Abstract
Background
Deficit syndrome (DS) schizophrenia patients have smooth pursuit eye movement (SPEM) dysfunction. We examined if they also had smell identification deficits, since social affiliation is related to olfaction in other mammals.
Methods
Sixty-seven patients had DS assessments: 31 patients had SPEM and 50 had Smell Identification Test (SIT) assessments, and 14 patients had both measurements.
Results
DS patients had worse SPEM and SIT performance than the non-DS patients. Areas under the receiver–operator characteristic (ROC) curves for SIT and SPEM were both fairly accurate in identifying the DS. The odds ratio (OR) for the DS for impaired versus normal SPEM was 6.21 (95% confidence interval [CI]: 1.21, 32.25) and for microsmia versus normosmia was 10.4 (95% CI: 1.23, 88.18). Further analyses showed that the association of SIT with both SPEM and the DS could account for the SPEM-DS association.
Conclusions
We found a strong association between the DS and SIT scores suggesting that the neural substrates of olfaction may be related to social affiliation in humans, as they are in other mammals. These data further support the notion that the DS defines a homogeneous subgroup of schizophrenia patients and further suggest that dysfunction in the neural circuitry of olfaction may contribute to its pathophysiology.
Keywords: Schizophrenia, negative symptoms, social function, deficit syndrome, odor identification, eye tracking
Introduction
Positive symptoms in schizophrenia patients tend to fluctuate in magnitude and severity over time, but a subgroup of patients have enduring negative symptoms that persist during remission and that are not secondary to positive symptoms, medication side effects, depression, or environmental deprivation. These patients have been designated as having the deficit syndrome (DS). As reviewed by Carpenter et al (1999), the deficit syndrome DS can be reliably assessed, has construct validity, and is related to course and biological measures; it also shows long-term reliability within patients (Amador et al 1999). Key features of this syndrome reflect impairments in social affiliation, such as diminished expression of emotions through affect and language and a low motivation or potential for social interactions. Many clinical correlates of the DS are also related to social performance, including greater impairments in premorbid functioning and a lower degree of social and occupational achievement in comparison with other schizophrenia patients (Bottlender et al 1999; Fenton and McGlashan 1994; Kirkpatrick et al 1996).
Because deficit symptoms are persistent traitlike features of a patient’s condition, they are assumed to reflect a primary neuropathology. The finding that the DS was associated with disrupted smooth pursuit eye movements (SPEM; Ross et al 1996, 1997) prompted the idea that SPEM and the DS of schizophrenia may share a common underlying pathophysiology. Nonetheless, the neurobiological basis for an association between oculomotor performance and social skills is not evident. Although this does not preclude a shared neurobiology for the DS and SPEM, the association is enigmatic.
Impairments in odor identification are also consistently reported in schizophrenia patients and have been associated with both negative symptoms and social behavioral dysfunction (Brewer et al 1996). These deficits are not a consequence of impaired odor detection and are presumed to reflect central olfactory circuitry dysfunction, distinct from the olfactory incapacity that can result from external trauma (which damages the nerves passing through the cribiform plate) or congestion (which impairs the odorants passage through the nasal mucosa). A connection between the DS and olfactory errors would be consistent with animal studies that relate the neurobiology of olfactory processing to the capacity for social affiliation. Indeed, olfactory stimuli are related to behavioral responses among conspecific animals in a wide array of species. A decade ago, Kirkpatrick and Buchanan (1990) hypothesized, upon review of ethologic studies, that DS patients might have an abnormality in the brain circuitry subserving social affiliation. Although they did not discuss olfactory function in their article, it is of note that the regions they proposed to underlie social drive also participate in olfactory processing, particularly the orbitofrontal cortex and amygdala. In addition to facilitating reciprocal social interactions, olfactory neurocircuitry also underlies motivated behavior in many mammals. Olfactory lesions in social rodents are known to decrease sexual, parental, and other social behaviors (Beauchamp et al 1977; Kirkpatrick et al 1994), and olfactory bulbectomy has been used as an animal model of depression; however, the behavioral profile of bulbectomized rodents could be viewed as corresponding to the DS as well.
Humans clearly employ visual and auditory information for socialization and may be only minimally dependent on olfactory cues in comparison with other mammals. Overall, research on the significance of olfactory communication among humans is limited. It has been shown that human infants identify their mothers through smell, and that human menstrual synchrony, which is related to social factors among women who share proximity (Weller and Weller 1995), is modulated by a direct olfactory mechanism. But even if olfaction plays only a minimal role in human socialization, the phylogenetically primitive limbic neural circuitry that initially evolved to associate olfactory cues with food, mating, and danger may continue to be central to the function of the later evolving higher cortical centers that modulate social interactions.
