Abstract
Over 40% of cancer patients will require radiation therapy during management of their disease. Although radiation therapy improves the survival of a significant number of cancer patients, both acute radiation toxicity (that which manifests during a course of clinical radiotherapy or shortly thereafter), and late toxicity (developing months to years after completion of radiotherapy) compromise overall outcomes for successfully treated cancer patients.
Keywords: radiation damage, protector drugs
Introduction
Despite improvements in the development of clinical radiotherapy treatment planning and treatment delivery technologies (Table I), there remains a significant toxicity of radiotherapy to normal tissues and organs.(1–3) Improved local control of cancer by radiotherapy dose escalation in patients with, for example, lung, esophagus, colorectal, pancreas or pelvic malignancies is associated with significant acute toxicity and normal tissue damage; however, higher radiation doses which are likely to be more effective cannot be used in these patients owing to acute toxicities occurring during the clinical course of radiotherapy.(4–7) These acute toxicities are associated with the tissue inflammatory response and are not always limited to the normal tissue in the irradiation beam. Acute toxicity can extend beyond the treated area. Examples include esophagitis (difficulty swallowing) and pneumonitis (cough, fever, lung fluid accumulation) in the lung cancer patient, and intestinal and rectal irradiation-induced inflammation (diarrhea, cramps, abdominal pain) in the colorectal cancer patient. Acute toxicities are usually transient and symptoms resolve weeks after completion of treatment. Indeed, acute side effects may limit the patient’s capacity to comfortably complete a treatment course. Furthermore, there is renewed concern, about the occurrence of late-manifesting toxicities (defined as those appearing months to years after completion of a successful treatment course) in patients treated with radiotherapy. Late toxicity is usually limited to tissues treated and does not usually affect survival; however, late effects including fibrosis (scarring) and organ functional failure may ensue depending on the volume of tissue treated and dose of irradiation delivered.(5–7) Therefore, to ameliorate these toxicities and thereby improve the therapeutic ratio (that is ratio of cancer cell killing to normal tissue toxicity caused by a given dose), radioprotective drugs are receiving significant interest.(1–2, 8–11)
Table I.
Radiotherapy Technologies Currently Utilized
|
The molecular pathways that are utilized for radiation protection follow on current knowledge regarding the molecular biological mechanisms of ionizing irradiation induced cell killing at the level of single cells, tissues and organs (Figure 1). Ionizing irradiation, hits oxygen and water molecules in cells and results in production of radical oxygen species (ROS) such as superoxide and hydroxyl radical which deplete cellular antioxidant stores, most prominently glutathione.(8, 9) Replacement of cellular antioxidant capacity by increasing levels of the enzyme Manganese Superoxide Dismutase is an example of one radioprotector strategy at the cellular level.(10–11) Both dying and surviving cells within an irradiated tissue cell release inflammatory cytokines which can act as cytotoxins at both the local tissue level and also through the blood circulation, can affect distant sites via action on specific cellular surface receptors.(3–4) Agents which limit cytokine binding or action at the cellular receptor level include the TLR5(12) receptor agonist and provide another strategy for radiation protection. Therefore, recognition of potential radioprotective pathway targets is based on understanding the underlying molecular biology of irradiation cellular killing.
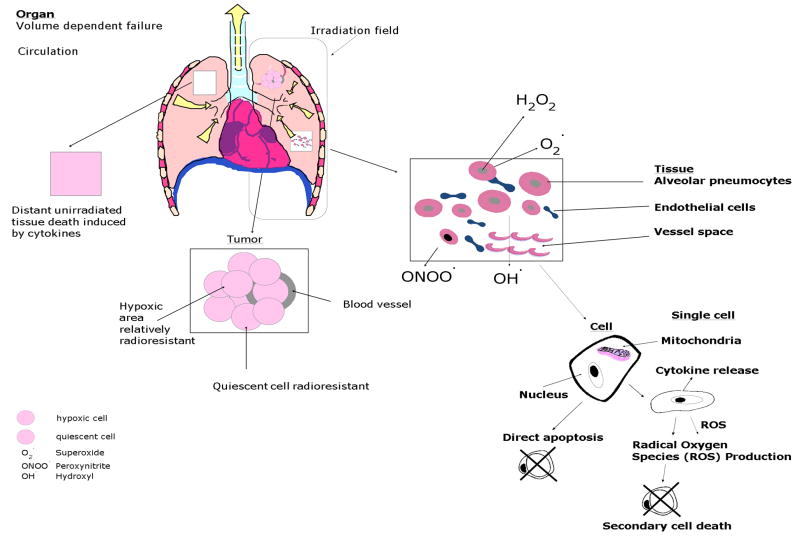
Figure 1. Cell, tissue and organ specific pathways of ionizing irradiation toxicity.
Subtotal lung irradiation is shown as an example. Single alveolar pneumoncytes from an area within lung tissue are shown relative to total lung irradiation. Individual cells experience ionizing irradiation-induced production of radical oxygen species (ROS) including superoxide, hydroxyl radical, nitric oxide and peroxynitrite from the interaction of ionizing irradiation with oxygen and water. ROS interaction with pyrimidine and purine bases nuclear DNA produces single and double strand breaks, initiation of DNA repair, communication of DNA damage through the cell cytoplasm to the mitochondrial membrane via (stress activated protein (SAP) kinases) and then translation of pro-apoptotic, BCL2 family members from nucleus to mitochondria.(86) Then follows mitochondrial membrane permeability, cytochrome c disassociation from cardiolipin, and cytoplasmic leakage of cytochrome c which leads to activation of the caspase-3 pathway and apoptosis.(17–18, 86) Both dying and irradiated but recovering cells release ROS and inflammatory cytokines including IL-1, TNFα and TGFβ, which directly (through cell to cell contact with other cells in the tissue), and indirectly (via the circulation to cells at distant sites), produce acute local tissue and systemic effects respectively. Within the irradiated tissue differences in radiosensitivity of different cell phenotypes (endothelial cells, alveolar pneumocytes, alveolar macrophages and bronchopulmonary stem cells) contribute to the magnitude of tissue damage. The volume of tissue within the lung that is in the irradiation beam determines as does irradiation dose the magnitude of acute and chronic normal tissue effects.
This article will address current and future strategies for development of radioprotective agents, both systemically delivered and organ specific targeted radioprotectors. Radioprotectors are being developed for the purpose of both reducing acute radiotherapy side effects and minimizing late chronic radiation toxicity in the cancer patient.
Ionizing Irradiation Clinical Effects
Ionizing irradiation causes significant toxicity at the single cell, tissue and organ level, and the clinical effects of therapeutic irradiation depend upon the dose delivered and the volume of tissue exposed.(1, 3, 6–7) For example, if a tumor volume is large, this necessitates ionizing irradiation delivery to a significant volume of normal tissue. There is a non-linear relationship between dose of irradiation and cell death.(3) Cell phenotype within a tissue and tissue specific differences in irradiation response determine the shape of the cell killing curve. For example lymphoid tissues such as the thymus are relatively radioresponsive compared to skeletal muscle tissue. Dose rate (the quantity of irradiation delivered per minute), fraction size (dose delivered per treatment session) and level of oxygenation of the tissues treated directly increase cell death.(3) Irradiation not only kills tumor cells, but also proliferating normal cells. Both normal and tumor tissue contain a subset of dividing cell populations, and quiescent or non-dividing subsets. Quiescent cells are relatively resistant to ionizing irradiation killing.(3) Rapidly dividing normal and tumor cell populations are more susceptible than those cells which are either slowly proliferating or non-proliferative. However, unlike normal tissues, rapidly dividing tumor cell subsets can outdistance their blood supply and become hypoxic.(3, 13, 14) Since oxygen is a main molecular target for irradiation production of ROS, hypoxic cells in tumors show relative radioresistance.(13–14 )
Release of cytotoxic inflammatory cytokines from irradiated tissue can also recruit inflammatory cells including lymphocytes, macrophages and polymorphonuclear leukocytes which then infiltrate tissues and cause further normal cell killing(4,15–16) through the generation of yet other inflammatory cytokines and byproducts, including more ROS.(17–18) The cellular and tissue specific pathways involved in irradiation killing are shown in Figure 1.
Ionizing Irradiation Toxic Effects
Acute Effects of Ionizing Irradiation
Depending on the anatomic site treated (Figure 2) acute effects may include: nausea and vomiting, tiredness, fatigue, diarrhea, headache, as well as normal tissue swelling, skin erythema, cough, difficulty swallowing and difficulty breathing.
