Abstract
Zearalenone is a mycoestrogen that is produced in the fungi Fusarium graminearum, Fusarium culmorum, Fusarium equiseti, and Fusarium crookwellense. These fungi commonly exist in agricultural products. Human pregnane X receptor (hPXR) is a ligand-activated transcription factor that regulates the expression of numerous hepatic drug-metabolizing enzymes, including several clinically important cytochrome P450s. In this report, we show that zearalenone is an efficacious ligand for hPXR. We also describe the creation and validation of a novel adenoviral-mediated transduction protocol used to express functional FLAG-tagged-hPXR protein in a transformed cell line (HepG2) and primary cell types (cultured hepatocytes). Treatment of hPXR-transduced HepG2 cells with zearalenone induces expression of CYP3A4, the “prototypical” PXR-target gene in human liver. Treatment of hPXR-transduced cultured hepatocytes isolated from PXR-knockout mice with zearalenone induces the expression of Cyp3a11, the prototypical murine hepatic PXR-target gene. Using mammalian two-hybrid assays, we show that zearalenone displaces the nuclear receptor corepressor protein N-CoR from hPXR, while it recruits coactivator proteins steroid receptor coactivator-1, Glucocorticoid Receptor-Interacting Protein 1 and PPAR-Binding protein (GRIP1) and PBP to hPXR. Concentration-response analysis using a PXR-responsive reporter gene assay reveals that zearalenone activates hPXR with an EC50 value of approximately 1.5 μM. Because activation of hPXR represents the molecular basis of an important class of drug interactions, our findings suggest that studies to investigate the potential of zearalenone to induce the metabolism of other drugs in humans are warranted. In addition, due to the limited availability of primary human hepatocytes, our adenoviral-mediated hPXR expression protocol will likely prove useful in studies of the xenobiotic response.
Keywords: PXR, CYP3A, zearalenone, drug interaction, cofactor, nuclear receptor
Zearalenone (6-[10-hydroxy-6-oxo-trans-1-undecenyl]-B-resorcyclic acid lactone) is a mycoestrogen that activates the estrogen receptor (ER) with an efficacy comparable to that of 17β-estradiol, the principle endogenous ER ligand. Zearalenone is biosynthesized by the fungi Fusarium graminearum, Fusarium culmorum, Fusarium equiseti, and Fusarium crookwellense (Bennett and Klich, 2003). These fungi are commonly found in cereal crops worldwide. Zearalenone has also been patented as an oral contraceptive (Bennett and Klich, 2003). While most of its biological activities are attributed to the agonist effect on the ER, zearalenone also produces certain biological reactions that cannot be explained by its estrogenic activity (Hidy et al., 1977; Utian, 1973). To further explore the biological activities of zearalenone, we screened a panel of nuclear receptor family members for their ability to respond to this compound. We found that in addition to ERα, zearalenone also efficaciously activates the human xenobiotic receptor—pregnane X receptor (PXR, NR1I2).
PXR is a member of the nuclear receptor superfamily of ligand-activated transcription factors (Blumberg et al., 1998; Kliewer et al., 1998). It is a key regulator of xenobiotic-inducible CYP3A gene expression (Goodwin et al., 2002; Kliewer et al., 2002). In addition, it regulates other genes involved in the metabolism of xenobiotic and endobiotic compounds such as CYP2B, CYP2C, CYP24, glutathione S-transferases, sulfotransferases, and Uridine diphosphate (UDP)-glucuronosyltransferases (Chen et al., 2003; Maglich et al., 2002; Pascussi et al., 2005; Sonoda et al., 2002; Wei et al., 2002). PXR also regulates the expression of the drug transporter genes Oatp2, Mdr1, Mrp2, and Mrp3 (Geick et al., 2001; Kast et al., 2002; Staudinger et al., 2003). Therefore, PXR activation has a dual nature. On one hand, it protects cells from toxic insults. On the other, it represents the molecular basis for an important class of drug interactions.
In the present report, we show that zearalenone activates human PXR (hPXR) and induces CYP3A4 in HepG2 cells in a PXR-dependent manner. The PXR-dependent induction of CYP3A was further confirmed by utilizing primary mouse hepatocytes isolated from PXR-KO mice and transduced with adenovirus carrying either Green Flourescent Protein (GFP) (blank virus) or hPXR. Moreover, we demonstrate that at a molecular level, zearalenone activates PXR by displacing the corepressor N-CoR and by recruiting the coactivator proteins steroid receptor coactivator-1 (SRC-1), PBP, and GRIP1. Our data suggest that the exposure to zearalenone likely increases the metabolism of coadministered drugs and potentially causes food-drug interaction.