It thus seemed reasonable to propose that olfactory identification deficits and social functioning might be associated in schizophrenia. We investigated odor identification and the DS in schizophrenia patients, also examining whether or not we could replicate earlier reports that had associated SPEM and the DS.
Methods and Materials
The study sample included 67 patients diagnosed with DSM III-R schizophrenia from an inpatient schizophrenia research unit who were characterized as to the presence of the DS. All subjects provided informed consent for this institutional review board–approved study. The patients were physically healthy, as indicated by recent physical examinations, laboratory evaluation of complete blood chemistry profile, complete blood count, urinalysis, and thyroid function tests. Diagnosis was made considering data from the Diagnostic Interview for Genetic Studies (Nurnberger et al 1994), past records, and symptom ratings, and reflected an agreement between clinical and research staff. Demographic data included age, gender, education, and the age of onset of positive symptoms.
We assessed the DS with the Schedule for the Deficit Syndrome (SDS; Kirkpatrick et al 1989), a scale that uses historical data rather than cross-sectional data to assess negative symptoms. Information about the patient was obtained from patient and family interviews, chart review, and discussions with the clinical staff on restricted affect, diminished emotional range, poverty of speech, curbing of interests, diminished sense of purpose, and diminished social drive. The DS was rated as present if at least two of these negative symptoms were rated as both primary and stable illness features. All patients were evaluated by raters who were trained to reliability (kappa = .71). The raters of the DS were trained at the Maryland Psychiatric Research Center, where interrater reliability was established under the supervision of Brian Kirkpatrick, a developer of the SDS.
Odor identification was assessed using the University of Pennsylvania Smell Identification Test (SIT; Doty et al 1984), which we have previously found to be stable and reliably measured in schizophrenia patients (Malaspina et al 1994). It is a standardized, multiple-choice, scratch-and-sniff test consisting of four test booklets with 10 items each. Subjects scratched the scent-impregnated area and were asked to make a selection from one of four possible answers for each item. If the patient could not identify an odor and the examiner observed that the area was not sufficiently scratched, the examiner either rescratched the area or suggested that the patient do so until an odor was selected.
We assessed SPEM in patients with corrected monocular and binocular vision while they were receiving stable doses of typical antipsychotic medications (Amador et al 1995). Briefly, we evaluated SPEM through electrooculographic (EOG) recordings of eye movement quality. Subjects’ heads were positioned on a chin rest 57 cm from an AMDEK 310 visual monitor, and a Grass Model 7-D polygraph with an AC coupler and a 3-sec time constant was used. Electrode impedances were kept below 10 kohms, sensitivity was set at 15 mv/mm, and the band pass was 1.2 to 90 Hz with a 60-Hz notch filter. Miniature (9-mm) Ag/AgCl electrodes were placed bilaterally at the outer canthi to record horizontal movement (forehead ground). The target subtended 1.5° of visual arc, traversed a 20° horizontal visual angle, had a period of 2.5 sec (0.4 Hz), and a maximum velocity of 27.78°/sec. Electrodes placed above and below the left eye recorded eye blinks, and paper recordings were made of horizontal eye movements and blinks. To maximize performance, SPEM was performed while subjects monitored a nonvowel letter target that changed every 5 sec. Quality of SPEM was assessed by visual matching of the paper polygraph tracings (with blinks subtracted) to a 9-point scale, ranging from 1 to 5 in 0.5 intervals, with 1 representing the best tracking quality (Shagass et al 1976). Three trials of 30 sec each were recorded and the best tracing was used for the analysis.
Smooth pursuit eye movement scores ≥ 2.5 (where 1 was the best and 5 was the worst eye tracking quality) evidenced the high-frequency intrusions that have been associated with schizophrenia (Holzman et al 1976) and were considered to represent poor SPEM. University of Pennsylvania Smell Identification Test scores ≥ 33 were considered to reflect normal olfaction, normosmia; scores below 33 denoted impaired olfaction, microsmia, as defined by Doty et al (1994) from normative data. Evaluations of the SIT, SPEM, and DS were performed independently, without knowledge of the other measures reported herein or the aims of this analysis.