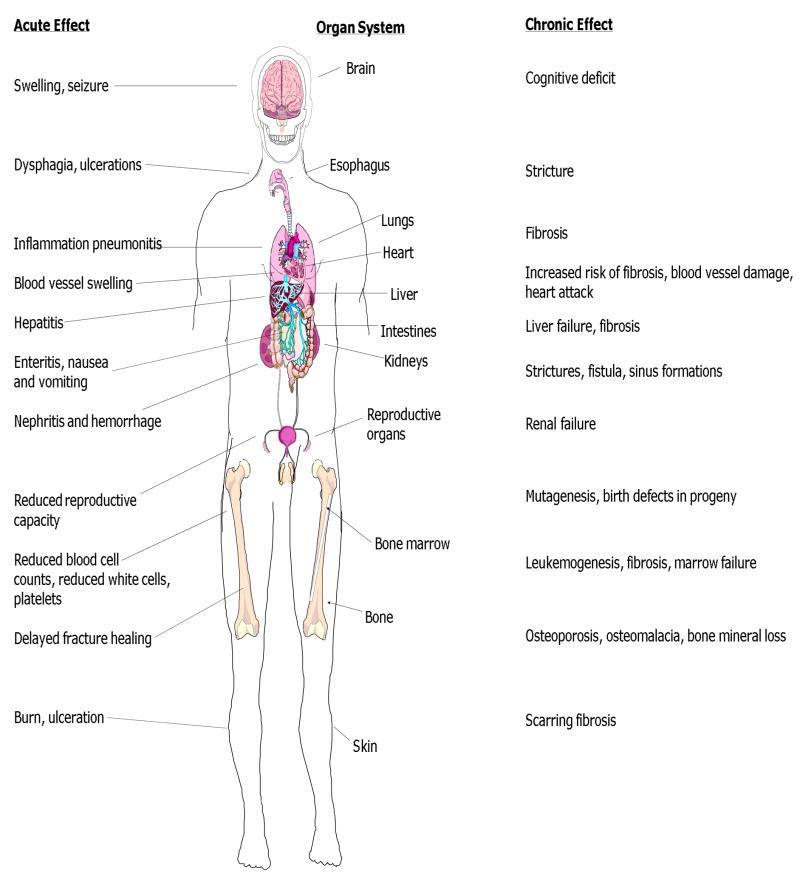
Figure 2. Organ specific acute and chronic radiation toxicities.
Acute tissue toxicity experienced during a radiotherapy treatment course or shortly thereafter is described as symptoms and signs of tissue damage for each organ (left side). Chronic radiation side effects occurring months to years later are also shown (right). Severity and duration of both acute and chronic side effects depends on radiation dose, dose rate, quality of irradiation (greater for high LET radiation beams – See Box 1) and volume of tissue treated.
The acute effects of irradiation are based on both normal tissue response and tumor cell killing following on the underlying molecular biological effects of ionizing irradiation. Within tissues and organs, ionizing irradiation kills dividing cells by both stochastic (random) and determinative (microenvironment induced) mechanisms.(19) Dividing cells in the DNA synthetic or (S) phase of the cell cycle are relatively less sensitive to radiation killing compared to those in mitosis (M) or in the second gap (rest phase between DNA synthesis and mitosis).(3) Those cells in metabolic quiescence (non-dividing) and those in relatively hypoxic areas are less sensitive to irradiation killing.(3, 13) Direct irradiation killing leads to elimination of those cells from the tissue and organ. Both direct and indirect (mediated by cytokine and ROS release from dying cells) irradiation killing effects are significant in influencing the magnitude and duration of acute side effects. In cells that repair irradiation damage and survive, release of inflammatory cytokines including transforming growth factor β1 (TGFβ1), Interleukin 1 (IL-1), and tumor necrosis factor α (TNFα) act both locally within the irradiated tissue/tumor, and enter the systemic circulation where tissues outside the irradiation beam can experience cell killing.(4, 15, 20) Inflammatory cytokine binding to specific receptors on sublethally irradiated or unirradiated cells (outside the irradiation volume), leads to cell death through the apoptosis (programmed cell death) pathway(17–18, 21), autophagy(22) and necrosis(4) death pathways.
Cells and tissues recover from irradiation acute effects in a variety of ways. Surviving cells within irradiated tissue and those in adjacent unirradiated tissue, particularly in the primitive or stem cell compartments are induced to proliferate and repopulate areas in the tissue that were depleted by irradiation killing.(3) In addition, stem cell or progenitor populations from outside the irradiated tissue migrate into the irradiated volume and facilitate repopulation or replenishment of tissue function.(23–25) Stem cell populations involved in regeneration of irradiated tissue (epithelial progenitor cells for example in the irradiated oral cavity, esophagus or intestine) include those which home to sites in the vacated tissue microenvironment, depleted by irradiation killing. For example, endothelial cell progenitors of bone marrow origin migrate in and repopulate blood vessel endothelial cells killed by irradiation.(25) Repair and replenishment of irradiated tissue is also facilitated by migration into the irradiated area of lymphocytes, macrophages and neutrophils which elaborate reparative cytokines including vascular endothelial growth factor I (VEGF-1), hepatocyte growth factor (HGF), fibroblast growth factor (FGF) and epidermal growth factor (EGF).(26)
Thus, recovery from acute irradiation effects occurs at the cellular level by restoration of antioxidant pools through biochemical synthesis of glutathione and upregulation of antioxidant enzymes(8, 17–18), and at the tissue level by stem cell mediated repopulation through both proliferation of in situ cells and by migration into tissues via the circulation of progenitor cells from distant sites.(3, 23–25)
Chronic Effects of Ionizing Irradiation
Chronic irradiation effects are critically important in all patients, but particularly in those who receive total body irradiation (TBI). Total body irradiation is utilized in some cancer therapies particularly for patients who require a bone marrow transplant.(27–29) TBI is delivered either in single fraction or in multiple fractionated courses designed in part to clear space in the bone marrow by causing apoptotic death of a sufficient number of hematopoietic stem cells and their progeny for homing and proliferation of donor hematopoietic stem cells that are injected intravenously. Observing responses to total body irradiation in both experimental animal models and in clinical radiotherapy patients demonstrates multiple chronic effects including features common to premature aging such as hair graying, skin thinning and dryness, formation of cataracts, early myocardial fibrosis, myocardial infarction, neurodegeneration and osteopenia/osteomalasia.(3, 30) In pediatric and adult long term cancer survivors, who received total brain irradiation, neurocognitive defects have been detected.(3, 31–33) Radiation may induce life shortening by decreasing endocrine function through glandular cell death or by system wide depletion of antioxidants which are exhausted by perpetual response to persistent ROS production in tissues.(34–35) During aging, ROS production is persistent, so the requirements for continual replenishment of antioxidant stores including glutathione, superoxide dismutase, catalase and glutathione peroxidase increase.(34) Furthermore, accumulation of increased levels of p53, p21 and BAX in naturally aging cells or those prematurely aging after irradiation may slow proliferation and return more cells to quiescence.(30, 35) Therefore, long-term consequences that follow the cellular, tissue and organ responses to ionizing irradiation, are common to those of aging.
Chronic or late effects depend upon the organ treated, but are also directly related to irradiation dose and volume treatment(3) (Figure 2). Response to irradiation acute effects may contribute towards the manifestation of chronic effects.(3) After recovery from the acute effects of irradiation, tissues appear normal in situ and under the microscope.(3, 30, 35) There may be no obvious sign of irradiation injury. Current experimental evidence suggests that both endothelial cells in blood vessels recovering within irradiated tissues and those supplemented by endothelial progenitors which migrate into the tissues from bone marrow origin are depleted of intracellular levels of thrombomodulin.(36) Irradiated tissues which recover from the acute effects continue to produce ROS which may continually deplete thrombomodulin.(20, 36) Upregulation of cell-destructive proteases can also initiate a second wave of apotosis.(36) Endothelial cell death has been associated temporally with accumulation in irradiated tissues of fibroblast progenitor cells migrating in from the bone marrow microenvironment.(37) The signals that elicit this migration are unknown, but accumulation of fibroblast progenitor cells leads to their proliferation in irradiated tissues, and functional inactivation in that organ by replacement of functioning areas with scar tissue.(4, 15, 20)
Mechanistic models for irradiation chronic effects have been aided by observations in animal models. For example, there is a relative decrease in histopathologic evidence of late irradiation fibrosis, in animal strains genetically altered to mask the expression of inflammatory cytokines such as (TGFβ)(38–40), or deleted for expression of enzymes required for generation of free radicals (nitric oxide synthase-1, neuronal NOS,) as in mitochondrial NOS knockout mice.(41) Animals deficient in the signaling response to irradiation induced TGFβ (SMAD3- deficient mice) demonstrate decreased irradiation-induced late fibrosis in skin and lung.(38–39) Chronic side effects are not limited to fibrosis, but include abnormal blood vessel formation called telangiectasias(19), ulcerations and organ failure.(42–44)
A prominent chronic effect of ionizing irradiation is carcinogenesis/leukemogenesis. The irradiated tissue microenvironment can prevent apoptosis of damaged proliferating cells by cell to cell contact.(30, 45–53) Irradiation induces cell cycle growth delay by both a G1 (gap in the cell cycle between mitosis and DNA synthesis) and a G2 (block in the cell cycle following mitosis) growth arrest.(3) Holding cells in growth arrest creates a condition of quiescence.(3, 16) Prolonged production of ROS in cells of the microenvironment of the irradiated lung(54) and bone marrow(55–56) months to years after irradiation can potentially induce genetic change in other quiescent cells. Furthermore, migration of a stem cell population from distant sites into an irradiated microenvironment can expose those homing stem cells to ROS released from irradiated stromal cells causing mutations and even malignant transformation.(24–25, 57–58) Therefore, the persistent elaboration of both ROS and humoral cytokines by surviving cells within an irradiated tissue/organ facilitate chronic interaction with other cells that are attempting to repopulate and restore that tissue and organ.