MATERIALS AND METHODS
Animal care
Generation of the PXR-knockout (PXR-KO) mice was previously described (Staudinger et al., 2001). All rodents were maintained on standard laboratory chow and were allowed food and water ad libitum. The studies reported here have been carried out in accordance with the Declaration of Helsinki and/or with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health.
Plasmids and chemicals
The full-length hPXR, mouse PXR, mouse PXR2, human CAR, mouse CAR, and human RXR mammalian expression vectors were described previously (Kliewer et al., 1998; Lehmann et al., 1997, 1998). Gal4-fused human ER-LBD, GR-LBD, and mouse PPARγ-LBD mammalian expression vectors were described previously (Goodwin et al., 2000; Kliewer et al., 1997; Oliver et al., 2001). XREM-Luc and RXRE-Luc were described previously (Goodwin et al., 1999; Kliewer et al., 1992). pFR-Luc is commercially available (Stratagene, La Jolla, CA). VP16-hPXR mammalian expression vector was described previously (Ding and Staudinger, 2005a). GAL4-SRC-1, GAL4-PBP, and GAL4-N-CoR were generous gifts from Dr. Barry Forman (Synold et al., 2001). GRIP1 RID was polymerase chain reaction (PCR) amplified from pGAD-424-GRIP1 (Hong et al., 1997) (a generous gift from Dr. Stallcup) using the following primers: left primer, 5′ gAC ggC GAATTC ATg CCC CAg gCg gCC AgC ggg 3′, and right primer, 5′ gAC ggC ggATCC TCA gAg TTT ggg ggT TAT TTC Cgg 3′. To generate GAL4-GRIP1, PCR-amplified GRIP1 RID was cloned into the EcoRI and BamHI sites of pM (BD Biosciences, Palo Alto, CA). The final construct was verified by DNA sequencing. The SV-β-Gal plasmid is commercially available (Invitrogen, Carlsbad, CA). All compounds were purchased from Sigma (St. Louis, MO) and were dissolved as 1000X stocks in DMSO.
Cell culture and transient transfection of CV-1 cells
CV-1 cells were plated on 96-well plates as described previously (Brobst et al., 2004).The XREM-LUC reporter gene assays and mammalian two-hybrid assays were performed as described previously (Brobst et al., 2004; Ding and Staudinger, 2005a,c). For RXRE-LUC and pFR-LUC assays, each well was transfected with 20 ng of reporter gene, 5 ng of nuclear receptor expression vector (human RXR for RXRE-LUC, GAL4-human ER-LBD, GAL4-human GR-LBD, and GAL4-mouse PPARγ-LBD for pFR-LUC), and 40 ng of SV-β-gal and added with pBluescript to 110 ng of total DNA per well. Twenty-four hours posttransfection, cells were drug treated for 24 h. The luciferase activities were determined using the luciferase assay kit per the manufacturer’s instructions (Promega, Madison, WI) and normalized to β-galactosidase acivities. β-Galactosidase acivities were measured using o-nitrophenyl β-D-galactopyranoside (ONPG) assay (Sigma). For ONPG assay, 110 mg ONPG was dissolved in 100 ml 0.1 M NaHPO4 buffer, which was made by mixing 6.84 ml 1 M Na2HPO4, 3.16 ml 1 MNaH2PO4, and 90 ml H2O. Twenty microliters of cell lysate and 200 μl ONPG buffer were mixed and incubated at 37°C for 30–60 min and read at 420 nm.
Real-time quantitative PCR
Mouse hepatocytes were isolated using a two-step perfusion as described previously (Ding and Staudinger, 2005b). About 48 h postplating, the hepatocytes were transduced with adenovirus. Twenty-four hours later, hepatocytes were treated with drugs in maintenance medium for additional 24 h before RNA isolation. HepG2 cells were plated on a 12-well plate at a density of 5 × 105 cells per well in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Herndon, VA) supplemented with penicillin-streptomycin (100 units/ml penicillin and 100 μg/ml streptomycin) and l-glutamine (2 mM). About 24 h postplating, the hepatocytes were transduced with adenovirus. Twenty-four hours later, HepG2 cells were treated with drugs in maintenance medium for additional 24 h before RNA isolation. Total RNA was isolated using Trizol reagent (Invitrogen) according to the manufacturer’s instructions. RNA (10 μg/lane) was resolved on 3.7% formaldehyde and 1% agarose gel to verify the integrity of the RNA. One microgram of DNaseI-treated RNA was reverse transcribed using random primers following the manufacturer’s instructions (Promega). Equal amounts of cDNA were used in real-time quantitative PCRs. Reactions included 200 nM fluorogenic probe and 300 nM primers specific for each gene. The fluorogenic probe and primer sets were designed using the Primer3 program (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). BioSearch Technologies (Novato, CA) synthesized the fluorogenic probes. The sequences (5′ to 3′) for the primers and probes are as follows: CYP3A4, forward primer (CAg gAg gAA ATT gAT gCA gTT TT), fluorogenic probe (FAM-CCC AAT AAg gCA CCA CCC ACC TAT gA-BHQ1), and reverse primer (gTC AAg ATA CTC CAT CTg TAg CAC AgT); Cyp3a11, forward primer (CAA ggA gAT gTT CCC TgT CA), fluorogenic probe (FAM-AgA Agg CAA AgA AAg gCA AgC CTg-BHQ1), and reverse primer (CCA CgT TCA CTC CAA ATg AT). For 18S, 1X SybrGreen (BioWhittaker Molecular Applications, Rockland, ME) was included in the reaction instead of fluorogenic probe. The sequences (5′ to 3′) for the 18S primers are as follows: forward primer (AgT CCC TgC CCT TTg TAC ACA) and reverse primer (CgA TCC gAg ggC CTC ACT A). Cycling conditions were 95°C for 2 min followed by 45 cycles of 95°C for 15 s, 60°C for 15 s, and 68°C for 15 s using the Cepheid Smart Cycler system (Sunnyvale, CA). Fold induction was calculated as described (Schmittgen et al., 2000).
