Graphical abstract
.
Research highlights
▶ Thorax temperature was regulated from 37.0–45.3 °C (ambient temperature: 3–39 °C). ▶ Solar heat gain was used to increase thorax temperature by about 1–3 °C. ▶ High thorax temperature allowed regulation of an optimal head temperature. ▶ Flexible thermal strategy enabled foraging in a broad ambient temperature range.
Keywords: Honeybee, Thermoregulation, Foraging, Crop load, Water
Abstract
Foraging honeybees are subjected to considerable variations of microclimatic conditions challenging their thermoregulatory ability. Solar heat is a gain in the cold but may be a burden in the heat. We investigated the balancing of endothermic activity with radiative heat gain and physiological functions of water foraging Apis mellifera carnica honeybees in the whole range of ambient temperatures (Ta) and solar radiation they are likely to be exposed in their natural environment in Middle Europe.
The mean thorax temperature (Tth) during foraging stays was regulated at a constantly high level (37.0–38.5 °C) in a broad range of Ta (3–30 °C). At warmer conditions (Ta = 30–39 °C) Tth increased to a maximal level of 45.3 °C. The endothermic temperature excess (difference of Tbody − Ta of living and dead bees) was used to assess the endogenously generated temperature elevation as a correlate of energy turnover. Up to a Ta of ∼30 °C bees used solar heat gain for a double purpose: to reduce energetic expenditure and to increase Tth by about 1–3 °C to improve force production of flight muscles. At higher Ta they exhibited cooling efforts to get rid of excess heat. A high Tth also allowed regulation of the head temperature high enough to guarantee proper function of the bees’ suction pump even at low Ta. This shortened the foraging stays and this way reduced energetic costs. With decreasing Ta bees also reduced arrival body weight and crop loading to do both minimize costs and optimize flight performance.
1. Introduction
A honeybee colony needs water to thermoregulate the hive on hot days by evaporative cooling, to dilute stored honey, and for the consumption of nurse bees to produce jelly for feeding the larval brood (Park, 1946; Lindauer, 1955; Johansson and Johansson, 1978; Seeley, 1995; Kühnholz and Seeley, 1997). Some honeybees in the colony are specialized on water collection (Lindauer, 1952; Robinson et al., 1984). If they have to fly longer distances to water sources, they fuel their foraging flights with more sucrose (Visscher et al., 1996; Woyciechowski, 2007). Therefore, they prefer to collect water in the vicinity of the hive. In contrast to nectar, water is not stored in combs. Water foraging is regulated according to the current demand in the colony. The regulation of water collection is similar to that for nectar. The rate of unloading of water foragers indicates the colonies’ demand for it (Seeley, 1995; Kühnholz and Seeley, 1997).
During foraging honeybees have high energetic costs to maintain flight muscle temperature at an appropriate level above the minimum threshold of about 30 °C (e.g. Heinrich, 1979b, 1980b; Harrison and Hall, 1993; Harrison et al., 1996; Kovac and Schmaranzer, 1996; Woods et al., 2005). Water collecting bees regulate thorax temperature (35–41 °C on average) at a high level in a broad range of ambient temperatures (Schmaranzer, 2000). Water collecting does not provide a gain in energy, and therefore high thoracic temperatures in water foragers are especially interesting in comparison to nectar foragers where honeybees always endeavour to maximize energetic efficiency (gain/cost). As a rule, the energy expenditure of individual foragers is balanced with the net energetic gains to the colony (Seeley et al., 1991; Seeley, 1995; Schmidt-Hempel et al., 1985). The bees minimize the thermoregulatory costs during foraging by adapting their thorax temperature in response to the sucrose concentration of the available food source (Stabentheiner and Schmaranzer, 1986; Dyer and Seeley, 1987; Schmaranzer and Stabentheiner, 1988; Waddington, 1990; Stabentheiner and Hagmüller, 1991; Underwood, 1991; Stabentheiner, 2001). Thoracic temperature varies in a broad range (∼30–44 °C) depending on sucrose concentration and some other parameters. In the Ta range of 20.9–27.2 °C water collecting honeybees (max Tth = 38.1–40.7 °C; Schmaranzer, 2000) exhibited thorax temperatures similar to 0.5 M sucrose foraging bees (max Tth = 39.3–40.8 °C; Schmaranzer and Stabentheiner, 1988). The high energetic investment of water foragers pronounces the suggestion that water is crucial for the survival of the colony.
The body temperature of foraging insects is influenced by several environmental factors like ambient air temperature, solar radiation, and convection. The energy gain from solar radiation is important for the thermoregulation of foraging bees. An increase of the thorax temperature with increasing insolation was reported in Western honeybees arriving at the nest entrance after their foraging flights (Cena and Clark, 1972; Heinrich, 1979a; Cooper et al., 1985) and during nectar foraging (Heinrich, 1979a). Underwood (1991) reported the same for Indian honeybees collecting sugar syrup under sunny and overcast skies.
Kovac et al. (2009) investigated the influence of solar radiation on the thermoregulation of water foraging wasps in more detail. Vespula and Polistes did both, increase the thorax temperature and reduce active heat production, as solar heat gain increased. In honeybees, the relative contribution of endothermic heat production and heat gain from solar radiation on the body temperature is unknown. We here report on the balancing of endothermic activity with radiative heat gain in water foraging honeybees. However, honeybees forage in the cold as well as at high temperatures. The thermoregulatory challenge, therefore, differs considerably in dependence on ambient conditions. Solar heat is a gain in the cold but may be a burden in the heat. We expected differences in the thermoregulatory behavior to occur. In order to give a comprehensive overview of all mechanisms of thermoregulation and optimization of endothermic efforts, our investigation covers the whole range of ambient temperatures water foraging bees exhibit during their foraging trips in their natural environment under Middle European climate conditions. Infrared thermography allowed the non-invasive, undisturbed measurement of the temperatures of thorax, head and abdomen. This revealed new findings on the balancing of thermoregulation with functional requirements during foraging.
2. Materials and methods
2.1. Animals, field site and measuring conditions
Measuring location was an apiary with 20 honeybee colonies (Apis mellifera carnica) in an orchard on a farm in Gschwendt near Graz/Austria, Middle Europe. We investigated honeybees foraging water from a rainwater barrel, covered with a swimming wooden grate, located 3–10 m beside the colonies. In order not to impair their behavior during foraging, we refrained from marking the individuals. Because there foraged always at least 10 and up to 50 bees from 20 colonies nearby, the probability of pseudoreplication is very low. To cover the whole range of ambient temperatures of honeybees foraging in Middle European climate conditions, measurements of foragers were performed on 14 days (2000, 2003, and 2006). Additional measurements concerning the operative temperature and weight of foragers were conducted in 2009 and 2010.
2.2. Measurements
The bees were filmed during their complete foraging stay (from landing until take off) at the water barrel with an infrared camera (ThermaCam SC2000 NTS, FLIR Inc.) without disturbing them. The infrared camera was calibrated periodically by slotting in a self-constructed peltier driven reference source of known temperature and emissivity (for details of calibration see Stabentheiner and Schmaranzer, 1987; Schmaranzer and Stabentheiner, 1988). Thermographic data were stored digitally with 14-bit resolution on a portable computer (DOLCH Flexpac-400-XG) at a rate of 3–5 frames s−1.
The ambient air temperature (Ta) and relative humidity was measured near the foraging and dead bees with NTC-sensors or thermocouples. The solar radiation was measured with a Dirmhirn-global radiation-pyranometer (range: 0.3–3.3 μm; NP-42, NEO Inc.) or with a miniature global radiation sensor (FLA613-GS mini spezial, AHLBORN) in the immediate vicinity of the insects. The temperature and radiation data were stored every 2 s with ALMEMO data loggers (AHLBORN). During body temperature calculation from the infrared thermograms they were automatically extracted from the logger files.
To determine the crop loading of the foraging honeybees, bees were individually marked with small paint marks on the abdomen and were trained to collect water on a balance (AB104, METTLER-TOLEDO). The amount of collected water was calculated from the weight difference between arrival and departure.
2.3. Operative temperature
To take into consideration the effects of ambient air temperature, solar radiation and air convection on the measurement site we determined the insects’ operative (environmental) temperature (Te; e.g. Bakken, 1976, 1980, 1992; Bishop and Armbruster, 1999; Coelho et al., 2007; Kovac et al., 2009). On 6 of the 14 measuring days 2 bees (except 10.04.2003 one bee) were taken from the hive entrance, killed by freezing and afterwards fixed with needles on their wings on the wooden grate about 0.4–0.8 cm above the strips of wood beside the foraging bees. Measurements started after temperature equilibration of the dead bees (about 1 h) and lasted about 3–4 h. Dead bees were measured simultaneously or alternating with the living bees (Fig. 1). The same dead bees were used for one measuring period.
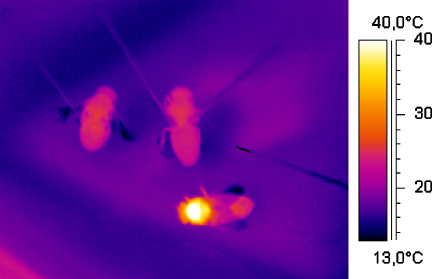
Fig. 1.