Systemic Effects
Systemic effects of ionizing irradiation have been well described in subtotal body as well as total body irradiated experimental animals and in humans.(3, 27–30) Systemic effects include both acute and chronic effects as described above, but with several unique features. In particular, systemic effects include symptoms in areas that were not irradiated including overall tiredness and easy fatigability, and are probably caused by the persistent elaboration through the circulation of inflammatory cytokines.(4, 16, 20)
Systemic effects apply to the response to subtotal body or regional irradiation such as a thoracic, abdominal or pelvic irradiation volume, as well as total body irradiation effects and are described as syndromes. The experience from clinical radiotherapy, principally total body irradiation to prepare patients with leukemia, lymphoma or disseminated cancer for a life saving marrow transplant, led to description of several syndromes of radiation toxicity.(59) A common principle with many of these syndromes is that partial body shielding greatly decreases the severity and the experience of the syndrome. A second basic principle is that protection from each syndrome is associated directly with reduced radiation dose rate and total dose.(3)
The central nervous system syndrome is associated with doses above 800 cGy total body dose or higher doses to the head and presents with signs and symptoms of brain swelling including nausea and vomiting, headache, sweating, rapid heart rate and rapid death. The gastrointestinal syndrome associated with TBI doses above 500 cGy presents with nausea, vomiting and diarrhea, and is associated with destruction of intestinal crypt and endothelial cells in the intestine, dehydration, severe abdominal pain, infection and blood loss.(69) The hematopoietic syndrome is associated with TBI doses above 300 cGy and a decrease in peripheral white blood cell count, platelet count, red blood cell count, and in the absence of source of bone marrow transplantation to replace damaged hematopoietic stem cells, may lead to death from infection, hemorrhage, weakness and fatigue.(59) The immunosuppression syndrome is associated with TBI doses as low as 100 cGy. Lymphocytes are the most radiosensitive cells in the peripheral blood, and thus a basic radiological biomarker dose sustained involves the magnitude of a decrease in slope of a peripheral blood lymphocyte count. Lymphocyte decrease can be associated with immunosuppression and susceptibility to infection, weakness and fatigue.(3)
Other systemic clinical effects are associated with partial body irradiation, such as the high dose irradiation induced cutaneous syndrome skin burns (beta burns) caused by local high doses above 30 Gy by electron irradiation or from the accumulation of radioactive isotopes on the skin. This syndrome is associated with erythema/redness, ulceration of the skin, heat loss, extravasation of fluids, lymphedema, hemorrhage and secondary infection.(3)
Details of the Hematopoietic Syndrome reveal many important radiobiologic principles. It is a collection of symptoms and signs associated with suppression of bone marrow function. This results in reduction of the number of peripheral blood red cells, platelets, and leukocytes (white cells). Individuals experience tiredness associated with anemia (low red cell count), propensity for bleeding (associated with low platelets), and inability to fight infections (associated with decreased white blood cell count). Shielding of as little as 10% of the bone marrow volume during total body irradiation can result in successful repopulation of the entire hematopoietic system by bone marrow stem cells that were in the protected microenvironment and can ameliorate or even prevent the Hematopoietic Syndrome.(3, 59) The production of inflammatory cytokines including TNFα, TGFβ1 and IL-1 correlates with the severity of suppression of hemopoiesis.(4, 20) Within the dose range required to cause the hematopoietic syndrome in humans (200 – 600 cGy total body dose) there are individuals who show reduced severity of depression of hematopoiesis (less of a decrease in white cell, platelet and red cell counts as well as immunosuppression by reduction of T-cell and B-cell numbers). These individuals may have less systemic cytokine production by irradiated tissues compared with others that develop severe hematopoietic depression.(59) The reason for individual patient variation in susceptibility to the Hematopoietic Syndrome is unknown, but genetic factors tend to make some individuals more sensitive. These include individuals with ataxia, telangiectasia(60–63), Fanconi anemia (64–66), Werner’s Syndrome(67), Blooms Syndrome(67–68) and other categories of radiation sensitive individuals also termed “hyper-responders”(61) with no known genetic defect, but with an exacerbated response to irradiation doses compared to other individuals.
With all syndromes, genetic predisposition to irradiation toxicity can shift the radiation dose response curve to the left in effect giving the individual a greater chance of experiencing the toxicity at a lower sustained dose of irradiation. Other conditions known to increase sensitivity of individuals to side effects of irradiation include those associated with DNA strand break repair such as ataxia telangiectasia(60), Werner’s syndrome(67), Bloom’s Syndrome(68) and Fanconi Anemia.(66) Of importance to the physician, there are subsets of individuals with no genetic markers, but with greater sensitivity to ionizing irradiation called “hyper-responders”.(3, 61) Whether these individuals have a greater irradiation induction of inflammatory cytokines or defect in regulation of endothelial cell function is unknown. Patients likely to develop pulmonary complications of lung irradiation include those with increased serum levels of TGFβ detected within the first weeks of radiotherapy.(15)
Acute, chronic and systemic responses to ionizing irradiation illustrate many common pathways in normal cellular, tissue and organ tissue repair. Knowledge of the underlying molecular biological pathways initiated by irradiation-induced DNA strand breaks, cellular apoptosis, and cell to cell interaction, including the elaboration of inflammatory cytokines, helps define several pathways for development of radioprotective agents.
Radioprotective Pathways
The mechanistic/biological basis for development of a radioprotective strategy necessitates an understanding of the molecular biology underlying the mechanism of the cellular, tissue and organ specific radiation damage response. Examples of the pathways for focus are shown in Figure 3 and include: nuclear DNA strand breaks, communication of nuclear stress responses through the cell cytoplasm to mitochondria, mitochondrial response to nuclear signaling, and mitochondrial initiation of apoptosis.(47–48, 69) Finally other cells respond to the inflammatory cytokine cascade that follows cell killing in a second wave of cell death.(3) This second wave may slowly persist or may occur in a delayed but severe fashion leading to the rapid onset of what is called chronic effects described above.
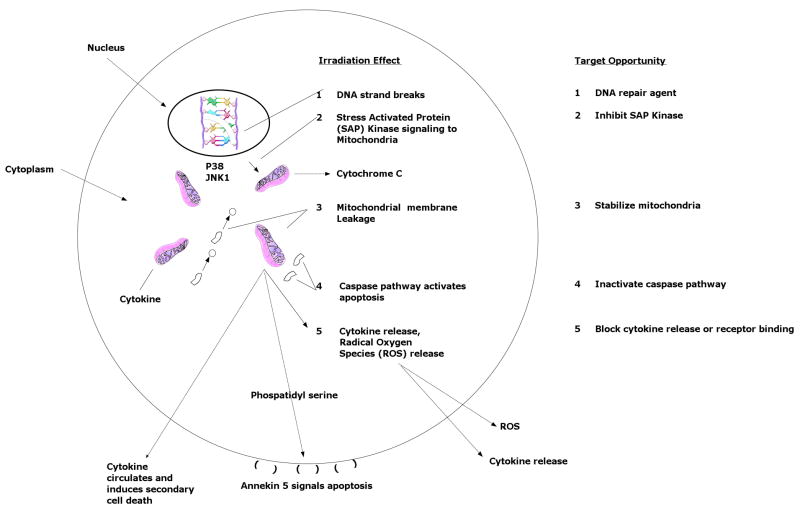
Figure 3. Targets for development of radioprotector agents based on molecular pathways of the irradiation response.
The molecular mechanism of irradiation damage in single cells (effects #1 – 4) and released inflammatory cytokines (effect #5) is defined by several target points where efforts for development of radioprotector drugs can focus. Radioprotectors could target the DNA damage step(121–122): (1); molecular translation of the DNA damage event to the mitochondria through the cytoplasm, (2); mitochondrial stabilization by preventing membrane permeability and leakage of cytochrome c, (3); activation of caspases to cause apoptosis(123), (4); or intracellular communication of cellular and tissue damage by elaboration of cytokines, (5). Examples of radioprotector drugs currently under development or in clinical trial are correlated to each irradiation effect and are shown in Table I.
Agents delivered prior to the initiation of radiotherapy would be the ones expected to target critical biochemical pathways in cells yet to be exposed to irradiation, and to either decrease the magnitude of a response pathway or convert the response to an alternate biochemical pathway.(70) Use of such an agent would be critically dependent on time of delivery, specificity of uptake in the tissues to be protected and delivery to the intracellular sites of interest. Organ specific targeted delivery of an antioxidant therapy is one example of such an agent.(11, 55) Intraorgan administration of MnSOD-PL to the oral cavity, esophagus, lung, bladder and intestine has been shown to be a potentially successful approach to localized radioprotection.(71) Other antioxidant agents which can be delivered locally or systemically are listed in Table II.