Adenovirus transduction and Western blot assay
Ad-GFP (Lehman et al., 2000) was a generous gift from Dr. Daniel Kelly (Washington University). To generate Ad-hPXR, full-length hPXR was PCR amplified from pSG5-hPXR. PCR product was then cloned into the BglII and XhoI sites of pShuttle-IRES-hrGFP (Stratagene), which produced an in-frame fusion of PXR and the FLAG tag in the vector. The final construct was verified by DNA sequencing. Recombination was performed using the AdEasy system according to the manufacturer’s instruction (Stratagene). Adenovirus was amplified in HEK293 cells as described (He et al., 1998). After amplification, virus was purified using two-step CsCl gradient centrifugation. Since the cesium chloride has a toxic effect on cell cultures, the virus suspension was dialyzed extensively against four changes of buffer (10 mM Tris-HCL pH 8.0, 100 mM NaCl, 0.1% bovine serum albumin, 20% glycerol), for 4 h each of dialysis. Cells were transduced for 48 h before protein isolation. Whole cell lysate was prepared in the lysis buffer containing 10% glycerol, 1% NP-40, 20 mM Tris (pH = 8), 137 mM NaCl, 1 mM NaVn, and 1X Complete (Roche Diagnostics, Indianapolis, IN). After freezing at −80°C, cells were thawed and centrifuged at 20,000 g for 10 min to remove cellular debris. Proteins were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to Nylon+ membrane (Novex, San Diego, CA) overnight at 100 mA. Membranes were probed in the primary antibody polyclonal antibody against the FLAG epitope (1:2000 dilution, Covance, CA) and the secondary antibody goat anti-rabbit IgG-HRP (1:5000 dilution, Santa Cruz, CA). PIERCE ECL Western Blotting Substrate (PIERCE, Rockford, IL) was used to visualize the secondary antibody.
RESULTS
Zearalenone Activates PXR and ERα
To investigate the regulation of nuclear receptors by zearalenone, we performed a series of reporter gene assays in CV-1 cells. As shown in Figure 1B, among all the nuclear receptors investigated, PXR and ERα are the most responsive. For the purpose of positive control, all nuclear receptors were treated with their cognate prototypical ligands, except for hPXR.2, of which no ligand has been identified. As expected, each receptor was activated by the cognate ligand (Fig. 1C).
FIG. 1.
