discussion
This is to our knowledge the first systematic review and meta-analysis of risk prediction rules in paediatric febrile neutropaenia. It describes 20 studies producing 16 separate models, assessing a variety of outcomes, with individual differences in definitions, covering five main categories: death, critical care requirement, serious medical complication, significant bacterial infection and bacteraemia. Despite the inclusion of nearly 8000 episodes of FNP, this review cannot conclude that any system is more effective or reliable than any other.
A clinical decision rule for febrile neutropaenic episodes can be broadly considered to have two uses. Primarily it is to decide if the risk of an episode is ‘low enough’ to allow reduced intensity therapy (e.g. outpatient management), but at the opposite end of the risk scale, a CDR may be helpful to direct increasingly close observation and more aggressive management. The patients at ‘high risk’ do not have such clear management options: there are no effective truly prophylactic measures to prevent sepsis syndrome but early recognition may prevent progression to septic shock.39
The majority of CDR in this review focus upon defining a group at ‘low risk’ of complications. Two rules in particular have been subject to greater verification, other rules show promise and have clinical/physiological similarities, but have had less validation.
The performance of only one rule could be reasonably assessed across multiple datasets; that of absolute monocyte count and temperature criteria proposed by Rackoff36 to exclude bacteraemia. This CDR, tested in 1171 episodes over five datasets, in three different groups across time and in different centres, has the greatest strength of evidence. The most appropriate pooled estimate of the rule’s effectiveness shows limited discriminatory ability, LR [low] = 0.22 (95% CrI 0.03–1.85), LR [medium] = 0.79 (95% CrI 0.12–2.06) and LR [high] = 3.41 (95% CrI 0.24–18.7). The marked uncertainty in these estimates is best demonstrated by the post-test probabilities of bacteraemia: Low risk = 6% (95% CrI 1–34%), Mid risk = 18% (CrI 3–37%) and High risk = 49% (95% CrI 6–84%).
Of the other rules the Santolaya model33 shows a moderate ability to differentiate between groups at low and high risk of ‘serious infection’, but again with marked uncertainty (LR [low] = 0.17 (95% CI 0.12–0.23) and LR [high] = 2.87 (95% CI 2.43–3.38), post-test probabilities for the low risk group = 13% (95% CI 9–13%) and in the high risk group 72% (95% CI 68–75%)). The rule has been developed and tested in Chile, which may limit its applicability in Western Europe and North America. The proportion of patients with bacteraemia (∼25%) is similar to the other studies in this review, but their broad definition of adverse medical outcomes, as found in ∼50% of cases, does not have a direct comparator among the Western European/North American studies reviewed, therefore no accurate conclusion can be reached. This reflects an uncertainty in the selection of the desired and measurable outcome. An ideal study would consider not just death, critical care requirement, serious medical complication, significant bacterial infection or bacteraemia but ‘an absence of adverse consequences’.
Any adaptation or development of a new rule should primarily look to assess the variables shown in the many CDR reviewed to be of predictive value, over those found purely by ‘p-value’ sampling of bivariable testing. In addition to reducing random error, building upwards from the simple clinical variables of age, disease and basic clinical examination will ensure any complex tests add significant value to the fundamentals of patient assessment.
An analysis of the techniques used to build the CDR was incorporated into this review. The studies are spread across a number of years, and during that time there have been significant methodological developments and technological improvements which have made previously complex computation within the reach of many health researchers. However, a series of previously described methodological problems with diagnostic/prognostic model papers were present in this review. These included: small event-per-variable ratios leading to models more likely to be overfitted to their original dataset and disappointing in clinical practice40; overestimation of accuracy from derivation studies; failure to examine for non-linear relationships, which may misjudge a predictor as unimportant,41 for example, there are plausible reasons to assume that patient age may have a non-linear ‘U’-shaped relationship with infection and outcome,42 as should time-from-chemotherapy; use of data-driven stepwise variable selection and cutpoint determination techniques which may give spurious results43,44; premature categorisation of continuous data; lack of examination of missing data and suboptimal examination of clustered data.