Table II.
Categories of Radiation Dose Modifying Agents
| Protectors | Examples | References | Irradiation Effect Target Opportunity In Fig. 3 |
|---|---|---|---|
| Sulfhydryl Compounds | Cysteine WR2721 | (1, 83, 85) (84–85) (2) |
1–3 1–3 |
| Antioxidants | Tempol | 3 | |
| Targeted Mitochondrial | MnSOD Mimics mn – | (79, 99) | 3 |
| Agents | porphryn based | ||
| GS-Nitroxides, | (80–81) | 3 | |
| Eukaryon-134-SOD mimic | (99) | 3 | |
| Molecules | |||
| Cryoprotective Agents | DMSO | (100) | |
| Immunomodulators | Histamine H2 receptor | (101) | 5 |
| Antagonists | 5 | ||
| Polysaccharides | (102–103) | 4–5 | |
| Heat killed lactobacillis | (103) | 1–5 | |
| Synthetic chemicals | (102) | 3–5 | |
| Flagillin | (12) | 3–5 | |
| B-Glucan | (104) | 3–5 | |
| Prostaglandins | (106) | ||
| Plant extracts | Curcumin | (105) | 3 |
| Orjentin | (105) | 3 | |
| Viciden | (105) | 3 | |
| Ngella sativa | (103) | 3 | |
| Podophyllum hovandrum | (107) | 3 | |
| Vitamins | Vitamin E | (108) | 3 |
| Vitamin C | (109) | 3 | |
| Cytokines | IL-1 | (110) | 5 |
| Stem Cell Factor | (111) | 5 | |
| G-CSF | (112) | 5 | |
| Gene Therapy Delivered | |||
| Antioxidants | MnSOD-PL | (70–71) | 3 |
| Mitigators | Examples | References | |
| ACE Inhibitors | Captopril | (113–115) | 5 |
| All Type-1, Type-2 | Clanipril | (113–114) | 5 |
| Receptor Antagonists | Penicillamine | (105) | 5 |
| Pentoxyphilline | (116) | 5 | |
| Endothelial Cell | Vascular endothelium | (45–46) | 5 |
| Infusion | |||
| Treatments | Examples | References | |
| Pentoxyfilline | (117) | 5 | |
| a-tochoferol | |||
| caloric restriction | (118–120) | 5 |
There are several possible targets for design and application of a radioprotector. These are shown in Figure 3.
Blocking nuclear DNA damage and its communication to the mitochondria
Overlapping pathways of cellular protection from ionizing irradiation, ultraviolet irradiation and heat have been revealed in the discovery of damage repair genes, genes for induction of antioxidant proteins(72–76), free radical scavengers, and by study of the evolution of heat shock proteins.(72) A common pathway in defense against ionizing irradiation involves protection of single and double strand nuclear DNA breaks, which lead to induction of the self-destructive pathways of apoptosis, autophagy and mitotic arrest(3, 73) as well as delayed mutations. There is evidence that all phyla in both the plant and animal Kingdoms maintain common genetic functions for adjusting to conditions of low level ionizing irradiation.(72–76) A radioprotector could well be one that protects against DNA strand breaks.
Mitochondrial Stabilization
Development of radioprotectors has also followed on knowledge of the intrinsic radiation resistance of specific transgenic mice that display overproduction of a mitochondrial localized antioxidant protein.(77) Also of importance was the observed relative radiosensitivity of a knockout strain of mice deficient in production of an antioxidant radioprotective protein such as MnSOD.(70–71) Agents which increase the cellular antioxidant pool anticipating large quantities of irradiation induced ROS, thus anticipate the need to neutralize these molecules. Such radioprotective agents include MnSOD transgene therapy(70–71, 78), and small molecules MnSOD mimics.(79) Other strategies to elevate cellular antioxidant stores, would be to deliver the immunostimulant TLR5-Flagellin(11) or another biological agent or derived product that elicits a stress response in cells including upregulation of MnSOD gene transcription and its protein production to achieve the goal of increasing the cellular antioxidant response capacity. Yet other relevant approaches would include small molecules that could act as ROS scavengers.(80–83) (Table II)
Other examples of therapeutic agents which have been developed along the lines of protecting the mitochondria in cells from initiating apoptosis doso by elevating antioxidant levels in response to irradiation such as WR2721 (Amifostine) which was designed as a ROS scavenger molecule.(83–85) (Table II)
Blocking caspase activation and poly ADP-ribosyl-polymerase (PARP) cleavage
These are two theoretical targets for future research, based upon knowledge of the post- mitochondrial events in single irradiated cells.(86) (Figure 3)
Decreasing systemic cytokine mediated cell death
Experimental approaches to ameliorate late irradiation effects have been identified in animal model systems administration of novel counteracting cytokines, anti-cytokines and immune stimulation with or without stem cell transplantation as well as dietary antioxidant strategies.(15, 20, 87)
Radiosensitizers
Reversing the strategy described for radiation protectors could result in development of radiosensitizers or agents that increase the cellular capacity to respond to ionizing irradiation. This strategy has been utilized in the development of tumor radiosensitizers designed to deliver specifically drugs that would sensitize the tumor relative to normal tissue.(3) An agent which specifically sensitizes tumor cells can also appropriately affect the therapeutic ratio (greater tumor toxicity compared to normal tissue toxicity). Such tumor radiosensitizing agents include: Bromo-deoxyuridine, BUDR, Taxol, Cis-Platin, Cytoxin and analogs, and recent anti-angiogenic drugs designed to target tumor vasculature or tumor cells.(3, 88–89) A major challenge for the development of tumor radiosensitizers has been the difficulty in finding tumor cell specific targets that do not overlap significantly with normal tissue functions. Currently available radiosensitizers have exploited tumor cell deficiencies or their overexpression of specific radiation damage response proteins.(90) The overlap between normal tissue and tumor cells has been significant and application of these new agents to experimental models or clinical trials has met with significant normal tissue toxicity.
Strategies and issues of concern
Long term side effects of radioprotectors have been a concern. Since the irradiation response of cells and tissues cause many cells to remain in a quiescent state protected from cell division by their residence in the microenvironment, there is a possibility that uptake of a radioprotective agent in those cells might alter their biological behavior while in the quiescent state. During the transition from recovery from the acute irradiation effect, radioprotective agents could have a second function that might be deleterious. For example, a small molecule capable of neutralizing ROS might be metabolized intracellularly to another molecule that could function as a carcinogen. Recent data indicates that MnSOD-PL administration systemically for protection against the hematopoietic syndrome, leads to an increase in survival of C57BL/6J mice with no detectable increase in carcinogenesis.(35)
New areas of research are showing particular promise including an understanding of the difference in redox chemistry and metabolism of oxygen between hypoxic regions within tumors and surrounding normal well oxygenated tissue. Oberley and colleagues(91–97) first described the difference in redox chemistry between tumor cells and normal tissues. Particularly in patients with large greater than 1 cm diameter squamous cell tumors of the lung, head and neck region, esophagus, and other bulky tumors of the pelvis such as cervical and endometrial cancer, and large abdominal tumors such as pancreas cancer or colon cancer, there has been appreciation of a shift in tumor cell metabolism from oxic to hypoxic conditions. Any unregulated growth of cancer cells beyond blood vessels produces areas of hypoxia and anoxia leading to necrosis.(3) Delivery of radioprotector drugs by the intravenous route may not reach a significant portion of the tumor volume. In addition, delivery of antioxidant drugs including some compounds that scavenge free radicals including superoxide, hydrogen peroxide, and peroxynitrite may halt the production of hydrogen peroxide products in normal cells.(94) Normal cells have an increased capacity to neutralize hydrogen peroxide through catalase and glutathione peroxidase while tumor cells, particularly in hypoxic or anoxic regions show down-regulation of these enzymes.(91–92)
Entry of antioxidant agents into tumor cells which result in the generation of hydrogen peroxide, can produce additional tumor selective toxicity through limited capacity for metabolism of hydrogen peroxide.(94–97) Furthermore, limited effectiveness of cancer blood vessels, as well as altered tumor redox metabolism in the cancer cells, may enhance relative levels of antioxidant drug delivery to normal tissues for radioprotection in the cancer patient.(88) An increased understanding of the metabolic differences between tumor cells and normal tissue with respect to capacity to activate radioprotectors could lead to the same strategy used in the development of the hypoxic cell cytotoxin Tirapazamine.(98) With this drug, normal oxygen concentration metabolizes the drug into a non-toxic moiety while hypoxic regions of tumors suffer the toxic effects of the unmodified drug.