Thermogram of a water foraging honeybee (bottom, Tthorax = 40.2 °C, Thead = 29.9 °C, Tabdomen = 26.9 °C) and dead bees (top left, Tthorax = 24.4 °C, Thead = 21.0 °C, Tabdomen = 24.6 °C; top right, Tthorax = 24.3 °C, Thead = 23.1 °C, Tabdomen = 22.9 °C) fixed with needles beside the foraging site of the bees at an ambient temperature of 15.1 °C (solar radiation: 684 W m−2). Black tip right of the bees, thermocouple for measurement of the ambient temperature.
In insect thermoregulation research Te has been determined using both dried (Klok and Chown, 1999; Coelho et al., 2007) or fresh carcasses (Bishop and Armbruster, 1999; Sformo and Doak, 2006; Kovac et al., 2009). We decided for fresh carcasses because they brought several advantages against dried specimens. Evaporative cooling of the dead bees was determined to be low under our experimental conditions. Within 3 h the fresh weight of dead bees was only reduced by 2.3% in sunshine (from 103.5 ± 9.8 mg (mean ± SD) to 101.1 ± 9.9 mg, 8 bees, Ta = 22.8 °C, radiation = 790 W m−2), and by 0.9% in shade (from 99.7 ± 13.0 to 98.8 ± 12.9 mg, 8 bees, Ta = 18.5 °C, radiation = 180 W m−2). Therefore, the dead bees’ heat capacity remained rather constant in our measuring periods. Relative humidity in shade was 47.2% in immediate vicinity to the bees and 39.1% about 1 m beside the water barrel (measured with 5 mm diameter miniature sensors, AHLBORN FHA646-R). Weight loss per bee equals an evaporative heat loss of 0.5 mW in sunshine and 0.2 mW in shade.
A main disadvantage of dried carcasses is their strongly reduced heat capacity, which influences their reaction to convection. In insects (bees) with a weight smaller than 30–40 mg the cooling rate increases especially steep (Bishop and Armbruster, 1999). Drying bees in turbulent air at a temperature of 65 °C for 26 h (until they reached a constant weight) reduced their weight from 96.4 ± 16.7 mg to 30.0 ± 5.3 mg (12 bees). This reduced their heat capacity by about 69.9% (from about 0.323 to 0.101 J °C−1, using a specific heat of 3.35 J °C−1 g−1 for biological tissues). This is much higher than the decrease in fresh carcasses within a measurement period of 3 h (2.3% in sunshine and 0.9% in shade, see above).
Another disadvantage of dried bees is their reduced body surface area. Drying reduced the cross-sectional area by 25.6% (from 52.6 ± 3.2 to 39.1 ± 2.6 mm−2, 12 bees), mainly because of a strong shrinking and bending of the abdomen. This means a reduction of absorbed radiation of roughly 10.6 mW per bee (at 790 W m−2). In dried specimens we were not able to expand the abdomen to its original length. However, in our freshly killed bees we could do this. If one assumes a partly (50%) restoration of the dried specimens’ absorbing area, there remains a loss of about 5.3 mW per bee (at 790 W m−2). This is about 10 times the error caused by evaporative heat loss of fresh carcasses (see above).
Hadley et al. (1991) demonstrated that even in a desert cicada which is able to exhibit considerable evaporative cooling at high ambient temperatures, evaporation causes just a small temperature depression (<0.4 °C) at ambient temperatures below 37.5 °C. When we integrate the evaporative heat loss of fresh carcasses in sunshine and shade into Fig. 6 by reducing the radiative heat gain (W m−2) accordingly (considering the honeybee body surface area, Woods et al., 2005), the resulting shift of the regression lines increases the Tth − Ta values at a given radiation by only about 0.1 °C. Therefore, we conclude that any evaporative temperature “error” in our dead bees is below ∼0.2 °C. In conclusion it can be said that the use of fresh carcasses improved the accuracy of the Te measurements considerably!
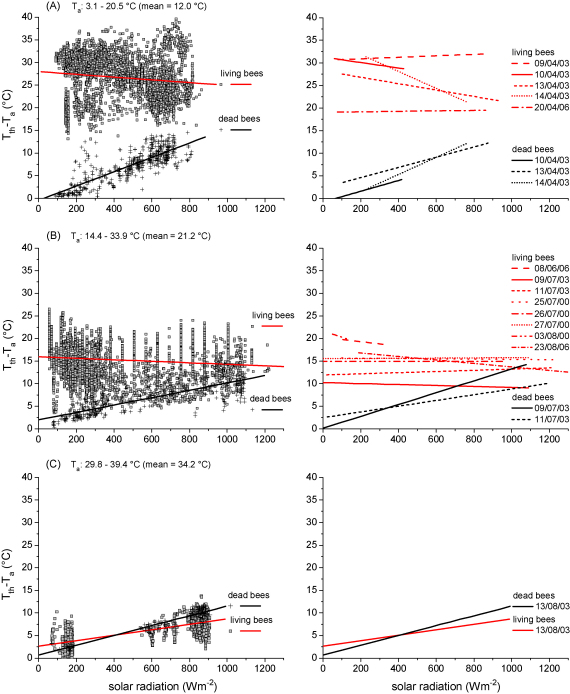
Fig. 6.
Thorax temperature excess (Tth − Ta) of living honeybees during water foraging, and of dead bees beside them, in dependence on solar radiation at three different ranges of ambient temperature (Ta, A–C). Left side, single measurements and linear correlation fit of all measuring days, right side, linear correlation fits of single measuring days. Number of observations and regression statistics in Tables 2 and 3.
2.4. Data evaluation and statistics
The temperature of the three body parts and of the water surface (in close vicinity to the spot where the bees’ mouthparts were in contact with the wet substrate) was calculated from the infrared thermograms by means of the AGEMA Research software (FLIR Inc.) controlled by a self-written Excel VBA-macro (Microsoft Corporation). Values of the body temperature during foraging were taken in regular intervals of about 3 s immediately after the landing of the insects until their take off. The surface temperatures of head (Thd), thorax (Tth) and abdomen (Tab) were calculated with an infrared emissivity of 0.97, determined for the honeybee cuticle (Stabentheiner and Schmaranzer, 1987; Schmaranzer and Stabentheiner, 1988). Because the ThermaCam is working in the long-wave infrared range (7.5–13 μm) the reflected radiation from the bees’ cuticle produced only a small measurement error (0.2 °C for 1000 W m −2) which was compensated for. In this way we reached an accuracy of 0.7 °C for the body surface temperature of the bees at a sensitivity of <0.1 °C.
The temperature gradient between the thorax and the ambient air (thorax temperature excess = Tthorax − Ta) is often used as a measure to judge the endothermic capability of insects. In sunshine, however, this is not a reliable measure of the endogenously generated temperature excess because of additional heating of the bees’ body by the solar radiation. Therefore, we compared the living bees’ temperature excess of thorax, head and abdomen with that of the dead bees (endothermic temperature excess = [Tbody − Ta]living − [Tbody − Ta]dead).
The relationship between body temperature, temperature excess, crop loading and Ta or solar radiation was described by simple linear, sigmoidal or exponential regression functions and tested with ANOVA. Data analysis and statistics were performed by using the Statgraphics package (Statistical Graphics Corporation) and ORIGIN software (OriginLab Corporation).
3. Results
Fig. 1 shows a thermogram of a water foraging honeybee (Apis mellifera carnica) and of 2 dead bees fixed at the foraging site on a wooden grate. We analyzed 879 foraging stays of bees at the water barrel. From 12,377 thermograms we evaluated body surface temperatures of head (Thd, n = 11,290), thorax (Tth, n = 11,340) and abdomen (Tab, n = 11,334) of water foragers, of all body parts of dead bees (n = 1037 each), and of the water surface (Twater, n = 4957). Fig. 2 shows representative body temperature curves of bees at low, medium and high ambient temperature (Ta). From these curves the mean value of each body part for each foraging stay was calculated and plotted in Fig. 3. It contains 3–45 measuring points per stay (including arrival and departure values) depending on the duration of foraging.
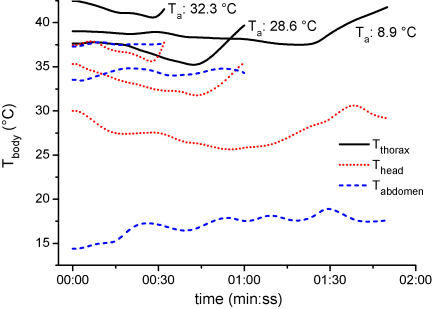
Fig. 2.
Temperature curves of thorax, head and abdomen of 3 water foraging honeybees at different ambient temperatures (Ta) from arrival till departure at the water barrel. Solar radiation 854, 824, 520 W m−2 from highest to lowest Ta, respectively.
Fig. 3.
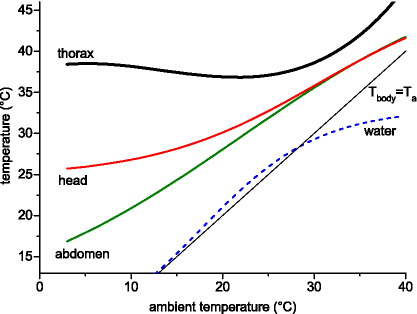
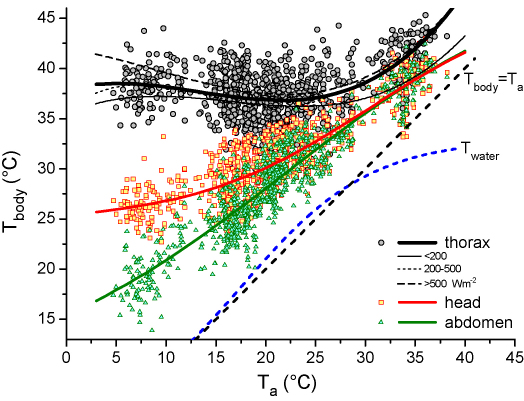
Water and body temperature of honeybees (means per stay) during water collecting in dependence on ambient temperature (Ta). Equations for non-linear regressions in Table 2. For the thorax curves are shown for all values and for three ranges of solar radiation. N = 879 foraging stays (11,340 temperature values). For Twater see Fig. 4.