Data Analysis
Patient demographics were compared by t tests among the subgroups defined by DS, SIT performance, and SPEM. Even though smoking has not been associated with olfactory identification impairments in schizophrenia, the proportion (chi-square) of current smokers in the deficit and non-DS groups and the mean SIT scores of the smoking and nonsmoking patients were compared (t test). Associations among the DS, SIT, and SPEM were conducted using the continuous SIT and SPEM scores and by dichotomizing the patients into those with better and worse performance on the physiologic measures, as defined above. The relationship of SIT and SPEM to the individual DS items was also explored. The odds ratio and 95% confidence intervals for having the DS were calculated for abnormal SPEM and for microsmia, and receiver–operator characteristic (ROC) curves were generated to compare the measures in their ability to identify DS patients. The relationships between SPEM and SIT in the patient sample were examined, and finally, we used partial correlations to examine the interrelationships among the three variables: DS, SIT, and SPEM.
Results
The 67 patients included 44 men and 23 women of mean age 32.3 ± 8.9 years. We assessed SPEM in 31 patients (21 men and 10 women, mean age 31.5 ± 7.8 years) and SIT in 50 patients (33 men and 17 women, mean age 33.2 ± 9.2 years); 14 patients had both SPEM and SIT measurements. The subgroups with SPEM or SIT, or with both assessments, did not differ significantly from the entire group in age, gender, education, or age of onset. The patients with the DS (Table 1) (including the subsamples having SPEM and SIT assessments) had a longer duration of illness, but the DS groups did not otherwise differ in age, education, or the age of onset of psychosis. The longer duration of illness in the DS patients may be a consequence of their present age because both groups had similar ages of onset.
Table 1.
Demographic Measures for 21 Deficit Syndrome Patients and 46 Non Deficit Syndrome Patients
| Deficit syndrome | M (SD) years | Statistical test | |
|---|---|---|---|
| Age | Deficit | 34.2 (10.1) | t = 1.19, df = 65, p = .24 |
| Non deficit | 31.5 (8.3) | ||
| Education | Deficit | 11.4 (2.0) | t = −1.72, df = 65, p = .091 |
| Non deficit | 12.5 (2.6) | ||
| Age of onset of psychosis | Deficit | 17.4 (3.8) | t = −1.57 df = 50.3, p = .12 |
| Non deficit | 19.2 (5.3) | ||
| Age of onset of psychosis | Deficit | 17.3 (7.5) | t = 2.31, df = 65, p = .024 |
| Non deficit | 11.6 (7.3) |
A greater proportion of men than women had the DS: 19 of 44 (43.2%) versus 3 of 23 (13%): χ2 = 6.221, df = 1, p = .013). Similarly, a greater proportion of men than women had microsmia: 27 of 33 (81.8%) versus 9 of 17 (52.9%): χ2 = 4.64, df = 1, p = .031. In contrast, SPEM quality was unrelated to gender: 9 of 21 men (42.9%) had impaired SPEM versus 5 of 10 (50%) women (χ2 = .14, df = 1, p = .5). Similar numbers of patients from the deficit and non-DS groups were currently smoking cigarettes (8 of 25 vs. 2 of 10: χ2 = .51, df = 1, p = .47), although the frequency of daily smoking was decreased for all subjects because they were inpatients on a nonsmoking hospital unit. The SIT scores did not differ for the smoking and nonsmoking patients (31.2 ± 3.58 vs. 31.54 ± 4.25, t = .22, df = 32, p = .825).
The deficit group had significantly worse mean SPEM and SIT performance than nondeficit patients (Table 2). Conversely, categorizing the patients by better or worse SPEM or SIT scores also showed that those with greater SPEM or SIT impairments were more likely to have the DS: of those with impaired SPEM, 8 of 14 (57.1%) had the DS, compared with 3 of 17 (17.6%) of those with normal SPEM (χ2 = 5.23, df = 1, p = .022); of microsmic patients, the DS was present in 17 of 36 (47.2%), versus 1 of 14 (7.1%) of the normosmic patients (χ2 = 7.03, df = 1, p = .008).
Table 2.
Group Statistics
| Deficit syndrome |
n | M (SD) | 95% confidence interval of the difference between the groups |
Statistical test | |
|---|---|---|---|---|---|
| SPEM | Deficit | 11 | 3.68 (1.1) | .102, 1.76 | t = 2.30, df = 29 |
| Non deficit | 20 | 2.75 (1.1) | p = .029 | ||
| UPSIT | Deficit | 17 | 28.1 (4.8) | −6.67,−1.03 | t = −2.742, df = 48 |
| Non deficit | 33 | 31.9 (4.6) | p = .009 |
SPEM, smooth pursuit eye movement; UPSIT, University of Pennsylvania Smell Identification Test.