This review has demonstrated a wide range of rules for the prediction of adverse outcomes during episodes of febrile neutropaenia in children. None of the rules identified has been subject to the extensive geographical and temporal discriminatory validity assessments that mark the highest quality CDRs, and many potential difficulties with model building have been identified. Practical application of many of these CDR within an in-patient environment is likely to be safe but without further research uncertainty will remain as to the efficiency of the CDR in use. To provide this information and maximise the value of the information already collected by these and other cohorts of children with febrile neutropaenia, an individual-patient-data (IPD) meta-analysis is being undertaken to develop and test new or existing prediction models and provide a firmer basis for stratified treatment trials in this common and occasionally fatal complication of therapy.45
Contributions
R.S.P. conceived the idea and co-ordinated and led the review, developed the protocol, undertook screening and data extraction, synthesis and drafted the manuscript. He has had full access to the data and has final responsibility for the paper and the decision to submit. R.W. developed the protocol, undertook screening and data extraction and read and modified the manuscript. L.A.S. developed the protocol, reviewed the results and read and modified the manuscript. A.J.S. developed and checked the synthesis and read and modified the manuscript.
Funding
R.S.P. is supported by an MRC Research Training Fellowship G0800472, which also supported R.W. for this review. A.J.S. and L.A.S. received no external funding for their work in this study. The funder had no role in the design or conduct of the study nor the production of, or decision to submit, this manuscript.
Conflict of interest statement
None declared.
Acknowledgements
The authors wish to acknowledge Lindsey Myers and Melissa Hardman (CRD) for their search support.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ejca.2010.05.024.
Appendix A. Supplementary data
Web Appendix.
References
- 1.Pritchard-Jones K., Kaatsch P., Steliarova-Foucher E., Stiller C.A., Coebergh J.W.W. Cancer in children and adolescents in Europe: developments over 20 years and future challenges. European Journal of Cancer. 2006;42(13):2183–2190. doi: 10.1016/j.ejca.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Freycon F., Trombert-Paviot B. Trends in treatment-related deaths (TRDs) in childhood cancer and leukemia over time: a follow-up of patients included in the childhood cancer registry of the Rh“ne-Alpes region in France (ARCERRA) Pediat Blood Cancer. 2008;50(6):1213–1220. doi: 10.1002/pbc.21506. [DOI] [PubMed] [Google Scholar]
- 3.Hargrave D.R., Hann I.M. Progressive reduction in treatment-related deaths in Medical Research Council childhood lymphoblastic leukaemia trials from 1980 to 1997 (UKALL VIII, X and XI) Brit J Haematol. 2001;112(2):293–299. doi: 10.1046/j.1365-2141.2001.02543.x. [DOI] [PubMed] [Google Scholar]
- 4.Hann I., Viscoli C., Paesmans M., Gaya H., Glauser M. A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC) Br J Haematol. 1997;99(3):580–588. doi: 10.1046/j.1365-2141.1997.4453255.x. [DOI] [PubMed] [Google Scholar]
- 5.Phillips B., Selwood K., Lane S.M. Variation in policies for the management of febrile neutropenia in United Kingdom Children’s Cancer Study Group centres. Arch Dis Child. 2007;92(6):495–498. doi: 10.1136/adc.2006.102699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boragina M., Patel H. Management of febrile neutropenia in pediatric oncology patients: a Canadian survey. Pediat Blood Cancer. 2007;48(5):521–526. doi: 10.1002/pbc.20810. [DOI] [PubMed] [Google Scholar]
- 7.McGinn T.G., Guyatt G.H., Wyer P.C. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284(1):79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 8.Klastersky J., Paesmans M., Rubenstein E.B. The Multinational Association for Supportive Care in Cancer risk index: a multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol: Off J Am Soc Clin Oncol. 2000;18(16):3038–3051. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 9.Vidal L, Paul M, Ben-Dor I, et al. Oral versus intravenous antibiotic treatment for febrile neutropenia in cancer patients. Cochrane Database of Systematic Reviews (Online) 2004(4):CD003992-CD003992. [DOI] [PubMed]
- 10.Centre for Reviews and Dissemination. Systematic review: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009.