The administration of a radioprotective agent to a target tissue must take advantage of the time course for reaching target cells at risk and must include a delivery system designed to penetrate deep enough into the tissue to reach the proliferating stem cell populations. For example, in the prevention of irradiation-induced esophagitis, or oral cavity mucositis, intravenous administration of a radioprotector drug might be expected to reach all tissues, but may not provide a high enough level of uptake in the stem cell populations in those critical target tissues. In contrast, intra-oral or intra-esophageal localized administration using a delivery system known to penetrate several cell layers into a particular tissue, should provide a higher concentration of drug or transgene product to that site.(23) In contrast, the goal of protection of all normal tissues from total body irradiation might require intravenous administration or transdermal administration with effective absorption such that blood levels would reliably achieve effective systemic levels within the appropriate time prior to irradiation.(35)
Overproduction of a radioprotective antioxidant transgene product, or DNA repair enzyme, if administered too far in advance of irradiation can lead to compensatory down regulation of other antioxidants or DNA repair pathways and potentially remove the desired radioprotective effect.(82) Similarly, the administration of a radioprotective agent after radiation exposure might be ineffective due to the overwhelming amount of ROS produced by the oxidative stress of irradiation.(70–72) A list of representative radiation protectors, radiation damage mitigators and radiation damage treatment agents currently under consideration or development is shown in (Table II).
Conclusions
The success in development of radioprotective agents will depend increasingly on an understanding of the molecular biology of radiation damage, cellular, tissue, organ responses to irradiation, the effect of comorbid factors, and differences between tumor and normal cell biology. Strategies for developing tumor radiosensitizers and normal tissue radioprotectors have in the past relied upon known differences in tumor specific vs. normal cell biology in terms of cell cycle, expression of specific growth factor receptors, cell surface adhesion molecules, or other biological or immunological characteristics. Molecular targets of new radioprotectors should concentrate on the mechanisms of action on irradiation-induced damage, after nuclear DNA strand breaks are repaired, focusing instead on distal steps in the cellular response, including nuclear to mitochondrial transport of signaling molecules, and steps in induction of the cell death pathways including autophagy, apoptosis and necrosis. New strategies to identify metabolic differences between normal tissue and tumor cells will also be critical to the design of new classes of radioprotectors for clinical use.
Of equal importance is the concern of potential delayed deleterious effects of radioprotective agents in preventing the removal of irradiation damaged cells the survival of which may lead to an increase in unacceptable chronic side effects including organ failure and carcinogenesis.
Acknowledgments
Supported by Grant #U19A1068021 from NIAID/NIH
References
- 1.Nair CKK, Parida DK, Nomura T. Radioprotectors in Radiotherapy. J Radiat Res. 2001;42 :21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 2.Hahn SM, Krishna CM, Samuni A, DeGraff W, Cuscela DO, Johnstone P, Mitchell JB. Identification of nitroxide radioprotectors. Radiation Research. 1992;132:87–93. [PubMed] [Google Scholar]
- 3.Hall E, Giaccia A. Radiobiology for the Radiologist. 6. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 4.Rubin P, Casarett GW. In: Clinical Radiation Pathology. Saunders WB, editor. Philadelphia, PA: 1968. [DOI] [PubMed] [Google Scholar]
- 5.Hauer-Jensen M, Wang J, Denham JW. Bowel injury: Current and evolving management strategies. Semin Radiat Oncol. 2003;13:357–371. doi: 10.1016/s1053-4296(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 6.Gopal R, Tucker SL, Komaki R, Liao Z, Forster KM, Stevens C, Kelly JF, Starkschall G. The relationship between local dose and loss of function for irradiation lung. I J R O B P. 2003;56(1):106–113. doi: 10.1016/s0360-3016(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 7.Marks LB. Dosimetric predictors of radiation-induced lung injury. I J R O B P. 2002;54(2):313–316. doi: 10.1016/s0360-3016(02)02928-0. [DOI] [PubMed] [Google Scholar]
- 8.Epperly MW, Osipov AN, Martin I, Kawai KK, Borisenko GG, Tyurina YY, Jefferson M, Bernarding M, Greenberger JS, Kagan VE. Ascorbate as a “redox-sensor” and protector against irradiation-induced oxidative stress in 32D cl 3 hematopoietic cells and subclones overexpressing human manganese Superoxide Dismutase. I J R O B P. 2004;58(3):851–861. doi: 10.1016/j.ijrobp.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Turella P, Cerella C, Filomeni G, Bullo A, DeMaria F, Ghibelli L, Ciriolo MR, Cianfriglia M, Mattei M, Frederici G, Ricci G, Caccuri AM. Proapoptotic activity of new glutathione S-Transferase inhibitors. Cancer Res. 2005;65:3751–3761. doi: 10.1158/0008-5472.CAN-04-3903. [DOI] [PubMed] [Google Scholar]
- 10.Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. I J R O B P. 2003;57(4):1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 11.Carpenter M, Epperly MW, Agarwal A, Nie S, Hricisak L, Niu Y, Greenberger JS. Inhalation delivery of manganese superoxide dismutase-plasmid/liposomes (MnSOD-PL) protects the murine lung from irradiation damage. Gene Ther. 2005;12:685–690. doi: 10.1038/sj.gt.3302468. [DOI] [PubMed] [Google Scholar]
- 12.Burdelva LG, Krivokrvsenko VI, Tallant TL, Strom E, Gleiberman AV, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Didonato JA, Feinstein E, Gudkov AV. An agonist of toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 14.Le Q-T. Identifying and targeting hypoxia in head and neck cancer: A brief overview of current approaches. Int J Radiation Oncology Biol Phys. 2007;69(2 Supplement):S56–S58. doi: 10.1016/j.ijrobp.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anscher MS, Kong FM, Andrews K, Clough R, Marks LB, Bentel G, Jirtle RL. Plasma TFG,1 as a predictor of radiation pneumonitis. I J R O B P. 1998;41:1029–1035. doi: 10.1016/s0360-3016(98)00154-0. [DOI] [PubMed] [Google Scholar]
- 16.Mothersill C, Seymour C. Review: Radiation-induced bystander effects: Past history and future directions. Rad Res. 2001;155:759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Bayir H, Fadeel B, Palladino MJ, Witasp E, Kurnikov IV, Tyurina YY, Tyurin VA, Amoscato AA, Jiang J, Kochanek PM, DeKosky ST, Greenberger JS, Shvedova AA, Kagan VE. Apoptotic interactions of cytochrome c: Redox flirting with anionic phospholipids within and outside of mitochondria. Biochimica et Biophysica Acta. 2006;1757:648–659. doi: 10.1016/j.bbabio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Kagan VE, Tyrina YY, Bayir H, Chu CT, Kapralov AA, Vlasova II, Belikova NA, Tyurin VA, Amoscato A, Epperly M, Greenberger J, Dekosky S, Shvedova AA, Jiang J. The “pro-apoptotic genies” get out of mitochondria: Oxidative lipidomics and redox activity of cytochrome c/cardiolipin complexes. Chem Biol Interact. 2006;163:15–28. doi: 10.1016/j.cbi.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Safwat A, Bentzen SM, Turesson I, Hendry JH. Deterministic rather than stochastic factors explain most of the variation in the expression of skin telangiectasia after radiotherapy. Int J Radiat Oncol Biol Phys. 2002;52:198–204. doi: 10.1016/s0360-3016(01)02690-6. [DOI] [PubMed] [Google Scholar]
- 20.Rubin P, Johnston CJ, Williams JP, McDonald S, Finkelstein JN. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Bio Phys. 1995;33:99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 21.Mendonca MS, Mayhugh BM, McDowell B, Chin-Sinex H, Smith ML, Dynlacht JR, Spandau DF, Lewis DA. A radiation-induced acute apoptosis involving TP53 and BAX precedes the delayed apoptosis and neoplastic transformation of CGL1 human hybrid cells. Rad Res. 2005;163:614–622. doi: 10.1667/rr3387. [DOI] [PubMed] [Google Scholar]