The OR for the DS for impaired versus normal SPEM was 6.21 (95% confidence interval [CI]: 1.21, 32.25); the OR for the DS for microsmia versus normosmia was 11.63 (95% CI: 1.37, 98.5). Receiver–operator characteristic curves showed that SPEM and SIT had similar attributes in discriminating the DS. Areas under the ROC curve for SIT scores (.73, 95% CI: .59, .87, SE = .07, p = .008) and for SPEM quality (.74, 95% CI: .56, .92, SE = .092, p = .032) indicated that both tests had a fair accuracy in categorizing patients based on the DS. Our SPEM cutoff score of < 2.5 for normal tracking showed a sensitivity of 73% and a specificity of 70%: increasing the sensitivity to 100% caused specificity to decline to 15%. Our SIT cutoff score of 33 had a sensitivity of 94% and a specificity of 41%: increasing the cutoff score to 34 resulted in a sensitivity of 100% and a specificity of 34%.
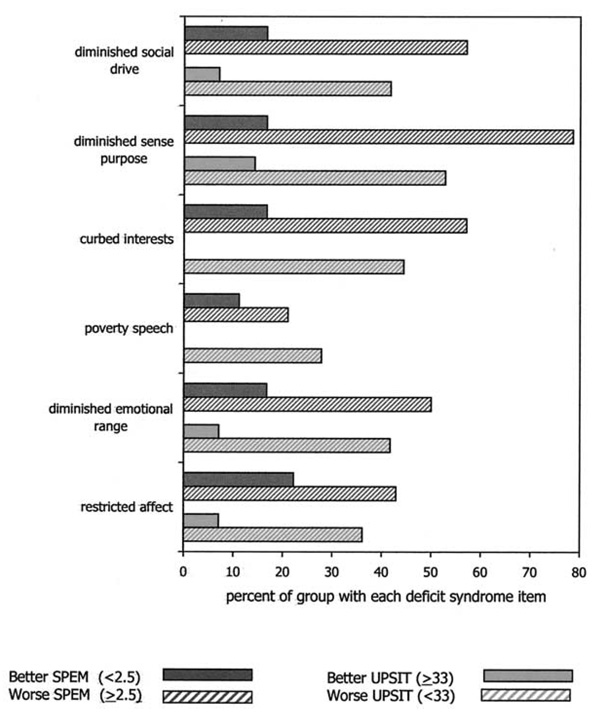
We also examined the proportion of patients in the groups with better and worse SPEM and the groups with better and worse SIT who met the criteria for the individual DS items. As depicted in Figure 1, those with impaired SPEM, compared with those with normal SPEM, were significantly more likely to have diminished emotional range, curbing of interests, diminished sense of purpose, and diminished social drive, but they were not significantly more likely to have a restricted affect or poverty of speech. Those with microsmia, compared with normosmia, were significantly more likely to have all of the DS symptoms.
Figure 1.
The proportion of subjects in whom the individual deficit syndrome symptoms were both primary and stable, with subjects dichotomized by better and worse performance on smooth pursuit eye movement (SPEM) and University of Pennsylvania Smell Identification Test (UPSIT). Chi-square group differences (all df = 1), Fisher’s Exact test for cells with 0. For SPEM (total n = 31): RA (1.56, p = .21), DER (4.07, p = .044), PS (.64, p = .43), CI (5.72, p = .017), P (12.26, p < .001), SD (5.72, p = .017). For UPSIT (total n = 50): RA (4.19, p = .041), DER (5.52, p = .019), PS (4.86, p = .027), CI (9.15, p = .002), S (6.13, p = .013), SD (5.52, p = .019).