- 11.Lijmer J.G., Mol B.W., Heisterkamp S. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA. 1999;282(11):1061–1066. doi: 10.1001/jama.282.11.1061. [DOI] [PubMed] [Google Scholar]
- 12.Whiting P., Rutjes A., Reitsma J., Bossuyt P., Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3(1) doi: 10.1186/1471-2288-3-25. 25–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centre for Reviews and Dissemination. Systematic reviews of clinical tests. In: Centre for Reviews and Dissemination, editor. Systematic review: CRD’s guidance for undertaking reviews in health care. York: University of York; 2009. p. 127.
- 14.Harbord R. METANDI: Stata module to perform meta-analysis of diagnostic accuracy. In. S456932 ed: Department of Social Medicine, University of Bristol; 2008.
- 15.Simel D.L., Bossuyt P.M. Differences between univariate and bivariate models for summarizing diagnostic accuracy may not be large. J Clin Epidemiol. 2009;62(12):1292–1300. doi: 10.1016/j.jclinepi.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Goodacre S., Sutton A.J., Sampson F.C. Meta-analysis: the value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med. 2005;143(2):129–139. doi: 10.7326/0003-4819-143-2-200507190-00012. [DOI] [PubMed] [Google Scholar]
- 17.Lunn D., Thomas A., Best N., Spiegelhalter D. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10(4):337. 325–337, 325. [Google Scholar]
- 18.Dukic V., Gatsonis C. Meta-analysis of diagnostic test accuracy assessment studies with varying number of thresholds. Biometrics. 2003;59(4):936–946. doi: 10.1111/j.0006-341x.2003.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adcock K.G., Akins R.L., Farrington E.A. Evaluation of empiric vancomycin therapy in children with fever and neutropenia. Pharmacotherapy. 1999;19(11):1315–1320. doi: 10.1592/phco.19.16.1315.30867. [DOI] [PubMed] [Google Scholar]
- 20.Alexander S.W., Wade K.C., Hibberd P.L., Parsons S.K. Evaluation of risk prediction criteria for episodes of febrile neutropenia in children with cancer. J Pediatr Hematol/Oncol. 2002;24(1):38–42. doi: 10.1097/00043426-200201000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Ammann R.A., Hirt A., Luthy A.R., Aebi C. Identification of children presenting with fever in chemotherapy-induced neutropenia at low risk for severe bacterial infection. Med Pediatr Oncol. 2003;41(5):436–443. doi: 10.1002/mpo.10320. [DOI] [PubMed] [Google Scholar]
- 22.Baorto E.P., Aquino V.M., Mullen C.A., Buchanan G.R., DeBaun M.R. Clinical parameters associated with low bacteremia risk in 1100 pediatric oncology patients with fever and neutropenia. Cancer. 2001;92(4):909–913. doi: 10.1002/1097-0142(20010815)92:4<909::aid-cncr1400>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Gala Peralta S., Cardesa Salzman T., Garcia Garcia J.J. Bacteraemia risk criteria in the paediatric febrile neutropenic cancer patient. Clin Transl Oncol. 2005;7(4):165–168. doi: 10.1007/BF02708754. [DOI] [PubMed] [Google Scholar]
- 24.Hann I., Viscoli C., Paesmans M., Gaya H., Glauser M. A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC) Br J Haematol. 1997;99(3):580–588. doi: 10.1046/j.1365-2141.1997.4453255.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones G.R., Konsler G.K., Dunaway R.P., Pusek S.N. Infection risk factors in febrile, neutropenic children and adolescents. Pediatr Hematol Oncol. 1996;13(3):217–229. doi: 10.3109/08880019609030820. [DOI] [PubMed] [Google Scholar]
- 26.Klaassen R.J., Goodman T.R., Pham B., Doyle J.J. “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000;18(5):1012–1019. doi: 10.1200/JCO.2000.18.5.1012. [DOI] [PubMed] [Google Scholar]
- 27.Lucas K.G., Brown A.E., Armstrong D., Chapman D., Heller G. The identification of febrile, neutropenic children with neoplastic disease at low risk for bacteremia and complications of sepsis. Cancer. 1996;77(4):791–798. [PubMed] [Google Scholar]