3.1. Body temperature, ambient and water temperature
We investigated the body temperature regulation of water foraging honeybees (Apis mellifera carnica) in the whole range of ambient temperatures (Ta = ∼3–40 °C) and solar radiation (50–1200 W m−2) they are likely to be exposed in their natural environment. In April 2003 for example, after a bad weather front, the ambient air temperature was very low (Ta = ∼3–15 °C). On this day we started measurements early in the morning at the onset of the bees’ water foraging, when they needed the water very urgently for the brood. Another measuring day in August 2003 was the hottest day of the year, with ambient air temperatures above 30 °C (Ta = ∼30–40 °C). On the other days we had moderate conditions in the range of about 15–30 °C (Table 1).
Table 1.
Summary statistics for the temperature of head, thorax and abdomen of water foraging honeybees and ambient temperature (Ta), relative humidity (Hum) and solar radiation (Rad) for each single measuring day. Data presented as means ± SD or only means. N = number of measurements, “+” indicates values for dead bees.
| Date | Thead (°C) | Tthorax (°C) | Tabdomen (°C) | Ta (°C) | Hum (%) | Rad (W m−2) | Bees | Nmeasurements (hd/th/ab) | Dead bees |
|---|---|---|---|---|---|---|---|---|---|
| 09.04.2003 | 26.8 ± 2.5 | 38.8 ± 2.3 | 20.0 ± 2.5 | 7.5 | 41.1 | 457 | 53 | 956/961/961 | |
| 10.04.2003 | 26.4 ± 1.7 | 37.9 ± 1.7 | 17.7 ± 2.1 | 7.7 | 55.5 | 181 | 23 | 273/274/273 | |
| 10.04.2003 | 8.5 ± 1.2 | 9.1 ± 1.3 | 8.9 ± 1.2 | 7.8 | 55.5 | 181 | 1 | 65/65/65 | + |
| 13.04.2003 | 28.0 ± 3.2 | 38.8 ± 2.5 | 23.1 ± 2.8 | 14.1 | 49.7 | 493 | 95 | 1417/1429/1431 | |
| 13.04.2003 | 19.6 ± 3.8 | 22.7 ± 4.7 | 22.1 ± 4.3 | 14.6 | 49.7 | 511 | 2 | 190/190/190 | + |
| 14.04.2003 | 28.8 ± 2.5 | 38.5 ± 2.5 | 26.3 ± 2.8 | 13.6 | 43.6 | 573 | 41 | 600/600/599 | |
| 14.04.2003 | 19.3 ± 3.2 | 22.2 ± 3.4 | 21.6 ± 3.2 | 13.9 | 43.6 | 558 | 2 | 305/305/305 | + |
| 20.04.2006 | 29.5 ± 2.1 | 37.2 ± 2.3 | 25.7 ± 2.6 | 18.0 | 43.6 | 195 | 100 | 246/250/247 | |
| 08.06.2006 | 25.7 ± 1.9 | 35.7 ± 2.7 | 23.7 ± 1.2 | 16.3 | 57.4 | 174 | 16 | 134/139/139 | |
| 09.07.2003 | 32.7 ± 2.4 | 36.8 ± 3.2 | 31.8 ± 2.5 | 27.0 | 31.9 | 495 | 55 | 602/609/606 | |
| 09.07.2003 | 33.5 ± 5.3 | 34.9 ± 5.8 | 34.4 ± 6.0 | 27.7 | 31.9 | 537 | 2 | 150/150/150 | + |
| 11.07.2003 | 31.9 ± 2.3 | 37.2 ± 3.1 | 30.5 ± 2.2 | 24.6 | 38.4 | 506 | 69 | 667/679/679 | |
| 11.07.2003 | 30.7 ± 4.2 | 32.2 ± 4.6 | 32.3 ± 5.1 | 26.1 | 38.4 | 562 | 2 | 217/217/217 | + |
| 25.07.2000 | 28.2 ± 1.9 | 35.0 ± 2.8 | 27.1 ± 2.0 | 19.5 | 58.2 | 410 | 37 | 1062/1062/1062 | |
| 26.07.2000 | 31.7 ± 2.1 | 36.9 ± 2.3 | 31.0 ± 2.1 | 21.9 | 54.0 | 610 | 97 | 1502/1502/1502 | |
| 27.07.2000 | 30.4 ± 2.6 | 36.0 ± 2.6 | 29.1 ± 2.6 | 20.4 | 50.4 | 606 | 133 | 2312/2312/2312 | |
| 03.08.2000 | 27.5 ± 1.5 | 36.9 ± 1.8 | 24.9 ± 1.7 | 16.4 | 86.8 | 84 | 45 | 549/549/549 | |
| 13.08.2003 | 38.1 ± 2.5 | 40.9 ± 2.5 | 38.1 ± 2.2 | 34.2 | 23.1 | 676 | 99 | 883/886/886 | |
| 13.08.2003 | 40.9 ± 3.9 | 43.0 ± 4.2 | 42.4 ± 4.0 | 34.9 | 23.1 | 686 | 2 | 110/110/110 | + |
| 23.08.2006 | 29.8 ± 1.6 | 36.9 ± 2.9 | 27.0 ± 1.3 | 21.1 | 58.8 | 435 | 16 | 87/88/88 |
Body temperatures varied in a wide range, Tth from 25.8 to 46.4 °C, Thd from 16.2 to 43.4 °C, and Tab from 13.0 to 44.0 °C. At ambient temperatures of about 3–30 °C, Tth was regulated rather independent of Ta. At Ta > ∼30 °C, however, it increased nearly linearly with Ta (Fig. 3). Head and abdomen exhibited a stronger dependence on Ta but both of them were regulated well above Ta, especially at low Ta. The head was warmer and better regulated than the abdomen (Fig. 3). The relation of body temperature and ambient air temperature could be described best with a polynomial function for the thorax (R2 = 0.28692; Fig. 3 and Table 2):
| (1) |
and with a sigmoidal function for the head and the abdomen (head: R2 = 0.75303, abdomen: R2 = 0.85623; Fig. 3 and Table 2):
| (2) |
where T is Thd, Tab or Twater.
Table 2.
Equations of non-linear regressions for the water temperature and the temperature of thorax (Tth), head (Thd) and abdomen (Tab) of honeybees during water foraging in dependence on ambient temperature (Ta) and solar radiation (Fig. 3). Thorax: Eq. (1), head and abdomen: Eq. (2); R2 = coefficient of regression, N = number of foraging stays.
| Part | Radiation | Equations | R2 | N |
|---|---|---|---|---|
| Thorax | 0–1200 | 0.28692 | 879 | |
| <200 | 0.12903 | 226 | ||
| 200–500 | 0.41394 | 414 | ||
| >500 | 0.81774 | 239 | ||
| Head | 0–1200 | Thd = 24.66888 + (22.82587/(1 + e3.38148−0.11099 × Ta)) | 0.75303 | 879 |
| Abdomen | 0–1200 | Tab = 7.84585 + (43.38589/(1 + e1.54937−0.07062 × Ta)) | 0.85623 | 879 |
| Water | 0–1200 | Twt = 3.67262 + (29.33682/(1 + e2.74058−0.15550 × Ta)) | 0.92742 | 4957 |
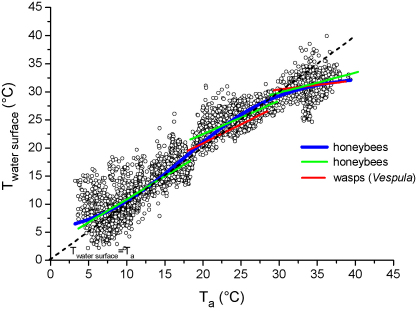
At the lowest mean Ta of about 4.7 °C the average values of Tth, Thd and Tab derived from these regression lines were 38.5, 25.9 and 17.8 °C, respectively. In the medium range of Ta, at about 20 °C, the Tth decreased to 37.0 °C, the Thd increased to 30.2 and the Tab to 28.1 °C. At the highest Ta measured (38.1 °C), Tth, Thd and Tab increased to 45.3, 40.6 and 40.8 °C, respectively. Plotting the Tth in dependence on three levels of solar radiation (<200, 200–500, >500 W m−2; Fig. 3) revealed, that bees foraging in sunshine were always warmer than bees foraging in shade. The water surface temperature measured closely beside the bees’ mouthparts increased in dependence on Ta (Fig. 4 and Table 3). Means per stay were in the range of 2.3–40.0 °C. It is noticeable, however, that it was somewhat higher than Ta at the low end, and lower than Ta at the high end of the investigated range of Ta. Therefore, not a linear but the sigmoidal Eq. (2) fitted the data best (R2 = 0.92742; Figs. 3 and 4 and Table 3). In order to allow comparison of the water temperature near our bees with that near vespine wasps (Vespula vulgaris, measured at the same time and place; Kovac et al., 2009), linear regression lines of corresponding ranges of Ta are plotted in Fig. 4 (regression statistics in Table 3). At a Ta of ∼20–30 °C bees and wasps differed significantly in intercepts (P < 0.00001, F-ratio = 87.31, Df = 1) but not in slopes (P = 0.2504, F-ratio = 1.32, Df = 1). At higher Ta (∼30–38 °C) they differed in both parameters (P < 0.05; intercepts: F-ratio = 4.65, Df = 1, slopes: F-ratio = 6.42, Df = 1).