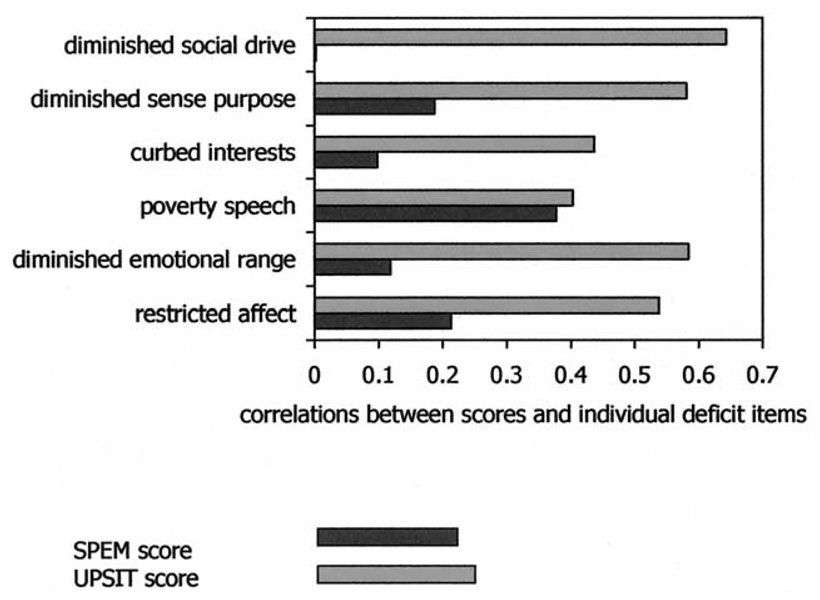
In addition to these categorical analyses, we also examined the absolute value of the correlation between the DS items and the continuous SIT scores and continuous SPEM quality ratings within the entire sample (Figure 2). These analyses showed that the actual magnitude of SPEM impairment only correlated significantly with poverty of speech (r = .377, n = 31, p = .036); in contrast, the magnitude of the SIT score deficits correlated significantly related with all of the DS symptoms except poverty of speech and curbing of interests (r’s > .54, n = 14, p’s < .047). The highest correlation for SIT performance was with diminished social drive (r = .64, n = 14, p = .013), which showed no relationship with SPEM quality whatsoever.
Figure 2.
Association of the deficit syndrome symptoms with the continuous smooth pursuit eye movement (SPEM) and University of Pennsylvania Smell Identification Test (UPSIT) scores (Pearson correlation coefficients).
The continuous SIT scores and SPEM quality scores were correlated (r = −.770, df = 12, p = .001), as were the dichotomous groupings of SPEM quality and SIT performance. Thus, SIT scores were significantly lower for those with abnormal versus normal SPEM (22.0 ± 4.3 vs. 31.9 ± 4.3: t = 3.88, df = 12, p = .002). Finally, we next used partial correlations to tease apart the relationships among SPEM, SIT, and the DS because the sample size was insufficient for multivariate statistical techniques. These analyses showed that SIT performance and DS continued to be significantly correlated after controlling for SPEM performance (r = .55, df = 11, p = .05) but that SPEM and the DS were no longer associated after controlling for SIT performance (r= −.17, df = 11, p = .56). Thus, the association of the DS and SPEM performance could be related to the association of both SPEM and the DS with olfactory identification performance.
Discussion
We found a robust relationship between SIT scores and the DS, suggesting that the neurocircuitry of olfaction, which is related to social affiliation in many species, might also be pertinent to the DS in schizophrenia. The quality of SPEM was also strongly related with the DS, as previously reported (Ross et al 1996, 1997), although our preliminary analyses on patients with both SPEM and SIT assessments suggest that SIT performance may have a more central relationship with the DS than SPEM. In any event, given the ease of administering and scoring the SIT and its shared properties with eye tracking quality in identifying the DS, odor identification may be a useful marker for the type of schizophrenia vulnerability that is associated with the DS.
The DS and microsmia were both more prevalent in the male patients, although there was no gender difference for eye tracking quality. Although cigarette smoking may cause small olfactory identification deficits in normal subjects, smoking has not been related to the olfactory deficits in schizophrenia (Brewer et al 2001; Houlihan 1994; Malaspina et al 1994; Moberg et al 1999). Instead, olfactory identification deficits may exist in schizophrenia because of dysfunction in central nervous system olfactory pathways (Malaspina et al 1998; Moberg et al 1999).