- 22.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nature Reviews: Mol Cell Bio. 2007;8:741–748. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 23.Niu Y, Epperly MW, Shen H, Smith T, Wang H, Greenberger JS. Intraesophageal MnSOD-plasmid liposome administration enhances engraftment and self-renewal capacity of bone marrow derived progenitors of esophageal squamous epithelium. Gene Therapy. 2008;15:347–356. doi: 10.1038/sj.gt.3303089. [DOI] [PubMed] [Google Scholar]
- 24.Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TL. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 25.Li X-M, Hu Z, Jorgenson ML, Wingard JR, Slayton WB. Bone marrow sinusoidal endothelial cells undergo nonapoptotic cell death and are replaced by proliferating sinusoidal cells in situ to maintain the vascular niche following lethal irradiation. Exper Hematol. 2008;36:1143–1156. doi: 10.1016/j.exphem.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 26.Ricardo SD, van Goor H, Hedí AA. Macrophage diversity in renal injury and repair. J Clin Inves. 2008;118:3522–3529. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belkacemi Y, Labopin M, Hennequin C, Hoffstetter S, Mungai R, Wygoda M, Lundell M, Finke J, Aktinson C, Lorchel F, Durdux C, Basara N. Reduced-intensity conditioning regimen using low-dose total body irradiation before allogeneic transplant for hematologic malignancies: Experience from the European group for blood and marrow transplantation. Int J Rad Oncol Biol Phys. 2007;67:544–551. doi: 10.1016/j.ijrobp.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 28.Wong JYC, Liu A, Schultheiss T, Popplewell L, Stein A, Rosenthal J, Essensten M, Forman S, Somlo G. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: An alternative to standard total body irradiation. Biol Blood Marrow Transplant. 2006;12:306–315. doi: 10.1016/j.bbmt.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 29.Sorror ML, Storer BE, Maloney DG, Sandmaier BM, Martin PJ, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–452. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotblat J, Lindop P. Long-term effects of a single whole-body exposure of mice to ionizing radiations. II. Causes of death. Proc R Soc Lond. 1961;154:350–368. [Google Scholar]
- 31.Haupt R, Fears TR, Robison LL, Mills JL, Nicholson HS, Zeltzer LK, Meadows AT, Byrne J. Educational attainment in long-term survivors of childhood acute lymphoblastic leukemia. JAMA. 1994;272:1427–1432. [PubMed] [Google Scholar]
- 32.Van Dongen-Melman JEWM, De Groot A, Van Dongen JJM, Verhulst FC, Hahlen K. Cranial irradiation is the major cause of learning problems in children treated for leukemia and lymphoma: A comparative study. Leukemia. 1997;11:1197–1200. doi: 10.1038/sj.leu.2400702. [DOI] [PubMed] [Google Scholar]
- 33.Laack NN, Brown PD, Ivnik RJ, Furth AF, Ballman KV, Hammack JE, Anusell RM, Shaw EG, Buckner JC. Cognitive function after radiotherapy for supratentorial low-grade glioma: A north central cancer treatment group prospective study. Int J Rad Oncol Biol Phys. 2005;63(4):1175–1183. doi: 10.1016/j.ijrobp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epperly MW, Dixon T, Wang H, Schlesselman J, Franicola D, Greenberger JS. Modulation of radiation-induced life shortening by systemic intravenous MnSOD-plasmid liposome gene therapy. Rad Res. 2008;170:437–443. doi: 10.1667/rr1286.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J, Zheng H, Ou X, Fink LM, Hauer-Jensen M. Deficiency of microvascular thrombomodulin and upregulation of protease-activated receptor-1 in irradiated rat intestine. Possible link between endothelial dysfunction and chronic radiation fibrosis. Am J Pathol. 2002;160:2063–2070. doi: 10.1016/S0002-9440(10)61156-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epperly MW, Sikora CA, Defilippi S, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Resp Mol Cell Biol. 2003;29 :213–224. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 38.Flanders KC, Sullivan CD, Fujii M, Sowers A, Anzano MA, Arabshahi A, Major C, Deng C, Russo A, Mitchell JB, Roberts AB. Mice lacking Smad3 are protected against cutaneous injury induced by ionizing radiation. Am J Pathol. 2002;160:1057–1068. doi: 10.1016/S0002-9440(10)64926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anzano MA, Mitchell JB, Russo A, Roberts AB. Interference with transforming growth factor-beta/Smad3 signaling results in accelerated healing of wounds in previously irradiated skin. Am J Pathol. 2003;163:2247–2257. doi: 10.1016/s0002-9440(10)63582-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dileto CL, Travis EL. Fibroblast radiosensitivity in vitro and lung fibrosis in vivo: Comparison between a fibrosis-prone and fibrosis-resistant mouse strain. Rad Res. 1996;146:61–67. [PubMed] [Google Scholar]
- 41.Kanai A, Epperly M, Pearce L, Binder L, Zeidel M, Meyers S, Greenberger J, deGroat W, Apodaca G, Peterson J. Differing roles of mitochondrial nitric oxide synthase in cardiomyocytes and urothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H13–H21. doi: 10.1152/ajpheart.00737.2003. [DOI] [PubMed] [Google Scholar]
- 42.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Rad Onco. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Current Pharm Design. 2007;13 :1317–1325. doi: 10.2174/138161207780618821. [DOI] [PubMed] [Google Scholar]
- 44.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, Juckett MB, Moulder JC. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Rad Oncol Biol Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muramoto GG, Chen B, Cui X, Chao NJ, Chute JP. Vascular endothelial cells produce soluble factors that mediate the recovery of human hematopoietic stem cells after radiation injury. Biol Blood Marrow Transplant. 2006;12:530–540. doi: 10.1016/j.bbmt.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 46.Chute JP, Muramoto GG, Salter AB, Meadows SK, Rickman DW, Chen B, Himburg HA, Chao NJ. Transplantation of vascular endothelial cells mediates the hematopoietic recovery and survival of lethally irradiated mice. Blood. 2007;109:2365–2372. doi: 10.1182/blood-2006-05-022640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 48.Gardai SJ, Bratton DL, Ogden CA, Henson PM. Recognition ligands on apoptotic cells: a perspective. J Leukoc Bio. 2006;79:896–903. doi: 10.1189/jlb.1005550. [DOI] [PubMed] [Google Scholar]
- 49.Seed TM, Kaspar LV. Probing altered hematopoietic progenitor cells of preleukemic dogs with JANUS fission neutrons. Rad Res. 1991;128:S81–S86. [PubMed] [Google Scholar]
- 50.Seed TM, Kaspar LV, Grdina DJ. Hematopoietic repair modifications during preclinical phases of chronic radiation-induced myeloproliferative disease. Exp Hematol. 1986;14 :433. [Google Scholar]
- 51.Seed TM, Kaspar LV. Changing patterns of radiosensitivity of hematopoietic progenitors from chronically irradiated dogs prone either to aplastic anemia or to myeloproliferative disease. Leukemia Res. 1990;14:299–307. doi: 10.1016/0145-2126(90)90156-4. [DOI] [PubMed] [Google Scholar]
- 52.Seed TM, Kaspar LV, Tolle DV, Fritz TE. Chronic radiation leukemogenesis : Postnatal hematopathologic effects resulting from in-utero irradiation. Leukemia Res. 1987;11 :171–179. doi: 10.1016/0145-2126(87)90023-3. [DOI] [PubMed] [Google Scholar]
- 53.Seed TM, Carnes BA, Tolle DV, Fritz TE. Blood responses under chronic low daily dose gamma irradiation: I. Differential preclinical responses of irradiated male dogs in progression to either aplastic anemia or myeloproliferative disease. Leukemia Res. 1989;13 :1069–1084. doi: 10.1016/0145-2126(89)90152-5. [DOI] [PubMed] [Google Scholar]
- 54.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Guang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. I J R O B P. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 55.Greenberger JS, Epperly MW. Pleiotrophic stem cell and tissue effects of ionizing irradiation protection by MnSOD-plasmid liposome gene therapy. In: Redberry GW, editor. Gene Therapy in Cancer. Nova Science Publishers, Inc; 2005. pp. 191–215. [Google Scholar]
- 56.Gorbunov NV, Poque-Geile KL, Epperly MW, Bigbee WL, Draviam R, Dav BW, Wald N, Watkins SC, Greenberger JS. Activation of the nitric oxide synthase 2 pathway in the response of bone marrow stromal cells to high doses of ionizing radiation. Rad Res. 2000;154:73–86. doi: 10.1667/0033-7587(2000)154[0073:aotnos]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 57.Constine LS, Tarbell N, Hudson MM, Schwartz C, Fisher SG, Muhs AG, Basu SK, Kun LE, Ng A, Mauch P, Sandhu A, Culakova E, Lyman G, Mendenhall N. Subsequent malignancies in children treated for Hodgkin’s disease: Associations with gender and radiation dose. Int J Rad Onc Biol Phys. 2008;72:24–33. doi: 10.1016/j.ijrobp.2008.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romanik EA, Leif J, Sakakeeny MA, Greenberger JS. Sequence analysis of mutational hot spots in the endogenous and transfected CSF-1 receptor in gamma-irradiation induced factor-independent subclones of clonal hematopoietic progenitor cell lines. Radiat Oncol Invest: Clin Bas Res. 1993;1:94–102. [Google Scholar]