Fig. 4.
Temperature of the water surface besides foraging bees in dependence on ambient temperature (Ta). The blue curve is fitted with Eq. (2) for the complete range of Ta. Green (bees) and red lines (vespine wasps, from Kovac et al., 2009) are subranges described with simple linear regressions (equations, number of observations and regression statistics in Table 3).
Table 3.
Equations of linear and non-linear (Eq. (2)) regressions of water surface temperature (Twater) beside the foraging bees and wasps (Fig. 4, for wasps see Kovac et al., 2009) at different ranges of ambient temperature (Ta). R2 = coefficient of regression, N = number of measurements.
| Ta (°C) | equations | R2 | N | P | |
|---|---|---|---|---|---|
| 3–40 | Bees | Twater = 3.67262 + (29.33682/(1 + e2.74058−0.1555 × Ta)) | 0.92742 | 4957 | |
| 3–20 | Bees | Twater = 2.65084 + 0.82006 × Ta | 0.64556 | 2681 | <0.0001 |
| 15–35 | Bees | Twater = 10.60586 + 0.59269 × Ta | 0.58942 | 1611 | <0.0001 |
| 30–40 | Bees | Twater = 19.17958 + 0.35532 × Ta | 0.05588 | 665 | <0.0001 |
| 20–30 | Wasps | Twater = 7.33290 + 0.67507 × Ta | 0.29501 | 181 | <0.0001 |
| 30–40 | Wasps | Twater = 25.54591 + 0.16190 × Ta | 0.02232 | 514 | <0.0001 |
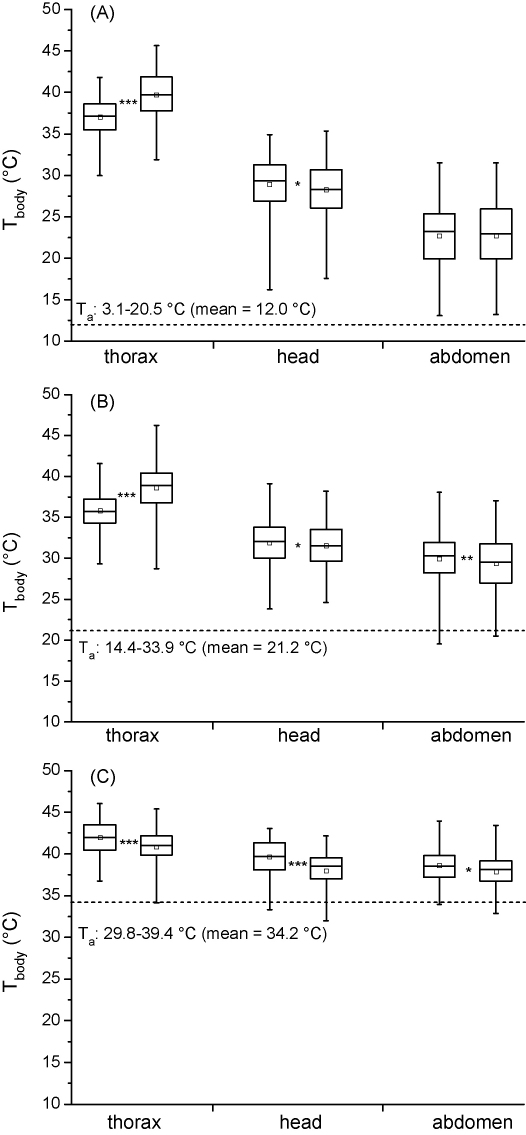
In Fig. 5A–C the temperatures of thorax, head and abdomen are shown immediately after arrival and before departure, at three different ambient temperature ranges. Towards departure, the Tth increased significantly at low and medium Ta. At a mean Ta of 12.0 °C from 37.0 to 39.7 °C, and at a mean Ta of 21.2 °C from 35.8 to 38.6 °C. At a high Ta of 34.2 °C, by contrast, Tth decreased towards departure from 42.0 to 40.8 °C (Mann–Whitney/Wilcoxon test, P < 0.001). The temperature of the head and the abdomen decreased significantly (P < 0.05) from landing till take off with one exception (abdomen at low Ta).
Fig. 5.
Temperature of thorax, head and abdomen of honeybees during arrival (left boxes) and departure (right boxes) at the water barrel at three different ambient temperatures (mean Ta, A–C). Box plots show mean, median, 25–75 percentile, minimum and maximum; number of measurements (arrival/departure) (A) thorax: 350/338, head: 349/337, abdomen: 350/338; (B) thorax: 349/348, head: 341/348, abdomen 349/348; (C) thorax: 117/111; head: 117/111, abdomen: 117/111. (*P < 0.05, **P < 0.01, ***P < 0.001; Mann–Whitney/Wilcoxon test).
3.2. Thorax temperature excess and solar radiation
The Tth of living and dead bees was always elevated above Ta, but to a different degree in the three different ranges of ambient temperature (Fig. 6A–C; regression statistics in Table 4). At low Ta (Fig. 6A; mean Ta = 12.0 °C) the thorax temperature excess (Tth − Ta, mean values of regression lines) of the living bees decreased from 27.7 to 25.4 °C as solar radiation increased from 90 to 862 W m−2 (−3.0 °C kW−1 m−2) whereas in dead bees it increased from 1.0 to 12.3 °C as solar radiation increased from 90 to 810 W m−2 (15.7 °C kW−1 m−2). Even at high radiation there remained a great difference between living and dead bees (11.4 °C at 900 W m−2).
Table 4.
Equations of linear regressions for the thorax temperature excess (Tth − Ta) of honeybees during water foraging, and of dead honeybees (Fig. 6A-C) in dependence on solar radiation (SolRad) at three different ambient temperatures (Ta). R2 = coefficients of regressions, N = number of measurements.
| Ta (°C) | Equations | R2 | N | P | |
|---|---|---|---|---|---|
| 12.0 | Living | Tth − Ta = 27.97033 − 0.00305 × SolRad | 0.01686 | 3514 | <0.0001 |
| Dead | Tth − Ta = −0.46607 + 0.01584 × SolRad | 0.67097 | 560 | <0.0001 | |
| 21.2 | Living | Tth − Ta = 15.96446 − 0.00169 × SolRad | 0.02300 | 6842 | <0.0001 |
| Dead | Tth − Ta = 1.99029 + 0.00817 × SolRad | 0.66869 | 367 | <0.0001 | |
| 34.2 | Living | Tth − Ta = 2.67510 + 0.00603 × SolRad | 0.45128 | 886 | <0.0001 |
| Dead | Tth − Ta = 0.70566 + 0.01083 × SolRad | 0.91013 | 110 | <0.0001 | |
At medium Ta (Fig. 6B; mean Ta = 21.2 °C) the thorax temperature excess of the living bees decreased from 15.9 to 13.9 °C (−1.7 °C kW−1 m−2) as solar radiation increased from 56 to 1221 W m−2 whereas in dead bees it increased from 2.6 to 11.1 °C (8.3 °C kW−1 m−2) as solar radiation increased from 78 to 1098 W m−2. The difference between living and dead bees was reduced to 5.2 °C at 900 W m−2 radiation.
At high Ta (Fig. 6C; mean Ta = 34.2 °C) by contrast, the thorax temperature excess increased with radiation in both living and dead bees. In living bees it increased from 3.2 to 8.2 °C as solar radiation increased from 70 to 905 W m−2 (6.0 °C kW−1 m−2), and in dead bees from 1.4 to10.5 °C as radiation increased from 68 to 909 W m−2 (10.8 °C kW−1 m−2). At a radiation value of 900 W m−2 the thorax temperature excess of the living bees was by 2.4 °C lower than that of the dead bees.