Central olfactory processing involves a distributed system with important connections to various brain centers implicated in the pathology of schizophrenia, particularly the prefrontal cortex and structures of the temporal lobe, limbic system, and thalamus. Olfactory information is unique among sensory modalities in that it may arrive directly in the prefrontal cortex, bypassing the thalamus altogether, enabling olfactory sensation to avoid influences from the reticular formation, which occur in the thalamus. Using the SIT as a neuroactivation task, we previously demonstrated frontal and medial temporal lobe hypometabolism for schizophrenia patients compared with control subjects (Malaspina et al 1998). Patients with the DS are likewise reported to show both frontal and medial lobe hypometabolism, whereas nondeficit patients show only the latter dysfunction (Gur et al 1995; Heckers et al 1999; Tamminga et al 1992). Thus, both microsmia and the DS could be a result of frontal hypofunction. Such dysfunction is also proposed to account for SPEM abnormalities in schizophrenia (Ross et al 1996, 1997). As described, it was the association of eye movements with the DS that prompted Ross et al to propose that dorsolateral prefrontal function might account for the DS, and our results provide no evidence to the contrary.
Frontal hypofunction could also be secondary to limbic system abnormalities, however. Deficits on the SIT could result from abnormalities in the olfactory bulb or other olfactory processing regions, such as the entorhinal area and its projections to the hippocampus or amygdala, which are reciprocally connected to higher level processing centers. Some postmortem schizophrenia studies have identified neuropathology in the entorhinal cortex and have found abnormal invaginations of the surface, disruption of cortical layers, displacement of neurons, and smaller entorhinal prealpha cell clusters (especially in men; Arnold et al 1991, 1995; Falkai et al 2000; Jakob and Beckmann 1986). A recent magnetic resonance imaging study also found reduced olfactory bulb volumes in schizophrenia patients (Turetsky et al 2000). These major structures for the relay and processing of olfactory information are also the convergence sites for higher level sensory information, which is intensely processed and then relayed to the hippocampus.
Among psychiatric disorders, odor identification deficits are relatively specific to schizophrenia-related psychoses (Moberg et al 1999; Stedman and Clair 1998), as they are not found in patients with major depression (Amsterdam et al 1987), bipolar disorder (Hurwitz et al 1988), or anorexia (Kopala et al 1995). Our findings suggest that the subgroup of schizophrenia patients with the DS overwhelmingly comprise the schizophrenia patients who are likely to have microsmia. These data shore up the concept that the DS comprises a homogeneous subgroup of schizophrenia patients, perhaps with a similar etiology.
The SIT might have utility as a marker in genetic studies if the DS and familial schizophrenia identify a similar group of patients (Dollfus et al 1998), although we found similar rates of the DS in familial and sporadic schizophrenia patents (Malaspina et al 2000); however, recent data suggests that de novo mutations might account for a significant proportion of sporadic schizophrenia (Malaspina et al 2001; Malaspina et al, in press). If these mutations are inherited by subsequent generations, the etiologic boundaries among familial and sporadic cases would be blurred. The older literature discerned a phenotype consistent with the DS in later born siblings, who are more likely to have older fathers. Last-born schizophrenia cases were found to have lower social competence, be less likely to recover, attain less education, be less likely to marry, and be more socially isolated than other patients of the same social class (Farina 1963; Schooler 1961, 1964). Genes accounting for the DS could arise in a proportion of sporadic cases and then be subsequently inherited by some familial cases. Then the etiopathophysiology of some sporadic and some familial cases would be the same, and the DS may be valuable at identifying this subtype.
Inasmuch as the DS is related to course and biological measures, our data suggest that SIT scores may have etiologic or prognostic implications for schizophrenia. These findings need to be replicated in a larger well-characterized patient sample, but dysfunction in the neural circuitry that evolved for olfactory processing might well be related to the deficit syndrome of schizophrenia. If so, then future studies should explore the association of odor identification, the deficit syndrome, and etiologic factors in the pathophysiology of schizophrenia.
Acknowledgments
This work was supported by the G. Harold and Leila Y. Mathers Foundation and Grant No. 1K24 MH01699 (DM) and by NIMH Grant No. 5P20 MH50727 (JG).