- 59.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, Dynan WS, Fike JR, Grdina DJ, Greenberger JS, Hauer-Jensen M, Hill RP, Kolesnick RN, Macvittie TJ, Marks C, McBride WH, Metting N, Pellmar T, Purucker M, Robbins ME, Schiesti RH, Seed TM, Tomaszewski JE, Travis EL, Wallner PE, Wolpert M, Zaharevitz D. Models for evaluating agents intended for the prophylaxis, mitigation, and treatment of radiation injuries. Report of an NCI Workshop, Dec. 3–4, 2003. Rad Res. 2004;162:711–718. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 60.Morgan JL, Holcomb TM, Morrissey RW. Radiation reaction in ataxia telangiectasia. Am J Dis Child. 1968;116:557–558. doi: 10.1001/archpedi.1968.02100020561022. [DOI] [PubMed] [Google Scholar]
- 61.Ho AY, Atencio DP, Peters S, Stock RG, Formenti SC, Cesaretti JA, Green S, Haffty B, Drumea K, Leitzin L, Kuten A, Azria D, Ozsahin M, Overgaard J, Andreassen CN, Trop CS, Park J, Rosenstein BS. Genetic predictors of adverse radiotherapy effects: The Gene-PARE project. Int J Radiat Oncol Biol Phys. 2006;65:646–655. doi: 10.1016/j.ijrobp.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 62.Shiloh Y. ATM and related protein kinases: Safeguarding genome integrity. Nat Rev Cancer. 2003;3:155–168. doi: 10.1038/nrc1011. [DOI] [PubMed] [Google Scholar]
- 63.Abraham RT. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001;15:2177–2196. doi: 10.1101/gad.914401. [DOI] [PubMed] [Google Scholar]
- 64.Casado JA, Nunez MI, Segovia JC, Ruiz de Almodóvar JM, Bueren JA. Non-homologous end-joining defect in fanconi anemia hematopoietic cells exposed to ionizing radiation. Rad Res. 2005;164:635–641. doi: 10.1667/rr3395.1. [DOI] [PubMed] [Google Scholar]
- 65.Taniguchi T, Garcia-Hiquera I, Xu B, Andreassen PR, Gregory RC, Kim ST, Lane WS, Kastan MB, D’Andrea AD. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109:459–472. doi: 10.1016/s0092-8674(02)00747-x. [DOI] [PubMed] [Google Scholar]
- 66.Marcou Y, D’Andrea A, Jeggo PA, Plowman PN. Normal cellular radiosensitivity in an adult Fanconi anemia patient with marked clinical radiosensitivity. Radiother Oncol. 2001;60 :75–79. doi: 10.1016/s0167-8140(01)00370-x. [DOI] [PubMed] [Google Scholar]
- 67.Imamura O, Fujita K, Itoh C, Takeda S, Furuichi Y, Matsumoto T. Werner and Bloom helicases are involved in DNA repair in a complementary fashion. Oncogene. 2002;21 :954–963. doi: 10.1038/sj.onc.1205143. [DOI] [PubMed] [Google Scholar]
- 68.Imamura O, Campbell JL. The human Bloom syndrome gene suppresses the DNA replication and repair defects of yeast dna2 mutants. Proc Natl Acad Sci USA. 2003;100:8193–8198. doi: 10.1073/pnas.1431624100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qiu Wei C-W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell, Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Greenberger JS, Epperly MW, Gretton J, Jefferson M, Nie S, Bernarding M, Kagan V, Guo HL. Radioprotective gene therapy. Curr Gene Ther. 2003;3:183–195. doi: 10.2174/1566523034578384. [DOI] [PubMed] [Google Scholar]
- 71.Greenberger JS, Epperly MW. Radioprotective antioxidant gene therapy: Potential mechanisms of action. Gene Ther Mol Biol. 2004;8:31–44. [Google Scholar]
- 72.Bennett CB, Lewis LK, Karthikeyan G, Lobachev KS, Jin YH, Sterling JF, Snipe JR, Resnick MA. Genes required for ionizing radiation resistance in yeast. Nat Genet. 2001;29 :426–434. doi: 10.1038/ng778. [DOI] [PubMed] [Google Scholar]
- 73.Lin Z, Nei M, Ma H. The origins and early evolution of DNA mismatch repair genes-multiple horizontal gene transfers and co-evolution. Nucleic Acids Res. 2007;35:7591–7603. doi: 10.1093/nar/gkm921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cox MC, Battista JR. Deinococcus radiodurans the consummate survivor. Nature Reviews: Micro. 2005;3:882–892. doi: 10.1038/nrmicro1264. [DOI] [PubMed] [Google Scholar]
- 75.Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, Hess M, Omeichenko MV, Kostandarithes HM, Makarova KS, Wackett LP, Fredrickson JK, Ghosal D. Accumulation of Mn(II) in deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306:1025–1030. doi: 10.1126/science.1103185. [DOI] [PubMed] [Google Scholar]
- 76.White O, Eisen JA, Heidelberg JF, Hickey EK, Peterson JD, Dodson RJ, Haft DH, Gwinn ML, Nelson WC, Richardson DL, Moffat KS, Qin H, Jiang L, Pamphile W, Crosby M, Shen M, Vamathevan JJ, Lam P, McDonald L, Utterback T, Zalewski C, Makarova KS, Aravind L, Daly MJ, Minton KW, Fleischmann RD, Ketchum KA, Nelson KE, Salzberg S, Smith HO, Venter JC, Fraser CM. Genome sequence of the radioresistant bacterium deinococcus radiodurans R1. Science. 1999;286:1571–1576. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wispe JR, Warner BB, Clark JC, Dey CR, Neuman J, Glasser SW, Crapo JD, Chang LY, Whitsett JA. Human MnSOD in pulmonary epithelial cells of transgenic mice confers protection from oxygen injury. J Biol Chem. 1992;267:23937–23941. [PubMed] [Google Scholar]
- 78.Zhang X, Epperly MW, Kay MA, Cheng ZY, Dixon T, Franicola D, Greenberger BA, Komanduri P, Greenberger JS. Radioprotection in vitro and in vivo by minicircle plasmid carrying the human manganese superoxide dismutase transgene. Hum Gene Ther. 2008;19:820–826. doi: 10.1089/hum.2007.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Batinic-Haberle I, Vujaskovic Z. Comparison of two Mn porphyrin-based mimics of superoxide dismutase in pulmonary radioprotection. Free Radic Biol Med. 2007;44:982–989. doi: 10.1016/j.freeradbiomed.2007.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fink M, Macias CA, Xiao J, Tyurina YY, Delude RL, Greenberger JS, Kagan VE, Wipf P. Hemigramicidin-TEMPO conjugates: Novel mitochondria-targeted antioxidants. Crit Care Med. 2007;35:5461–5470. doi: 10.1097/01.CCM.0000279192.96303.E7. [DOI] [PubMed] [Google Scholar]
- 81.Jiang J, Belikova NA, Hoye AT, Zhao Q, Epperly MW, Greenberger JS, Wipf P, Kagan VE. A mitochondria-targeted nitroxide/hemi-gramicidin S conjugate protects mouse embryonic cells against γ-irradiation. I J R O B P. 2008;70:816–825. doi: 10.1016/j.ijrobp.2007.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amstad P, Peskin A, Shah G, Mirault ME, Moret R, Zbinden I, Cerutti P. The balance between Cu, Zn-superoxide dismutase and catalase affects the sensitivity of mouse epidermal cells to oxidative stress. Biochemistry. 1991;30:9305–9313. doi: 10.1021/bi00102a024. [DOI] [PubMed] [Google Scholar]
- 83.Rix-Montel MA. Biophysical aspects of radioprotectors by aminothiols. Engineering in Medicine and Biology Society. 1988;2:1032–1033. [Google Scholar]
- 84.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed radioprotection by nuclear transcription factor kB-mediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to free radical scavenger WR1065. Free Radical Biol Med. 2006;40:1004–1016. doi: 10.1016/j.freeradbiomed.2005.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Herceg Z, Milosvic Z, Kljajic R, Radnic Z. The effects of use of radioprotective compound WR-2721 on haematological parameters in lethally irradiated swines. YRPA, Proceedings of the 3rd Italian-Yugoslav Symposium on Radiation Protection; Plitvice, Yugloslavia. 1990. pp. 30–35. [Google Scholar]
- 86.Epperly MW, Sikora CA, DeFilippi SJ, Gretton JA, Zhan Q, Kufe DW, Greenberger JS. MnSOD inhibits irradiation-induced apoptosis by stabilization of the mitochondrial membrane against the effects of SAP kinases p38 and Jnk1 translocation. Rad Res. 2002;157:568–577. doi: 10.1667/0033-7587(2002)157[0568:msdsir]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 87.Saunders LR, Verdin E. Sirtuins: critical regulators at the crossroads between cancer and aging. Oncogene. 2007;26:5489–5504. doi: 10.1038/sj.onc.1210616. [DOI] [PubMed] [Google Scholar]