- 28.Madsen K., Rosenman M., Hui S., Breitfeld P.P. Value of electronic data for model validation and refinement: bacteremia risk in children with fever and neutropenia. J Pediatr Hematol/Oncol. 2002;24(4):256–262. doi: 10.1097/00043426-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Paganini H.R., Aguirre C., Puppa G. A prospective, multicentric scoring system to predict mortality in febrile neutropenic children with cancer. Cancer. 2007;109(12):2572–2579. doi: 10.1002/cncr.22704. [DOI] [PubMed] [Google Scholar]
- 30.Petrilli A.S., Melaragno R., Bianchi A. Fever and neutropenia in children with cancer: a new therapeutic proposal. Amb; Rev Assoc Med Bras. 1991;37(4):173–180. [PubMed] [Google Scholar]
- 31.Rondinelli P.I.P., Ribeiro K.d.C.B., de Camargo B. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol/Oncol. 2006;28(10):665–670. doi: 10.1097/01.mph.0000212996.94929.0b. [DOI] [PubMed] [Google Scholar]
- 32.Santolaya M.E., Alvarez A.M., Avils C.L. Prospective evaluation of a model of prediction of invasive bacterial infection risk among children with cancer, fever, and neutropenia. Clinical Infectious Diseases. 2002;35(6):678–683. doi: 10.1086/342064. [DOI] [PubMed] [Google Scholar]
- 33.Santolaya M.E., Alvarez A.M., Becker A. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001;19(14):3415–3421. doi: 10.1200/JCO.2001.19.14.3415. [DOI] [PubMed] [Google Scholar]
- 34.Tezcan G., Kupesiz A., Ozturk F. Episodes of fever and neutropenia in children with cancer in a tertiary care medical center in Turkey. Pediatr Hematol Oncol. 2006;23(3):217–229. doi: 10.1080/08880010500506719. [DOI] [PubMed] [Google Scholar]
- 35.West D.C., Marcin J.P., Mawis R. Children with cancer, fever, and treatment-induced neutropenia: risk factors associated with illness requiring the administration of critical care therapies. Pediatr Emerg Care. 2004;20(2):79–84. doi: 10.1097/01.pec.0000113875.10140.40. [DOI] [PubMed] [Google Scholar]
- 36.Rackoff W.R., Gonin R., Robinson C., Kreissman S.G., Breitfeld P.B. Predicting the risk of bacteremia in children with fever and neutropenia. J Clin Oncol. 1996;14(3):919–924. doi: 10.1200/JCO.1996.14.3.919. [DOI] [PubMed] [Google Scholar]
- 37.Riikonen P., Jalanko H., Hovi L., Saarinen U.M. Fever and neutropenia in children with cancer: diagnostic parameters at presentation. Acta Paediatr Int J Paediatr. 1993;82(3):271–275. doi: 10.1111/j.1651-2227.1993.tb12658.x. [DOI] [PubMed] [Google Scholar]
- 38.Rojo L.C., Rodriguez Z.N., Tordecilla C.J. Low risk febrile neutropenia in oncological pediatric patients: clinical experience [Spanish] Rev Chilena Pediatr. 2008;79(2):157–162. [Google Scholar]
- 39.Rivers E., Nguyen B., Havstad S. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 40.Steyerberg E.W., Eijkemans M.J., Harrell F.E., Jr., Habbema J.D. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–1079. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Royston P., Sauerbrei W. [Multivariable model – building: a pragmatic approach to regression analysis based on fractional polynomials for modelling continuous variables]. John Wiley & Sons Inc. (E); 2008. [Google Scholar]
- 42.Watson R.S., Carcillo J.A., Linde-Zwirble W.T. The epidemiology of severe sepsis in children in the United States. Am J Respir Crit Care Med. 2003;167(5):695–701. doi: 10.1164/rccm.200207-682OC. [DOI] [PubMed] [Google Scholar]
- 43.Altman D.G., Lausen B., Sauerbrei W., Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst. 1994;86(11):829–835. doi: 10.1093/jnci/86.11.829. [DOI] [PubMed] [Google Scholar]
- 44.Riley R.D., Abrams K.R., Sutton A.J. Reporting of prognostic markers: current problems and development of guidelines for evidence-based practice in the future. Br J Cancer. 2003;88(8):1191–1198. doi: 10.1038/sj.bjc.6600886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips R. Predicting infectious complications in febrile neutropenic children with cancer (PICNICC). <http://bit.ly/PICNICC> [accessed May 2010].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web Appendix.