References
- Amador XF, Kirkpatrick B, Buchanan RW, Carpenter WT, Marcinko L, Yale SA. Stability of the diagnosis of deficit syndrome in schizophrenia. Am J Psychiatry. 1999;156:637–639. doi: 10.1176/ajp.156.4.637. [DOI] [PubMed] [Google Scholar]
- Amador XF, Malaspina D, Sackeim HA, Coleman EA, Kaufmann CA, Hasan A. Visual fixation and smooth pursuit eye movement abnormalities in patients with schizophrenia and their relatives. J Neuropsychiatry Clin Neurosci. 1995;7:197–206. doi: 10.1176/jnp.7.2.197. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Settle RG, Doty RL, Abelman E, Winokur A. Taste and smell perception in depression. Biol Psychiatry. 1987;22:1481–1485. doi: 10.1016/0006-3223(87)90108-9. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Franz BR, Gur RC, Gur RE, Shapiro RM, Moberg PJ. Smaller neuron size in schizophrenia in hippocampal subfields that mediate cortical-hippocampal interactions. Am J Psychiatry. 1995;152:738–748. doi: 10.1176/ajp.152.5.738. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry. 1991;48:625–632. doi: 10.1001/archpsyc.1991.01810310043008. [DOI] [PubMed] [Google Scholar]
- Beauchamp GK, Magnus JG, Shmunes NT, Durham T. Effects of olfactory bulbectomy on social behavior of male guinea pigs (Cavia procellus) J Comp Physiol Psychol. 1977;91:336–346. doi: 10.1037/h0077327. [DOI] [PubMed] [Google Scholar]
- Bottlender R, Wegner U, Wittmann J, Strauss A, Moller HJ. Deficit syndromes in schizophrenic patients 15 years after their first hospitalisation: Preliminary results of a follow-up study. Eur Arch Psychiatry Clin Neurosci. 1999;249 suppl 4:27–36. doi: 10.1007/pl00014182. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Edwards J, Anderson V, Robinson T, Pantelis C. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021–1031. doi: 10.1016/0006-3223(95)00594-3. [DOI] [PubMed] [Google Scholar]
- Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- Carpenter WT, Arango C, Buchanan RW, Kirkpatrick B. Deficit psychopathology and a paradigm shift in schizophrenia research. Biol Psychiatry. 1999;46:352–360. doi: 10.1016/s0006-3223(99)00088-8. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Germain-Robin B, Chabot P, Brazo P, Delamillieure S, Langolois A, et al. Family history and obstetric complications in deficit and nondeficit schizophrenia: Preliminary studies. Eur Psychiatry. 1998;13:270–272. doi: 10.1016/S0924-9338(98)80034-5. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: A rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178. doi: 10.1288/00005537-198402000-00004. [DOI] [PubMed] [Google Scholar]
- Falkai P, Schneider-Axmann T, Honer WG. Entorhinal cortex pre-alpha cell clusters in schizophrenia: Quantitative evidence of a developmental abnormality. Biol Psychiatry. 2000;47:937–943. doi: 10.1016/s0006-3223(99)00250-4. [DOI] [PubMed] [Google Scholar]
- Farina A, et al. Birth order of recovered and nonrecovered schizophrenics. Arch Gen Psychiatry. 1963;9:224–228. doi: 10.1001/archpsyc.1963.01720150034005. [DOI] [PubMed] [Google Scholar]
- Fenton WS, McGlashan TH. Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry. 1994;151:351–356. doi: 10.1176/ajp.151.3.351. [DOI] [PubMed] [Google Scholar]
- Gur RE, Mozley PD, Resnick SM, Mozley LH, Shtasel DL, Gallacher F, et al. Resting cerebral glucose metabolism in first-episode and previously treated patients with schizophrenia relates to clinical features. Arch Gen Psychiatry. 1995;52:657–667. doi: 10.1001/archpsyc.1995.03950200047013. [DOI] [PubMed] [Google Scholar]
- Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, et al. Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry. 1999;56:1117–1123. doi: 10.1001/archpsyc.56.12.1117. [DOI] [PubMed] [Google Scholar]
- Holzman PS, Levy DL, Proctor LR. Smooth pursuit eye movements, attention, and schizophrenia. Arch Gen Psychiatry. 1976;33:1415–1420. doi: 10.1001/archpsyc.1976.01770120019001. [DOI] [PubMed] [Google Scholar]
- Houlihan DJ, Flaum M, Arnold SE, Keshavan M, Alliger R. Further evidence for olfactory identification deficits in schizophrenia. Schizophr Res. 1994;12:179–182. doi: 10.1016/0920-9964(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Hurwitz T, Kopala L, Clark C, Jones B. Olfactory deficits in schizophrenia. Biol Psychiatry. 1988;23:123–128. doi: 10.1016/0006-3223(88)90081-9. [DOI] [PubMed] [Google Scholar]