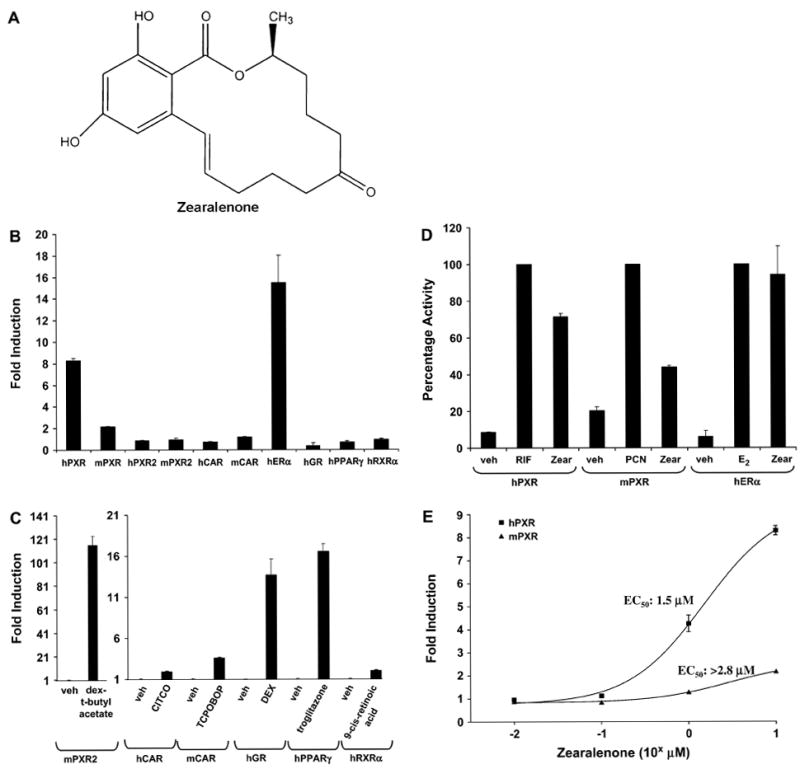
Zearalenone activates ER and PXR. (A) The chemical structure of zearalenone. (B) CV-1 cells were transfected with expression vectors encoding various nuclear receptors together with reporter genes as described in “Materials and Methods” section. Cells were treated with vehicle (0.1% DMSO) or 10 μM of zearalenone, except for human estrogen receptor (hER), which was treated with 1 μM zearalenone. (C) CV-1 cells were transfected as (B) and then treated with vehicle (0.1% DMSO) or various nuclear receptor ligands: dexamethasone-t-butylacetate (10 μM), 6-(4-chlorophenyl)imidazo(2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (1 μM), TCPOBOP (100 nM), dexamethasone (1 μM), troglitazone (2.5 μM), and 9-cis retinoid acid (1 μM). (D) CV-1 cells were transfected with expression vectors encoding hPXR, mouse PXR, and GAL4-ERαLBD together with luciferase reporter genes as described in “Materials and Methods” section. Transfected cells were treated with vehicle (0.1% DMSO) or 10 μM of the indicated compounds, except for hER, which was treated with 1 μM zearalenone or 100 nM 17β-estradiol. The reporter activity with rifampicin for hPXR, PCN for mPXR, or 17β-estradiol for hERα is defined as 100. (E) CV-1 cells were transfected with the expression vector encoding hPXR or mPXR together with the XREM-LUC reporter gene and treated with increasing concentrations of zearalenone. All cells were treated for 24 h. The data represent the mean of replicates ± SEM (n = 4) and are normalized against β-galactosidase activity.
Zearalenone’s activity against PXR exhibits species-selective properties, with hPXR being more responsive when compared with mouse PXR. The PXR.2 splice variant was not activated by zearalenone. Comparison to the classic species-selective PXR ligands revealed that zearalenone activated hPXR with about 70% efficacy of that of rifampicin, a prototypical hPXR ligand, while it only had about 40% efficacy on mouse PXR when compared to pregnenolene 16α-carbonitrile (PCN), a prototypical rodent PXR ligand (Fig. 1D). Reporter gene assays using ERα demonstrated that zearalenone activated ERα with an efficacy comparable to that of the full agonist 17β-estradiol. Full concentration-response analysis on PXR revealed that zearalenone was more potent and efficacious at activating hPXR when compared with mouse PXR (Fig. 1E).
Zearalenone Induces Endogenous Cytochrome P450 3A in a hPXR-Dependent Manner
To determine the extent to which zearalenone induces the expression of endogenous PXR-target genes in liver cells, we treated adenovirus-transduced HepG2 cells and performed real-time quantitative PCR analysis for CYP3A4, the pro-totypical PXR-target gene in human liver. Western blot analysis confirmed that adenoviral transduction resulted in the expres-sion of the FLAG-tagged hPXR protein (Fig. 2A). Notably, zearalenone treatment induced CYP3A4 gene expression in HepG2 cells that were transduced with hPXR adenovirus, while this effect was nearly absent in the HepG2 cells that were transduced with the GFP (blank) adenovirus (Fig. 2B). To confirm the results from HepG2 cells and to determine the biological activity of zearalenone in a normal hepatic cellular environment, we also performed similar experiments in primary cultures of mouse hepatocytes that were isolated from PXR-KO mice. Western blot analysis demonstrated the expression of the FLAG-tagged hPXR protein in primary mouse hepatocytes after adenoviral transduction (Fig. 3A). As shown in Figure 3B, in PXR-KO mouse hepatocytes that were transduced with the hPXR adenovirus, zearalenone induced the gene expression of Cyp3a11, the prototypical PXR-target gene in mouse liver. In primary cultures of PXR-KO mouse hepatocytes that were transduced with the blank virus, zearalenone had no effect on the Cyp3a11 gene expression. Taken together, these data strongly suggest that the induction of CYP3A by zearalenone is mediated by PXR.
FIG. 2.