3.3. Endothermic temperature elevation
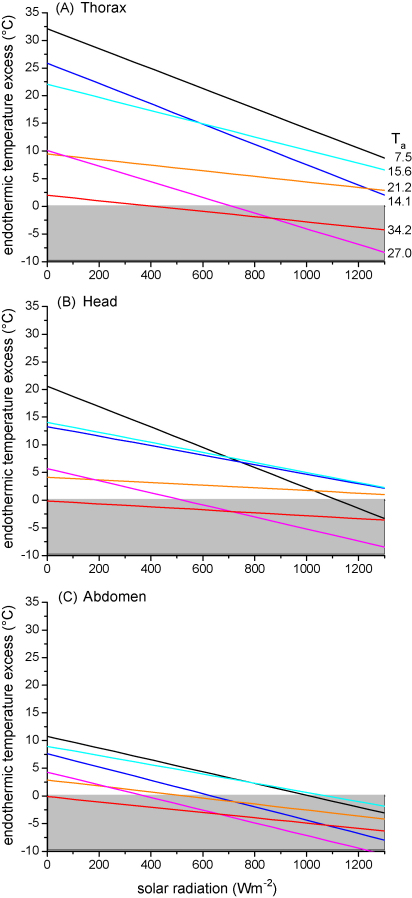
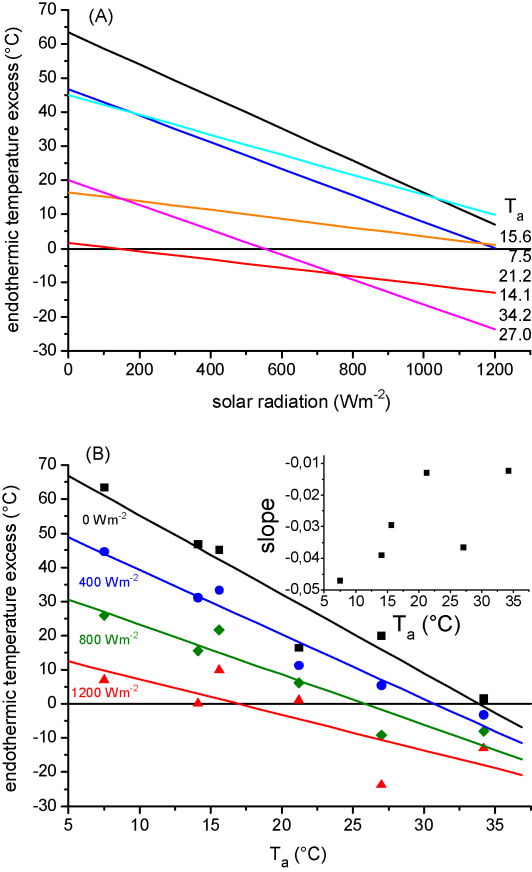
The thorax temperature excess (Tth − Ta) of our dead bees reveals the insects’ operative environmental temperature excess, integrating the heat gain from solar radiation minus the heat losses via radiation, external convection and evaporation. The difference between the living and the dead bees’ thorax temperature excess regression lines describes the active, endogenously generated part of the thoracic temperature excess. We here call it the ‘endothermic temperature excess’ (endothermic temperature elevation). In the same way curves for the head and the abdomen were calculated. Fig. 7A–C gives an overview of the endothermic temperature excess at six different ambient temperatures, when living and dead bees had been measured simultaneously. The endothermic temperature excess declined strongly with increasing solar radiation in most cases. At the two highest measuring temperatures (mean Ta = 27.0 and 34.2 °C) the endothermic temperature excess of the thorax became negative (Tth − Ta live < Tth − Ta dead), which means that cooling of the thorax was performed.
Fig. 7.
Endothermic temperature excess of water foraging honeybees ([Tbody − Ta]living − [Tbody − Ta]dead, difference of the gradient of thorax, head or abdomen to ambient temperature between living and dead bees), in dependence on solar radiation at six different ranges of ambient temperatures (shown centered Ta).
The endothermic temperature excess of head and abdomen decreased with increasing solar radiation in a similar way as in the thorax (Fig. 7A–C). It was higher in the head than in the abdomen. In the abdomen it decreased more often below zero (Tbody − Ta live < Tbody − Ta dead). On the warmest measuring day (mean Ta = 34.2 °C) the calculated curves of both head and abdomen remained below zero at all levels of radiation. This means that the living bees used the imbibed water for cooling.
Fig. 8A shows the endothermic temperature excess added up for all body parts, derived from the regression lines of Fig. 7. Fig. 8B shows the intercepts of the regressions lines of Fig. 8A at four levels of global radiation. This correlate of endothermic heat production increased with decreasing Ta. This increase was steep at low and flatter at high external heat gain. The insert in Fig. 8B reveals a weak trend but no significant correlation of the slopes of the regressions lines of Fig. 8A with the ambient temperature (R2 = 0.50954, P = 0.11113). A comparison of the slopes with the water temperature revealed a similar result slightly beyond significance (R2 = 0.62375, P = 0.06164). However, there are indications that the living bees reacted to the summed environmental conditions (Te). The slopes of the thorax temperature excess (in dependence on radiation) of the living bees decreased with increasing temperature excess of the dead bees (R2 = 0.62334, P = 0.06179). Elimination of the value from the hottest measuring day (13.08.2003, when the bees performed active cooling efforts) from the calculation resulted in a significant correlation (R2 = 0.77229, P = 0.04972).
Fig. 8.
(A) Summed endothermic temperature excess of all body parts from regressions in Fig. 7 in dependence on solar radiation at six different ambient temperatures (Ta). (B) Summed endothermic temperature excess of all body parts at four levels of radiation as depicted from the regression lines of (A), in dependence on Ta. Lines: R2 = 0.92421, 0.95273, 0.89166, 0.61122 from lowest to highest radiation; P < 0.00221, 0.00085, 0.00457, 0.06622; N = 6 each. Insert: slopes of summed regression lines of (A) (no correlation with Ta).
3.4. Duration of foraging stay and crop loading
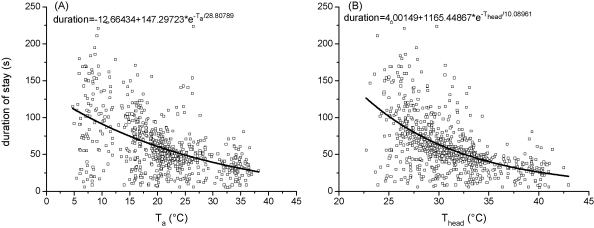
The duration of the foraging stays declined with increasing Ta (Fig. 9A). At a Ta of 5.0 °C the foragers stayed at the water barrel for 113 s (on average) but only for 27 s at 38.0 °C. However, there was a great variance of values, especially at low Ta. The relation between duration of stay and Ta or body temperatures could be described best with an exponential function of the type:
| (3) |
where T = Ta, Twater, Thd, Tth, Tab, or solar radiation.
Fig. 9.
(A) Duration of foraging stays of water foraging honeybees in dependence on ambient temperature (Ta) and (B) in dependence on the bees’ head (Thead) temperature. Fit statistics in Tables 5 and 6.
Fig. 9 shows the results of the calculation procedures. With regression analysis and ANOVA we tested which of the environmental factors (ambient air temperature, water temperature, solar radiation) and which of the bees’ body temperatures (thorax, head or abdomen) had the greatest influence on the duration of the foraging stays. Results revealed that the duration of the foraging stays correlated best with the bees’ head temperature (see Tables 5 and 6).
Table 5.
Statistical details of ANOVA for the bees’ duration of stay at the foraging site in dependence on body temperatures (thorax, head, abdomen) and environmental factors (Ta: ambient temperature, Twater: water temperature, SolRad: solar radiation).
| Source | Sum of squares | Df | Mean square | F-ratio | P |
|---|---|---|---|---|---|
| Tthorax | 14066.5 | 1 | 14066.5 | 14.92 | <0.0001 |
| Thead | 59511.6 | 1 | 59511.6 | 63.12 | <0.0000 |
| Tabdomen | 2579.35 | 1 | 2579.35 | 2.74 | <0.0981 |
| Ta | 8018.78 | 1 | 8018.78 | 8.50 | <0.0035 |
| Twater | 14873.9 | 1 | 14873.9 | 15.77 | <0.0001 |
| SolRad | 13428.7 | 1 | 13428.7 | 14.24 | <0.0002 |
| Residual | 682642.0 | 724 | 942.876 | ||
| Total | 0.000001 | 730 | |||
Table 6.
Statistical details of non-linear regressions for the bees’ duration of stay at the foraging site in dependence on body temperatures (thorax, head, abdomen) and environmental factors (Ta: ambient temperature; Twater: water temperature; SolRad: solar radiation) according to Eq. (3). Linear regression delivered lower values of R2.
| Source | α | β | γ | R2 | N |
|---|---|---|---|---|---|
| Tthorax | −18.41317 | 215.73481 | 38.02212 | 0.02320 | 743 |
| Thead | 4.00149 | 1165.44867 | 10.08961 | 0.30294 | 743 |
| Tabdomen | 316.77319 | −175.03882 | −77.0755 | 0.25663 | 743 |
| Ta | −12.66434 | 147.29723 | 28.80789 | 0.26593 | 743 |
| Twater | 195.96191 | −82.27684 | −45.8712 | 0.28926 | 731 |
| SolRad | 72.83873 | −0.92511 | −269.94261 | 0.07855 | 743 |
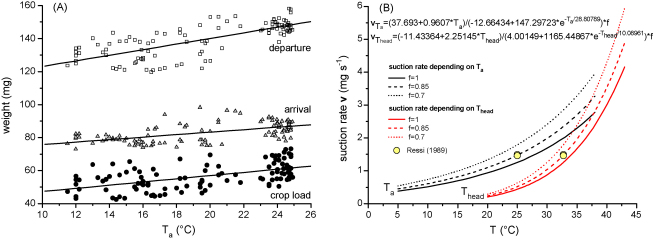
The mean crop loading of 15 individually marked bees increased linearly from 48.7 to 61.7 mg water as the ambient temperature increased from 11.5 to 25.0 °C (Fig. 10A; R2 = 0.31084, N = 110, P < 0.0001). From the amount of crop loading and the duration of the foraging stays we estimated the mean suction rate per stay (crop loading/duration of foraging stay), which increased exponentially with Ta (Fig. 10B). This increase was much steeper in dependence on Thd. However, we also noticed that the bees did not always drink continuously during the whole foraging stay. They made short interruptions and often showed periods of self-grooming and walking. Especially towards the end of their stays they filled in time for pre-flight warm-up to reach a sufficient thorax temperature for an optimal take off. Unfortunately, our thermographic sequences did not allow exact identification of drinking pauses. From our own observations and earlier measurements of Schmaranzer (2000) we estimated actual duration of suction to be about 85% of the total duration of a stay on average. The curves calculated with this assumption matches measurements of the suction rate of Ressi (1989) closely (conducted at Ta and Twater = 25 °C). The suction rate increased exponential from 0.6 to 2.2 mg s−1 as Thead increased from 26 to 36 °C (Q10 = 3.7; Fig. 10B). However, correlation with the ambient temperature in this range of Ta resulted in a smaller elevation of the suction rate, from 1.6 to 2.9 mg s−1 (Q10 = 1.8).