- 88.Rowinsky EK. Targeted induction of apoptosis in cancer management: The emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J Clin Oncol. 2005;23:9394–9407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 89.Grdina DJ, Murley JS, Kataoka Y, Calvin DP. Differential activation of nuclear transcription factor KB, gene expression, and proteins by amifostine’s free thiol in human microvascular endothelial and glioma cells. Semin Radiat Oncol. 2002;12:103–111. doi: 10.1053/srao.2002.31383. [DOI] [PubMed] [Google Scholar]
- 90.Baumann M, Krause M, Hill R. Exploring the role of cancer stem cells in radioresistance. Nature Reviews Cancer. 2008;8:545–552. doi: 10.1038/nrc2419. [DOI] [PubMed] [Google Scholar]
- 91.Oberley LW, Buettner GR. Role of superoxide dismutase in cancer: A review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 92.Oberley LW. Superoxide dismutase and cancer. In: Oberley LW, editor. Superoxide Dismutase. II, Chapter 6. CRC Press; 1982. [Google Scholar]
- 93.Spitz DR, Elwell JH, Sun Y, Oberley LW, Oberley TD, Sullivan SJ, Roberts RJ. Oxygen toxicity in control and H2O2-resistant Chinese hamster fibroblasts. Arch Biochem Biophys. 1990;279:249–260. doi: 10.1016/0003-9861(90)90489-l. [DOI] [PubMed] [Google Scholar]
- 94.Zhong W, Oberley LW, Oberley TD, St Clair DK. Suppression of the malignant phenotype of human glioma cells by overexpression of manganese superoxide dismutase. Oncogene. 1997;14:481–490. doi: 10.1038/sj.onc.1200852. [DOI] [PubMed] [Google Scholar]
- 95.Zhong W, Oberley LW, Oberley TD, Yan T, Domann FE, St Clair DK. Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Diff. 1996;7:1175–1186. [PubMed] [Google Scholar]
- 96.Yan T, Oberley LW, Zhong W, St Clair DK. Manganese-containing superoxide dismutase overexpression causes phenotypic reversion in SV40-transformed human lung fibroblasts. Cancer Res. 1996;56:2864–2871. [PubMed] [Google Scholar]
- 97.St Clair DK, Wan XS, Oberley TD, Muse KE, St Clair WH. Suppression of radiation-induced neoplastic transformation by overexpression of mitochondrial superoxide dismutase. Mol Carcino. 1992;6:238–242. doi: 10.1002/mc.2940060404. [DOI] [PubMed] [Google Scholar]
- 98.Evans JW, Chernikova SB, Kachnic LA, Banath JP, Sordet O, Delahoussaye YM, Treszezansky A, Chon BH, Feng Z, Gu Y, Wilson WR, Pommier Y, Olive PL, Powell SN, Brown JM. Homologous recombination is the principal pathway for the repair of DNA damage induced by tirapazamine in mammalian cells. Cancer Res. 2008;68:257–265. doi: 10.1158/0008-5472.CAN-06-4497. [DOI] [PubMed] [Google Scholar]
- 99.Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfrov B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science. 2000;289:1567–1569. doi: 10.1126/science.289.5484.1567. [DOI] [PubMed] [Google Scholar]
- 100.Epperly MW, Epperly L, Zhang X, Franicola D, Greenberger JS. Overexpression of MnSOD transgene product protects cryopreserved bone marrow hematopoietic progenitor cells from ionizing irradiation. Rad Res. 2007;168:560–566. doi: 10.1667/RR1071R.1. [DOI] [PubMed] [Google Scholar]
- 101.Ghorbani M, Mozdarani H. In vitro radioprotective effects of histamine H2 receptor antagonists against gamma-rays induced chromosomal aberrations in human lymphocytes. Iran J Rad Res. 2003;1:99–104. [Google Scholar]
- 102.Kalechman Y, Albeck M, Oron M, Sobelman D, Gurwith M, Seghal SN, Sredni B. Radioprotective effects of the immunomodulator AS101. J Immunol. 1990;145:1512–1517. [PubMed] [Google Scholar]
- 103.Cermek M, Enginar H, Karaca T, Unak P. In vivo radioprotective effects of nigella sativa L oil and reduced glutathione against irradiation-induced oxidative injury and number of peripheral blood lymphocytes in rats. Photochem Photobiol. 82:1691–1696. [PubMed] [Google Scholar]
- 104.Gu Y-H, Takagi Y, Nakamura T, Hasegawa T, Suzuki I, Oshima M, Tawarava H, Niwano Y. Enhancement of radioprotection and anti-tumor immunity by yeast-derived β-Glucan in mice. J Med Food. 2005;8:154–158. doi: 10.1089/jmf.2005.8.154. [DOI] [PubMed] [Google Scholar]
- 105.Arora R, Gupta D, Chawla R, Sagar R, Sharma A, Kumar R, Prasad J, Singh S, Samanta N, Sharma RK. Radioprotection by plant products: present status and future prospects. Phytother Res. 2005;19:1–22. doi: 10.1002/ptr.1605. [DOI] [PubMed] [Google Scholar]
- 106.Hanson WR, Houseman KA, Collins PW. Radiation protection in vivo by prostaglandins and related compounds of the arachidonic acid cascade. Pharmacol Ther. 1988;39 :347–356. doi: 10.1016/0163-7258(88)90082-4. [DOI] [PubMed] [Google Scholar]
- 107.Chawla R, Arora R, Singh S, Sagar RK, Kumar R, Sharma A, Tripathi RP, Puri SC, Khan HA, Shawl AS, Sultan P, Krishan T, Oazi GN. Podophyllum hexandrum offers radioprotection by modulating free radical flux: role of aryl-tetralin lignans. Evid Based Complement Alternet Med. 2006;3:503–511. doi: 10.1093/ecam/nel037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Srinivasan V, Weiss JF. Radioprotection by vitamin E: injectable vitamin E administered alone or with WR-3689 enhances survival of irradiated mice. Int J Radiat Oncol Biol Phys. 1992;23:841–845. doi: 10.1016/0360-3016(92)90657-4. [DOI] [PubMed] [Google Scholar]
- 109.El-Nahas SM, Mattar FE, Mohamed AA. Radioprotective effect of vitamins C and E. Mutat Res. 1993;301:143–147. doi: 10.1016/0165-7992(93)90037-v. [DOI] [PubMed] [Google Scholar]
- 110.Neta RN, Duches SD, Oppenheim JJ. Interleukin-1 as a radioprotector. J Immunol. 1986;136:2483–2485. [PubMed] [Google Scholar]
- 111.Zsebo KM, Smith KA, Harley CA, Greenblatt M, Cooke K, Rich W, McNiece IK. Radioprotection of mice by recombinant rat stem cell factor. Proc Natl Acad Sci USA. 1992;89 :9464–9468. doi: 10.1073/pnas.89.20.9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MacVittie TJ, Monroy RL, Patchen ML, Souza LM. Therapeutic use of recombinant human G-CSF in a canine model of sublethal and lethal whole-body irradiation. Int J Radial Biol. 1990;57:723–736. doi: 10.1080/09553009014550891. [DOI] [PubMed] [Google Scholar]
- 113.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors and AII type-1 and type-2 receptor antagonists. Current Pharm Design. 2007;13 :1317–1325. doi: 10.2174/138161207780618821. [DOI] [PubMed] [Google Scholar]
- 114.Cohen EP, Irving AA, Drobyski WR, Klein JP, Passweg J, Talano JA, Juckett MB, Moulder JE. Captopril to mitigate chronic renal failure after hematopoietic stem cell transplantation: a randomized controlled trial. Int J Rad Oncol Biol Phys. 2008;70:1546–1551. doi: 10.1016/j.ijrobp.2007.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Rad Oncol. 2007;17:141–148. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 116.Rube CE, Wilfert F, Uthe D, Schmid KW, Knoop R, Willich N, Schuck A, Rube C. Modulation of radiation-induced tumour necrosis factor α (TNF-α) expression in the lung tissue by pentoxifylline. Radiother Oncol. 2002;64:177–187. doi: 10.1016/s0167-8140(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 117.Lefaix J-L, Delanian S, Vozenin MC, Leplat JJ, Tricaud Y, Martin M. Striking regression of subcutaneous fibrosis induced by high doses of gamma rays using a combination of pentoxifylline and a-tocopherol : an experimental study. Int J Radiat Oncol Biol Phys. 1999;43:839–847. doi: 10.1016/s0360-3016(98)00419-2. [DOI] [PubMed] [Google Scholar]
- 118.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p43 pathway. Nature. 2007;448:375–381. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 119.Kaeberlein M, Powers RW, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1197. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- 120.Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin SJ, Sinclair DA. HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 2005;309:1861–1864. doi: 10.1126/science.1113611. [DOI] [PubMed] [Google Scholar]
- 121.Zhou P-K, Sproston AR, Marples B, West CM, Margison GP, Hendry JH. The radiosensitivity of human fibroblast cell lines correlates with residual levels of DNA double-strand breaks. Radiother Oncol. 1997;47:271–276. doi: 10.1016/s0167-8140(97)00200-4. [DOI] [PubMed] [Google Scholar]
- 122.Brock WA, Tucker SL, Geara FB, Turesson I, Wike J, Nyman J, Peters LJ. Fibroblast radiosensitivity versus acute and late normal skin responses in patients treated for breast cancer. Int J Radiation Oncology Biol Phys. 1995;32:1371–1379. doi: 10.1016/0360-3016(95)00068-A. [DOI] [PubMed] [Google Scholar]
- 123.Morita Y, Perez GI, Paris F, Miranda SR, Ehleiter D, Haimovitz-Friedman A, Fuks Z, Xie Z, Reed JC, Schuchman EH, Kolesnick RN, Tilly JL. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6:1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]