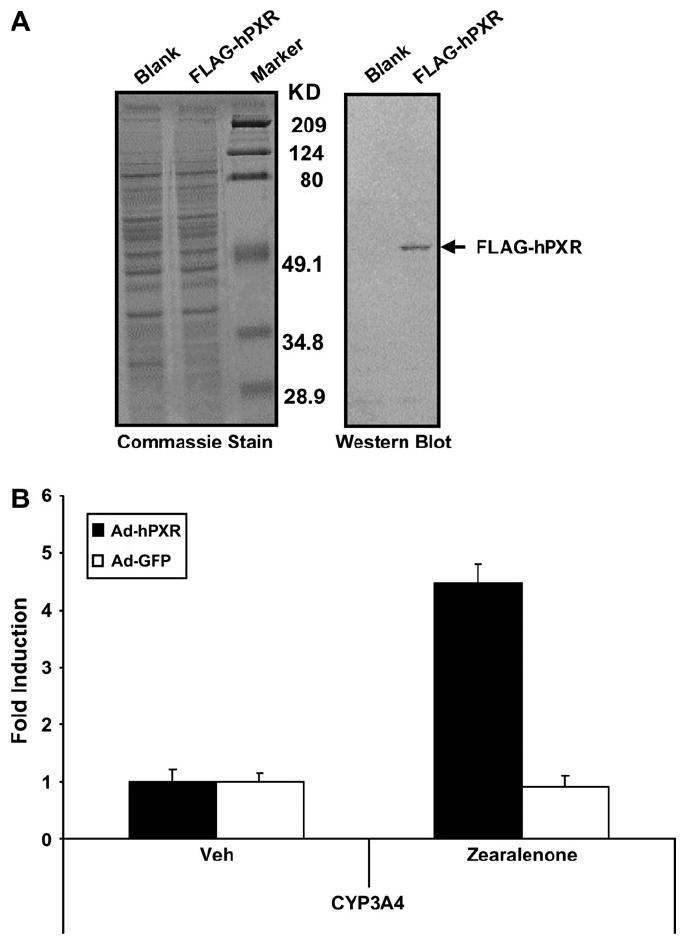
Zearalenone induces CYP3A4 gene expression through activation of PXR in HepG2 cells. (A) HepG2 cells were transduced with adenovirus carrying GFP (blank) or FLAG-tagged hPXR (FLAG-hPXR) and cultured for an additional 48 h before protein isolation. Total protein was subjected to Western blot analysis for the expression of FLAG-hPXR as described in “Materials andMethods” section. Greater than 90% transduction efficiency was achieved as determined by fluorescence microscopy. (B) HepG2 cells were transduced with adenovirus carrying GFP (Ad-GFP) or FLAG-tagged hPXR (Ad-hPXR). Twenty-four hours posttransduction, cells were treated with zearalenone (10 μM) for 24 h before RNA isolation. Total RNA was subjected to real-time quantitative PCR analysis to determine the relative expression levels of CYP3A4 as described in “Materials and Methods” section. The data are normalized to 18S levels and are expressed as average values ± SEM (n = 3).
FIG. 3.
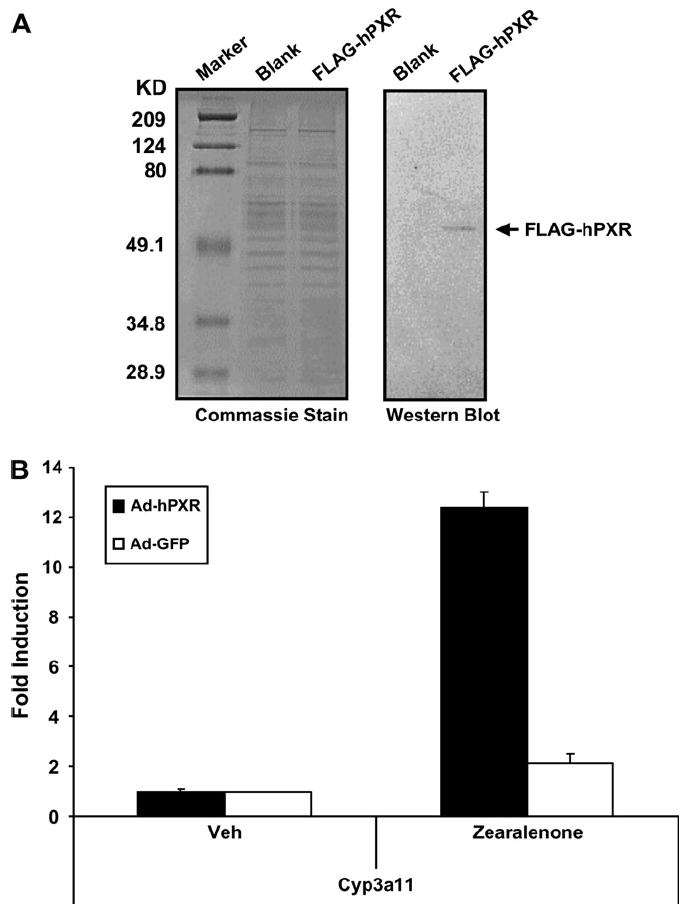
Zearalenone induces Cyp3a11 gene expression through activation of PXR in primary hepatocytes. (A) Primary hepatocytes isolated from PXR-KO mice were transduced with adenovirus carrying GFP (blank) or FLAG-tagged hPXR (FLAG-hPXR) and cultured for 48 h before protein isolation. Total protein was subjected to Western blot analysis for the expression of FLAG-hPXR as described in “Materials and Methods” section. Greater than 90% transduction efficiency was achieved as determined by fluorescence microscopy. (B) Primary hepatocytes isolated from PXR-KO mice were transduced with adenovirus carrying GFP (Ad-GFP) or FLAG-tagged hPXR (Ad-hPXR). Twenty-four hours posttransduction, cells were treated with zearalenone (10 μM)for another 24 h before RNA isolation. Total RNA was subjected to real-time quantitative PCR analysis to determine the relative expression levels of Cyp3a11 as described in “Materials and Methods” section. The data are normalized to 18S levels and are expressed as average values ± SEM (n = 3).