Fig. 10.
(A) Crop load, and weight upon arrival and departure of water foraging honeybees in dependence on ambient temperature (Ta) (arrival = 68.24759 + 0.75178 × Ta, R2 = 0.31235; departure = 106.05469 + 1.70236 × Ta, R2 = 0.54726; crop load = 37.693 + 0.9607 × Ta, R2 = 0.31084; for each curve N = 110 and P < 0.0001). (B) Suction rate in dependence on Ta and head temperature (Thead). Factor f (0–1) means portion of duration of stay used for suction. Mean Q10 values were 1.8 for Ta and 3.7 for Thead (f = 0.85).
4. Discussion
4.1. Body temperature, ambient and water temperature
Digby (1955) investigated the factors affecting the temperature excess of dead or anesthesized insects in artificial sunlight under laboratory conditions and found the temperature excess to vary directly with the radiation strength, similar to our dead bees. This applies to living insects only in the ectothermic state. Foraging honeybees, however, are always endothermic at medium to low Ta (Heinrich, 1979a; Schmaranzer and Stabentheiner, 1988; Kovac and Schmaranzer, 1996). In our water foragers endothermy was at a low level or absent only at high Ta (>∼30 °C; see below and Figs. 6–8). The same was observed in water foraging vespine wasps (Vespula; Kovac et al., 2009). However, the thermoregulatory behavior of our water foraging bees differed from that of vespine wasps (Fig. 6A–C, Table 3) at moderate Ta (∼20–30 °C). The bees’ thorax temperature excess decreased slightly with increasing radiation whereas it increased in Vespula. At high Ta (>∼30 °C), by contrast, the thorax temperature excess increased in both.
The relation between body temperature and ambient temperature shows impressively the thermoregulatory ability of the water foraging honeybees (Figs. 3 and 6). The thorax temperature was regulated independent of Ta (in sunshine and shade) in a broad range of Ta (∼3–30 °C). This resembles an investigation on honeybees collecting water in shade (Schmaranzer, 2000). Similar to our study he reported mean thoracic temperatures of 36.0–38.8 °C (Ta = 13.6–27.2 °C). Bees foraging from other natural resources like flowers regulate their thoracic temperature at a somewhat lower level. In shade Heinrich (1979a) reported means of about 30–35 °C at a Ta of 7–23 °C, and Kovac and Schmaranzer (1996) reported means of 35–38 °C at a Ta of 12–20 °C.
The thorax temperature and energy expenditure of sucrose foraging honeybees varies markedly in direct response to the richness of food rewards and distance (e.g. Stabentheiner and Schmaranzer, 1986, 1987, 1988; Dyer and Seeley, 1987; Schmaranzer and Stabentheiner, 1988; Waddington, 1990; Stabentheiner and Hagmüller, 1991; Underwood, 1991; Balderrama et al., 1992; Stabentheiner et al., 1995; Moffatt and Núñez, 1997; Moffatt, 2001; Stabentheiner, 1996, 2001). Highly motivated bees foraging concentrated sucrose solution increase body temperature with increasing energy gain from the food source. However, water does not provide a gain of energy. Rather, bees have to invest a lot of energy, especially to forage at low Ta. The high body temperatures observed (means ∼35–38 °C) are comparable with bees foraging 0.25–0.5 molar sucrose solution (Schmaranzer and Stabentheiner, 1988). Usually, honeybees avoid foraging at a Ta below about 12 °C. To our knowledge only Heinrich (1979a) reported foraging of a few bees on flowers at a Ta below 10 °C. In spring, when our colonies had to provide already a lot of brood, the bees collected water at very low and for them critical temperatures (down to 5 °C). At these very extreme conditions they exhibited thoracic temperatures of 33.5 °C above the ambient air on average. In some cases, mean Tth per stay was kept 36 °C above Ta. This extreme energetic investment for thermoregulation, therefore, emphasizes the water foragers’ highly motivated state despite the fact that water contains no usable energy. This is a good hint at the high importance of water for the survival of the colonies.
The temperature of the abdomen was below that of the head at low Ta (Fig. 3). However, Fig. 7C shows that at low Ta still a considerable amount of the thoracic heat production reached the abdomen. Heinrich (1980b, 1993) suggested that bees use a series of aortic loops in the petiole as a counter-current heat exchanger to prevent heat leakage to the abdomen. The heat still reaching the abdomen would be an inevitable result of the remaining hemolymph circulation. However, we presume that bees, beside the necessity to save energy, have to provide the abdomen with enough heat for proper function of physiological processes involved in energy supply and respiration.
Concerning the temperatures of head and abdomen, the head was the better-regulated body part (Figs. 2 and 3). Even at very low Ta the hemolymph circulation from the warm thorax (Heinrich, 1979b, 1980a; Coelho, 1991b) was kept at a level preventing the Thd from falling below 20 °C (mean per stay), which seems to bee necessary for a proper function of physiological and neural processes (see below). The similarity of Thd to Tab at high Ta (>30 °C) is suggested to be the result of the head cooling via the ingested water.
4.2. Endothermy and the use of external heat gain
Our water foragers kept mean Tth up to 36 °C above Ta during their stays at the water barrel. This means a very high energetic investment (e.g. Balderrama et al., 1992; Blatt and Roces, 2001; Moffatt, 2001; Stabentheiner et al., 2003). When they foraged in bright sunshine their Tth was about 1–3 °C higher than under shaded conditions (Fig. 3), i.e. they invested part of the external heat gain to increase the thorax temperature. In shade it is clear that any excess of body temperatures above Ta has to be generated by endothermic heat production with the flight muscles. In bees foraging in sunshine, however, the amount of the temperature elevation resulting from endothermy is not obvious. The Ta, even if measured close to the investigated insect, is often an inaccurate measure of its thermal environment. In addition, the solar radiation, and wind and other convective effects have to be considered. Therefore, we used the operative temperature (Te thermometer; Bakken, 1992) to quantify the summed influence of these environmental factors on the bees’ body temperature. The operative temperature was determined with freshly killed bees because in our investigations this brought clear advantages against dried specimens (see Section 2).
The difference between the living and dead bees’ body temperature excess (endothermic temperature excess = (Tbody − Ta)living − (Tbody − Ta)dead) was chosen to assess the bees’ endothermic activity (Figs. 6 and 7). Solar heat gain enabled the bees to reduce the own endothermic activity considerably though at the same time the Tth was increased (Fig. 3). The bees’ use of solar heat to reduce their own endothermic heat production was somewhat inconsistent at different ambient temperatures. At present we cannot explain the differences in the regressions’ slopes in Fig. 7 conclusively. Microclimatic effects not detectable by measurement of the operative temperature with the Te thermometer method, or microclimatic differences between the Te thermometers’ and the bees’ positions seem to have some importance. We also presume physiological or behavioral control mechanisms and reactions of the bees, allowing them to regulate their body temperature at different levels according to the environmental parameters and to their motivation.
Fig. 7 shows that a considerable amount of the endothermically generated heat was transferred to the head and the abdomen. The endothermic temperature excess added up for the three body parts (Fig. 8A) represents a correlate of the bees’ total amount of endothermic heat production. Fig. 8B reveals that the endothermic effort depended strongly on Ta. This resembles the dependence of energy metabolism of endothermic bees on Ta (e.g. Blatt and Roces, 2001; Moffatt, 2001; Stabentheiner et al., 2003). Our analysis also demonstrates that bees reduce energetic investment as insolation increases (Fig. 8). This reduction is probably smaller at high Ta. Such considerations, however, have to take into account that in our type of investigation any heat loss to ambience via respirative ventilation cannot be detected as temperature excess. The evaporative cooling due to (mainly cuticular) transpiration, on the other hand, is involved in our calculation as we used fresh dead bees for our operative temperature measurements. We presume the cuticular transpiration to be similar in living and fresh dead bees. In order to estimate the endothermic part of thermoregulation as accurate as possible, therefore, it is necessary to use fresh killed bees (with the same water content as living bees).
At high ambient temperatures (>∼30 °C), the bees’ thermoregulatory challenge was not the prevention of heat loss but the avoidance of overheating, especially in bright sunshine. One measure they took was a nearly completely reduction of endothermy (Fig. 7). In addition they cooled themselves via the ingested water. Fig. 4 also suggests that they actively seeked water patches with a temperature below Ta. A very similar behavior was observed in water foraging wasps (Vespula, Polistes; Kovac et al., 2009). We do not know whether bees (and wasps) increase respiratory ventilation to improve evaporative cooling in addition to cooling due to water ingestion.