- Jakob H, Beckmann H. Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm. 1986;65:303–326. doi: 10.1007/BF01249090. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Amador XF, Flaum M, Yale SA, Gorman JM, Carpenter WT, et al. The deficit syndrome in the DSM-IV Field Trial: I. Alcohol and other drug abuse. Schizophr Res. 1996;20:69–77. doi: 10.1016/0920-9964(95)00102-6. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW. The neural basis of the deficit syndrome of schizophrenia. J Nerv Ment Dis. 1990;178:545–555. doi: 10.1097/00005053-199009000-00001. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT. The Schedule for the Deficit Syndrome: An instrument for research in schizophrenia. Psychiatry Res. 1989;30:119–123. doi: 10.1016/0165-1781(89)90153-4. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): Behavioral and anatomical specificity. Behav Neurosci. 1994;108:501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kopala LC, Good K, Goldner EM, Birmingham CL. Olfactory identification ability in anorexia nervosa. J Psychiatry Neurosci. 1995;20:283–286. [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Corcoran C, Fahim C, Berman A, Harkavy-Freedman J, Yale Y, et al. Paternal age and sporadic schizophrenia: Evidence for de novo mutations. Am J Med Genet Psychiatr Genet. doi: 10.1002/ajmg.1701. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Goetz RR, Yale S, Berman A, Friedman JH, Tremeau Fl. Relation of familial schizophrenia to negative symptoms but not to the deficit syndrome. Am J Psychiatry. 2000;157:994–1003. doi: 10.1176/appi.ajp.157.6.994. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Harlap S, Fenig S, Heiman D, Nahon D, Feldman D, Susser E. Advancing paternal age and the risk of schizophrenia. Arch Gen Psych. 2001;58(4):361–367. doi: 10.1001/archpsyc.58.4.361. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Perera GM, Lignelli A, Marshall RS, Esser PD, Storer S, et al. SPECT imaging of odor identification in schizophrenia. Psychiatry Res. 1998;82:53–61. doi: 10.1016/s0925-4927(98)00008-0. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Wray AD, Friedman JH, Amador X, Yale S, Hasan A, et al. Odor discrimination deficits in schizophrenia: Association with eye movement dysfunction. J Neuropsychiatry Clin Neurosci. 1994;6:273–278. doi: 10.1176/jnp.6.3.273. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: A qualitative and quantitative review. Neuropsychopharmacology. 1999;21:325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Buchanan RW, Kirkpatrick B, Lahti AC, Medoff D, et al. Eye tracking disorder in schizophrenia is characterized by specific ocular motor defects and is associated with the deficit syndrome. Biol Psychiatry. 1997;42:781–796. doi: 10.1016/s0006-3223(96)00492-1. [DOI] [PubMed] [Google Scholar]
- Ross DE, Thaker GK, Buchanan RW, Lahti AC, Medoff D, Bartko JJ, et al. Association of abnormal smooth pursuit eye movements with the deficit syndrome in schizophrenic patients. Am J Psychiatry. 1996;153:1158–1165. doi: 10.1176/ajp.153.9.1158. [DOI] [PubMed] [Google Scholar]
- Schooler C. Birth order and schizophrenia. Arch Gen Psychiatry. 1961;4:91–97. doi: 10.1001/archpsyc.1961.01710070093013. [DOI] [PubMed] [Google Scholar]
- Schooler C. Birth order and hospitalization for schizophrenia. J Abnorm Social Psychol. 1964;69:574–579. doi: 10.1037/h0048880. [DOI] [PubMed] [Google Scholar]
- Shagass C, Roemer RA, Amadeo M. Eye-tracking performance and engagement of attention. Arch Gen Psychiatry. 1976;33:121–125. doi: 10.1001/archpsyc.1976.01770010077015. [DOI] [PubMed] [Google Scholar]
- Stedman TJ, Clair AL. Neuropsychological, neurological and symptom correlates of impaired olfactory identification in schizophrenia. Schizophr Res. 1998;32:23–30. doi: 10.1016/s0920-9964(98)00021-8. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, et al. Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry. 1992;49:522–530. doi: 10.1001/archpsyc.1992.01820070016003. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Moberg PJ, Yousem DM, Doty RL, Arnold SE, Gur RE. Reduced olfactory bulb volume in patients with schizophrenia. Am J Psychiatry. 2000;157:828–830. doi: 10.1176/appi.ajp.157.5.828. [DOI] [PubMed] [Google Scholar]
- Weller A, Weller L. Examination of menstrual synchrony among women basketball players. Psychoneuroendocrinology. 1995;20:613–622. doi: 10.1016/0306-4530(95)00007-b. [DOI] [PubMed] [Google Scholar]