Zearalenone Modulates the Interaction between PXR and Cofactors
The interaction between nuclear receptor corepressor proteins and nuclear receptors represses nuclear receptor activity in cells. On the other hand, the interaction between SRC proteins and nuclear receptors enhances nuclear receptor activity in cells. To investigate the molecular mechanism whereby zearalenone activates hPXR, we sought to determine the extent to which it differentially modulates the PXR-cofactor protein-protein interaction using the mammalian two-hybrid system. Cultures of CV-1 cells were transfected with the expression vector encoding the receptor-interacting domain from cofactor proteins fused to GAL4 DNA-binding domain and the expression vector encoding VP16-tagged hPXR together with the GAL4-responsive luciferase reporter gene pFR-LUC (Fig. 4A). As shown in Figure 4B, zearalenone efficaciously displaced the corepressor N-CoR from PXR. Similarly, zearalenone efficaciously recruited the coactivators SRC-1, PBP, and GRIP1 to PXR (Fig. 4C–E). These data suggest that zearalenone likely binds PXR and serves as a direct ligand for this receptor.
FIG. 4.
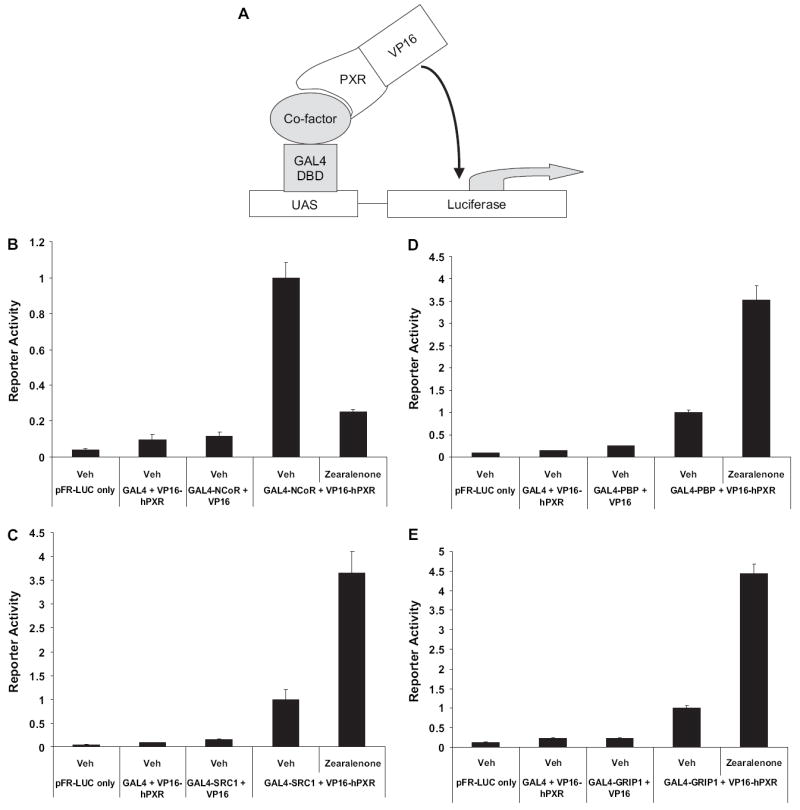
Zearalenone differentially modulates PXR-cofactor interactions. (A) Diagram illustrating the experimental strategy of mammalian two-hybrid system to measure the interaction between PXR and cofactor proteins. (B–E) CV-1 cells were transfected with the GAL4-responsive pFR-LUC reporter gene and the expression vectors encoding GAL4-cofactors and VP16-hPXR and treated with zearalenone (10 μM) for 24 h. Data points in reporter gene assays represent the mean ± SEM (n = 4). (B) Mammalian two-hybrid assay for the interaction between PXR and N-CoR. (C) Mammalian two-hybrid assay for the interaction between PXR and SRC-1. (D) Mammalian two-hybrid assay for the interaction between PXR and PBP. (E) Mammalian two-hybrid assay for the interaction between PXR and GRIP1.