4.3. Duration of foraging stay, crop load and strategies of optimization
Honeybees foraging from water sources obviously pursue a mixed strategy to use the heat gain from solar radiation. On the one hand they reduce energetic investment (Figs. 7 and 8), and on the other hand they increase the thorax temperature (Fig. 3).
At low to medium Ta (<∼30 °C) our bees had a higher Tth at take off than after landing. A similar relation was observed in bees gathering water (Schmaranzer, 2000) and in sucrose foragers (Schmaranzer and Stabentheiner, 1988; Waddington, 1990). Coelho (1991a) reported an increase of force production of flying bees with Tth up to a maximum at about 38–39 °C. Mean take off Tth of our water foragers was in this range (Fig. 5). We suggest that heavily loaded bees regulate flight muscle temperature to the optimum buoyancy temperature to facilitate take off and to improve flight performance in the initial phase after departure. Investing part of the external heat gain into a higher Tth, therefore, helps to optimize the function of flight muscles for the returning flight. At high Ta (>∼30 °C) Tth was already beyond the optimum value for take off at landing (∼42 °C) and a further increase was not necessary. Rather, the bees seemed to have troubles to get rid of excessive heat after flight and therefore cooled down to ∼41.0 °C towards departure.
However, the bees kept Tth at a high level throughout the whole stays at the water barrel even at the prolonged stays at low Ta (Figs. 2 and 9). Lowering Tth to the minimum for take off (∼30 °C; Esch, 1976; Coelho and Ross, 1996; Heinrich, 1993) would save much energy. Our analysis revealed that the suction rate depended especially strong on the head temperature (Fig. 10B and Tables 5 and 6). The increase was approximately exponential and in the range of 26–36 °C the rate was elevated at a factor of 3.7 (Q10)! This corresponds with a report of Núñez (1966) that cooling of the mouthparts prolonged the drinking time of sucrose foraging bees. Regulation of Tth at a high level even at low Ta allows the bees to keep Thd at a level high enough to guarantee a high suction speed. The shortened duration of stay (Fig. 9) in turn compensates at least in part for the higher energetic costs of a high Tth. In addition, it has to be kept in mind that cooling down would require an additional period of pre-flight warm-up (at ∼7.5 °C/min; Heinrich, 1979b; Stabentheiner et al., 2002) and this way would prolong the duration of stay.
Fig. 10A shows that the bees reduced crop loading as ambient temperature decreased. This resembles investigations on the amount of crop loading of sucrose foraging honeybees (Núñez, 1966; Pflumm, 1977; Marchl, 1986; Afik and Shafir, 2007). The question arises of whether this is an energetic or a functional optimization. Moffatt (2000) reported that a reduction of crop load is not an energetic optimization strategy as important as supposed by Schmidt-Hempel (1985). A smaller crop load surely reduces the drinking time, and this way the energetic costs per stay at the water barrel. This, however, means additional, costly foraging trips for the same amount of water. Therefore we suggest energetic optimization not to be the main purpose of the decreased crop load (compare Varjú and Núñez, 1991). Rather, optimization of the flight performance seems to be more important. Heinrich (1979b) and Woods et al. (2005) reported Tth in flight to decrease with Ta. In parallel, wingbeat frequency decreased. Coelho (1991a) observed a decline of flight force production with decreasing thorax temperature at a Tth below 39 °C. At low to medium Ta our water foragers displayed mean Tths of 36–37 °C at landing after flight (Fig. 5), which means that the unloaded water foragers seemed to fly with a suboptimal Tth concerning optimization of buoyancy (Coelho, 1991a). At the returning flight a high Tth is of higher importance because the bees are heavily loaded. Frisch and Lindauer (1955) observed that unloaded bees were able to increase flight speed with increasing foraging motivation (higher sucrose content of the gathered food) considerably on their flight to a food source. Loaded foragers lacked this regulatory ability completely at the returning flight. Therefore, we suggest that water foraging bees reduce crop load with decreasing Ta because otherwise they would have troubles to remain airborne. In addition, they reduce landing weight at about the same rate as crop loading (Fig. 10A). At present we do not know how this is accomplished, by reduction of provisioning or by increased egestion of the rectal bladder or the midgut. This we suggest to primarily be an energetic optimization because it allows them to load more water and to reach the water barrel faster at lower costs (Wolf et al., 1989; Feuerbacher et al., 2003).
5. Conclusion
A flexible thermal strategy allows honeybees to collect water at extremely variable environmental conditions. They are able to compensate for extreme heat loss in the cold and to prevent overheating in bright sunshine at high ambient temperature. Solar heat gain is used for a double purpose: to reduce energetic expenditure and to increase the thorax temperature to improve force production of flight muscles. A high thorax temperature also allows regulation of the head temperature high enough to guarantee proper function of the bees’ suction pump even at low ambient temperature. This shortens the foraging stays and in turn reduces energetic costs and improves efficiency.
Acknowledgements
Supported by the Austrian Fonds zur Förderung der Wissenschaftlichen Forschung (FWF, P16584-B06, P20802-B16). We greatly appreciate the help with electronics and software by G. Stabentheiner and S.K. Hetz, with data evaluation by M. Ablasser, B. Klug, B. Maurer and G. Rauter and for technical assistance by H. Käfer.
Contributor Information
Helmut Kovac, Email: he.kovac@uni-graz.at.
Anton Stabentheiner, Email: anton.stabentheiner@uni-graz.at.
References
- Afik O., Shafir S. Effect of ambient temperature on crop loading in the honey bee, Apis mellifera (Hymenoptera: Apidae) Entomologia Generalis. 2007;29:135–148. [Google Scholar]
- Bakken G.S. A heat transfer analysis of animals: unifying concepts and the application of metabolism chamber data to field ecology. Journal of Theoretical Biology. 1976;60:337–384. doi: 10.1016/0022-5193(76)90063-1. [DOI] [PubMed] [Google Scholar]
- Bakken G.S. The use of standard operative temperature in the study of the thermal energetics of birds. Physiological Zoology. 1980;53:108–119. [Google Scholar]
- Bakken G.S. Measurement and application of operative and standard operative temperatures in ecology. American Zoologist. 1992;32:194–216. [Google Scholar]
- Balderrama N.M., Almeida L.O., Nunez J.A. Metabolic rate during foraging in the honey bee. Journal of Comparative Physiology B. 1992;162:440–447. doi: 10.1007/BF00258967. [DOI] [PubMed] [Google Scholar]
- Bishop J.A., Armbruster W.S. Thermoregulatory abilities of Alaskan bees: effects of size, phylogeny and ecology. Functional Ecology. 1999;13:711–724. [Google Scholar]
- Blatt J., Roces F. Haemolymph sugar titres in foraging honey bees (Apis mellifera carnica): dependence on metabolic rate and in vivo measurements of maximal rates of trehalose synthesis. Journal of Experimental Biology. 2001;204:2709–2716. doi: 10.1242/jeb.204.15.2709. [DOI] [PubMed] [Google Scholar]
- Cena K., Clark J.A. Effect of solar radiation on temperatures of working honey bees. Nature. 1972;236:222–223. doi: 10.1038/newbio236222a0. [DOI] [PubMed] [Google Scholar]
- Coelho J.R. The effect of thorax temperature on force production during tethered flight in the honeybee (Apis mellifera) drones, workers and queens. Physiological Zoology. 1991;64:823–835. [Google Scholar]
- Coelho J.R. Heat transfer and body temperature in honey bee (Hymenoptera: Apidae) drones and workers. Environmental Entomology. 1991;20:1627–1635. [Google Scholar]
- Coelho J.R., Ross A.J. Body temperature and thermoregulation in two species of yellowjackets, Vespula germanica and V. maculifrons. Journal of Comparative Physiology B. 1996;166:68–76. [Google Scholar]
- Coelho J.R., Holliday C.W., Hastings J.M., Maty E., Swigart M., Mendell A. Thermoregulation in male western cicada killers (Sphecius grandis Say) in the Chihuahuan desert. Journal of Thermal Biology. 2007;32:270–275. [Google Scholar]
- Cooper P.D., Schaffer W.M., Buchmann S.L. Temperature regulation of honey bees (Apis mellifera) foraging in the Sonoran desert. Journal of Experimental Biology. 1985;114:1–15. [Google Scholar]
- Digby P.S.B. Factors affecting the temperature excess of insects in sunshine. Journal of Experimental Biology. 1955;32:279–298. [Google Scholar]
- Dyer C.D., Seeley T.D. Interspecific comparison of endothermy in honey-bees (Apis): deviations from the expected size-related patterns. Journal of Experimental Biology. 1987;127:1–26. [Google Scholar]