DISCUSSION
In this study, we have demonstrated that zearalenone activates the nuclear receptor PXR and induces the expression of the drug-metabolizing enzyme CYP3A4, a hepatic monooxygenase involved in the metabolism of about 60% of clinically used drugs (Guengerich, 1999). PXR activation has been shown by multiple groups to represent the molecular basis for drug-drug interactions and herb-drug interactions. Full-concentration analysis revealed that zearalenone activated hPXR at low micromolar levels. Although currently it is not known how high zearalenone concentration can reach in humans exposed to zearalenone-contaminated food, values up to 140 mg/kg and more of zearalenone in agricultural products have been reported (Schoental, 1983). In addition, high oral bioavailability (28.1%) of zearalenone has been reported in rats (Mallis et al., 2003). Thus, it is likely that zearalenone activates PXR in humans under certain circumstances. Because PXR regulates many drug-metabolizing enzymes and drug transporters in human liver and intestine, activation of PXR by zearalenone likely promotes the metabolism and elimination of many other coadministered drugs either through metabolism- or transport-mediated mechanism or both. Therefore, our findings indicate that the consequences of PXR activation potentially extends from drug-drug and herb-drug interactions to food-drug interactions. Moreover, further work to investigate zearalenone-drug interactions in vivo is warranted.
In addition to drug-metabolizing enzymes, PXR also regulates the 25-hydroxyvitamin D3-24-hydroxylase (CYP24), a mitochondrial enzyme responsible for inactivating vitamin D metabolites (Pascussi et al., 2005). Therefore, drugs that activate PXR have the potential to cause vitamin D deficiency and eventually osteomalacia and osteoporosis. Our findings suggest that the potential of long-term exposure to zearalenone to cause osteomalacia and osteoporosis is worth further investigation.
At the molecular level, agonist ligands bind to nuclear receptors and cause conformational changes in the receptor that favor the release of corepressor proteins and recruitment of coactivator proteins. The corepressor proteins repress the activity of nuclear receptors by recruiting the histone deacestylase complex (Alland et al., 1997). On the other hand, the coactivators either contain endogenous histone acetyltransferase (HAT) activity or help recruit HAT-containing components to nuclear receptors (Puigserver et al., 1999; Spencer et al., 1997). Our mammalian two-hybrid analysis demonstrates that zearalenone activates PXR by displacing the corepressor protein N-CoR and recruiting the coactivators SRC-1, PBP, and GRIP1, suggesting that zearalenone most likely directly binds to PXR and acts as classical nuclear receptor ligand.
In vitro studies of hepatic gene expression are often performed in primary hepatocytes. However, human hepatocytes are not readily available. On the other hand, studies performed in rodent hepatocytes are hard to translate to humans due to the existence of species-specific differences. For example, rifampicin is a potent inducer of CYP3A gene expression in human hepatocytes, but it has little effect on CYP3A gene expression in mouse hepatocytes, while the synthetic steroid PCN strongly induces CYP3A gene expression in mouse hepatocytes but not in human hepatocytes. Recent studies have revealed that this striking species-specific difference between humans and mice is due to the evolutionary divergence of the PXR ligand-binding domain in these two species (Kliewer, 2003; Xie et al., 2000). The development of an adenoviral-mediated expression system to deliver functional FLAG-tagged hPXR protein to cultured cell models represents a potentially important tool. This novel tool can be used to quickly and reliably characterize the extent to which new drug candidate molecules activate this clinically important and biologically relevant signaling pathway. Our studies using this expression system to deliver hPXR to PXR-KO cultures of mouse hepatocytes demonstrate that this model responds to PXR activators in a way similar to human hepatocytes. Therefore, this novel cultured hepatocyte “humanized” mouse model will likely prove useful in the studies of the human xenobiotic response and may serve as a valuable predictor of potential drug interactions in hepatocytes.
Acknowledgments
We thank Dan Brobst for his technical assistance throughout this project. We thank Dr. Daniel Kelly for Ad-GFP construct. We thank Dr. Michael Stallcup for pGAD424-GRIP1 and Dr. Barry Forman for GAL4-N-CoR, GAL4-SRC-1, and GAL4-PBP. This work was supported by the National Institutes of Health grant 1R01DK068443-01A1.