- Feuerbacher E., Fewell J.H., Roberts S.P., Smith E.F., Harrison J.F. Effects of load type (pollen or nectar) and load mass on hovering metabolic rate and mechanical power output in the honey bee Apis mellifera. Journal of Experimental Biology. 2003;206:1855–1865. doi: 10.1242/jeb.00347. [DOI] [PubMed] [Google Scholar]
- Esch H. Body temperature and flight performance of honey bees in a servomechanically controlled wind tunnel. Journal of Comparative Physiology. 1976;109:265–277. [Google Scholar]
- Frisch K.V., Lindauer M. Über die Fluggeschwindigkeit der Bienen und über ihre Richtungsweisung bei Seitenwind. Naturwissenschaften. 1955;42:377–385. [Google Scholar]
- Harrison J.F., Hall H.G. African-European honeybee hybrids have low intermediate metabolic capacities. Nature. 1993;363:258–260. [Google Scholar]
- Harrison J.F., Fewell J.H., Roberts S.P., Hall H.G. Achievement of thermal stability by varying metabolic heat production in flying honeybees. Science. 1996;274:88–90. doi: 10.1126/science.274.5284.88. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Thermoregulation of African and European honeybees during foraging, attack, and hive exits and returns. Journal of Experimental Biology. 1979;80:217–229. [Google Scholar]
- Heinrich B. Keeping a cool head: honeybee thermoregulation. Science. 1979;205:1269–1271. doi: 10.1126/science.205.4412.1269. [DOI] [PubMed] [Google Scholar]
- Heinrich B. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. I. Regulation of head temperature. Journal of Experimental Biology. 1980;85:61–72. [Google Scholar]
- Heinrich B. Mechanisms of body-temperature regulation in honeybees, Apis mellifera. II. Regulation of thoracic temperature at high air temperature. Journal of Experimental Biology. 1980;85:73–87. [Google Scholar]
- Heinrich B. Springer-Verlag; Berlin, Heidelberg, London, Paris: 1993. The Hot-Blooded Insects. [Google Scholar]
- Johansson T.S.K., Johansson M.P. Providing honeybees with water. Bee World. 1978;59:11–17. [Google Scholar]
- Klok C.J., Chown S.L. Assessing the benefits of aggregation: thermal biology and water relations of anomalous Emperor Moth caterpillars. Functional Ecology. 1999;13:417–427. [Google Scholar]
- Kovac H., Schmaranzer S. Thermoregulation of honeybees (Apis mellifera) foraging in spring and summer at different plants. Journal of Insect Physiology. 1996;42:1071–1076. [Google Scholar]
- Kovac H., Stabentheiner A., Schmaranzer S. Thermoregulation of water foraging wasps (Vespula vulgaris and Polistes dominulus) Journal of Insect Physiology. 2009;55:959–966. doi: 10.1016/j.jinsphys.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühnholz S., Seeley T.D. The control of water collection in honey bee colonies. Behavioral Ecology and Sociobiology. 1997;41:407–422. [Google Scholar]
- Lindauer M. Ein Beitrag zur Frage der Arbeitsteilung im Bienenstaat. Zeitschrift für vergleichende Physiologie. 1952;34:299–345. [Google Scholar]
- Lindauer M. The water economy and temperature regulation of the honeybee colony. Bee World. 1955;36 62–72; 81–92; 105–111. [Google Scholar]
- Marchl, R., 1986. Die Abflugmagenfüllung der Honigbiene in Abhängigkeit von verschiedenen äußeren Faktoren unter besonderer Berücksichtigung der Temperatur. Thesis, Karl-Franzens-Universität Graz, Austria.
- Moffatt L., Núñez J.A. Oxygen consumption in the foraging honeybee depends on the reward rate at the food source. Journal of Comparative Physiology B. 1997;167:36–42. doi: 10.1007/s003600050045. [DOI] [PubMed] [Google Scholar]
- Moffatt L. Changes in the metabolic rate of the foraging honeybee: effect of the carried weight or of the reward rate? Journal of Comparative Physiology A. 2000;186:299–306. doi: 10.1007/s003590050430. [DOI] [PubMed] [Google Scholar]
- Moffatt L. Metabolic rate and thermal stability during honeybee foraging at different reward rates. Journal of Experimental Biology. 2001;204:759–766. doi: 10.1242/jeb.204.4.759. [DOI] [PubMed] [Google Scholar]
- Núñez J. Quantitative Beziehungen zwischen den Eigenschaften von Futterquellen und dem Verhalten von Sammelbienen. Zeitschrift für vergleichende Physiologie. 1966;53:142–164. [Google Scholar]
- Park O.W. Activities of honeybees. In: Grout R.A., editor. The Hive and the Honeybee. Dadant and Sons; Hamilton, IL: 1946. [Google Scholar]
- Pflumm W. Welche Größen beeinflußen die Menge der von den Bienen und Wespen an der Futterquelle aufgenommenen Zuckerlösung. Apidologie. 1977;8:401–411. [Google Scholar]
- Ressi, R., 1989. Untersuchungen über Trinkmengen und Trinkgeschwindigkeiten von Carnica–und Ligustica–Bienen an einer künstlichen Futterquelle. Thesis, Karl-Franzens-Universität Graz, Austria.
- Robinson G.E., Underwood B.A., Henderson C.E. A highly specialized water-collecting honey bee. Apidologie. 1984;15:355–358. [Google Scholar]
- Schmaranzer S. Thermoregulation of water collecting honeybees (Apis mellifera) Journal of Insect Physiology. 2000;46:1187–1194. doi: 10.1016/s0022-1910(00)00039-1. [DOI] [PubMed] [Google Scholar]
- Schmaranzer S., Stabentheiner A. Variability of the thermal behaviour of honeybees on a feeding place. Journal of Comparative Physiology B. 1988;158:135–141. [Google Scholar]
- Schmidt-Hempel P., Kacelnik A., Houston A.I. Honeybees maximize efficiency by not filling their crop. Behavioral Ecology and Sociobiology. 1985;17:61–66. [Google Scholar]
- Seeley T.D., Camazine S., Sneyd J. Collective decision-making in honey bees—how 357 colonies choose among nectar sources. Behavioral Ecology and Sociobiology. 1991;28:277–290. [Google Scholar]
- Seeley T.D. Harvard University; Cambridge, USA: 1995. The Wisdom of the Hive. [Google Scholar]
- Sformo T., Doak P. Thermal ecology of Interior Alaska dragonflies (Odonata: Anisoptera) Functional Ecology. 2006;20:114–123. [Google Scholar]
- Stabentheiner A. Effect of foraging distance on the thermal behaviour of honeybees during dancing, walking and trophallaxis. Ethology. 1996;102:360–370. [Google Scholar]
- Stabentheiner A. Thermoregulation of dancing bees: thoracic temperature of pollen and nectar foragers in relation to profitability of foraging and colony need. Journal of Insect Physiology. 2001;47:385–392. doi: 10.1016/s0022-1910(00)00132-3. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A., Schmaranzer S. Thermografie bei Bienen: Körpertemperaturen am Futterplatz und im ‘Bienenbart’. Verhandlungen der Deutschen Zoologischen Gesellschaft. 1986;79:417–418. [Google Scholar]
- Stabentheiner A., Schmaranzer S. Thermographic determination of body temperatures in honey bees and hornets: calibration and applications. Thermology. 1987;2:563–572. [Google Scholar]
- Stabentheiner A., Schmaranzer S. Flight-related thermobiological investigations of honeybees (Apis mellifera carnica) In: Nachtigall W., editor. BIONA-report 6 Akademie der Wissenschaften und Literatur zu Mainz. Gustav Fischer; Stuttgart, New York: 1988. pp. 89–102. [Google Scholar]
- Stabentheiner A., Hagmüller K. Sweet food means ‘Hot Dancing’ in honey bees. Naturwissenschaften. 1991;78:471–473. [Google Scholar]
- Stabentheiner A., Kovac H., Hagmüller K. Thermal behavior of round and wagtail dancing honeybees. Journal of Comparative Physiology B. 1995;165:433–444. [Google Scholar]
- Stabentheiner A., Kovac H., Schmaranzer S. Honeybee nestmate recognition: the thermal behaviour of guards and their examinees. Journal of Experimental Biology. 2002;205:2637–2642. doi: 10.1242/jeb.205.17.2637. [DOI] [PubMed] [Google Scholar]
- Stabentheiner A., Vollmann J., Kovac H., Crailsheim K. Oxygen consumption and body temperature of active and resting honeybees. Journal of Insect Physiology. 2003;49:881–889. doi: 10.1016/S0022-1910(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Underwood B. Thermoregulation and energetic decision-making by the honeybees Apis cerana, Apis dorsata and Apis laboriosa. Journal of Experimental Biology. 1991;157:19–34. [Google Scholar]
- Varjú D., Núñez J. What do foraging honeybees optimize? Journal of Comparative Physiology A. 1991;169:729–736. [Google Scholar]
- Visscher P.K., Crailsheim K., Sherman G. How do honey bees (Apis mellifera) fuel their water foraging flights? Journal of Insect Physiology. 1996;42:1089–1094. [Google Scholar]
- Waddington K.D. Foraging profits and thoracic temperatures of honey bees (Apis mellifera) Journal of Comparative Physiology B. 1990;160:325–329. [Google Scholar]
- Wolf T.J., Schmidt-Hempel P., Ellington C.P., Stevenson R.D. Physiological correlates of foraging efforts in honey-bees: oxygen consumption and nectar load. Functional Ecology. 1989;3:417–424. [Google Scholar]
- Woods W.A., Heinrich B., Stevenson R.D. Honeybee flight metabolic rate: does it depend upon air temperature? Journal of Experimental Biology. 2005;208:1161–1173. doi: 10.1242/jeb.01510. [DOI] [PubMed] [Google Scholar]
- Woyciechowski M. Risk of water collecting in honeybee (Apis mellifera) workers (Hymenoptera: Apidae) Sociobiology. 2007;50:1059–1068. [Google Scholar]