References
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. doi: 10.1038/387049a0. [DOI] [PubMed] [Google Scholar]
- Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16:497–516. doi: 10.1128/CMR.16.3.497-516.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brobst DE, Ding X, Creech KL, Goodwin B, Kelley B, Staudinger JL. Guggulsterone activates multiple nuclear receptors and induces CYP3A gene expression through the pregnane X receptor. J Pharmacol Exp Ther. 2004;310:528–535. doi: 10.1124/jpet.103.064329. [DOI] [PubMed] [Google Scholar]
- Chen C, Staudinger JL, Klaassen CD. Nuclear receptor, pregnane x receptor, is required for induction of UDP-glucuronosyltransferases in mouse liver by pregnenolone-16{alpha}-carbonitrile. Drug Metab Dispos. 2003;31:908–915. doi: 10.1124/dmd.31.7.908. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005a;312:849–856. doi: 10.1124/jpet.104.076331. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. The ratio of constitutive androstane receptor to pregnane X receptor determines the activity of guggulsterone against the Cyp2b10 promoter. J Pharmacol Exp Ther. 2005b;314:120–127. doi: 10.1124/jpet.105.085225. [DOI] [PubMed] [Google Scholar]
- Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005c;69:867–873. doi: 10.1016/j.bcp.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Geick A, Eichelbaum M, Burk O. Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem. 2001;276:14581–14587. doi: 10.1074/jbc.M010173200. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol. 1999;56:1329–1339. doi: 10.1124/mol.56.6.1329. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Redinbo MR, Kliewer SA. Regulation of cyp3a gene transcription by the pregnane x receptor. Annu Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci U S A. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidy PH, Baldwin RS, Greasham RL, Keith CL, McMullen JR. Zearalenone and some derivatives: production and biological activities. Adv Appl Microbiol. 1977;22:59–82. doi: 10.1016/s0065-2164(08)70160-6. [DOI] [PubMed] [Google Scholar]
- Hong H, Kohli K, Garabedian MJ, Stallcup MR. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast HR, Goodwin B, Tarr PT, Jones SA, Anisfeld AM, Stoltz CM, Tontonoz P, Kliewer S, Willson TM, Edwards PA. Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J Biol Chem. 2002;277:2908–2915. doi: 10.1074/jbc.M109326200. [DOI] [PubMed] [Google Scholar]
- Kliewer SA. The nuclear pregnane X receptor regulates xenobiotic detoxification. J Nutr. 2003;133:2444S–2447S. doi: 10.1093/jn/133.7.2444S. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002;23:687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterstrom RH, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998;92:73–82. doi: 10.1016/s0092-8674(00)80900-9. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–3140. doi: 10.1074/jbc.272.6.3137. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998;102:1016–1023. doi: 10.1172/JCI3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin B, Hawkins-Brown D, Moore JT, Kliewer SA. Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Mallis LM, Sarkahian AB, Harris HA, Zhang MY, McConnell OJ. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;796:71–86. doi: 10.1016/j.jchromb.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Oliver WR, Jr, Shenk JL, Snaith MR, Russell CS, Plunket KD, Bodkin NL, Lewis MC, Winegar DA, Sznaidman ML, Lambert MH, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98:5306–5311. doi: 10.1073/pnas.091021198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P, et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest. 2005;115:177–186. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Adelmant G, Wu Z, Fan M, Xu J, O’Malley B, Spiegelman BM. Activation of PPARgamma coactivator-1 through transcription factor docking. Science. 1999;286:1368–1371. doi: 10.1126/science.286.5443.1368. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Zakrajsek BA, Mills AG, Gorn V, Singer MJ, Reed MW. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal Biochem. 2000;285:194–204. doi: 10.1006/abio.2000.4753. [DOI] [PubMed] [Google Scholar]
- Schoental R. Precocious sexual development in Puerto Rico and oestrogenic mycotoxins (zearalenone) Lancet. 1983;1:537. doi: 10.1016/s0140-6736(83)92229-8. [DOI] [PubMed] [Google Scholar]
- Sonoda J, Xie W, Rosenfeld JM, Barwick JL, Guzelian PS, Evans RM. Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR) Proc Natl Acad Sci U S A. 2002;99:13801–13806. doi: 10.1073/pnas.212494599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Onate SA, Tsai SY, Tsai MJ, et al. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31:523–527. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Utian WH. Comparative trial of P1496, a new non-steroidal oestrogen analogue. Br Med J. 1973;1:579–581. doi: 10.1136/bmj.1.5853.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD. Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J. 2002;2:117–126. doi: 10.1038/sj.tpj.6500087. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]


