Abstract
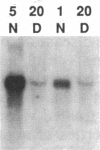
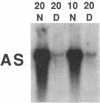
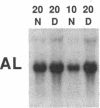
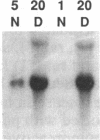
Citrullinemia is an inborn error of metabolism due to deficiency of the urea cycle enzyme, argininosuccinate synthetase [L-citrulline:L-aspartate ligase (AMP-forming), EC 6.3.4.5]. The disease was first described in humans but was recently reported in dairy cattle in Australia. Here we report the nucleotide sequence of the normal bovine cDNA for argininosuccinate synthetase and the mutation present in animals with citrullinemia. Analysis of DNA from affected animals by Southern blotting did not readily identify the mutation in the bovine gene. RNA (Northern) blotting revealed a major reduction in the steady-state amount of mRNA in the liver of affected animals to less than 5% of controls. The bovine cDNA was cloned and sequenced and revealed 96% identity with the deduced human sequence at the amino acid level. Starting with mutant bovine liver, the mRNA was reverse-transcribed; the cDNA product was amplified with the polymerase chain reaction, cloned, and sequenced. The sequence revealed a C----T transition converting arginine-86 (CGA) to a nonsense codon (TGA). A second C----T transition represented a polymorphism in proline-175 (CCC----CCT). The mutation and the polymorphism were confirmed by amplification of genomic DNA and demonstration with restriction endonuclease enzymes of both the loss of an Ava II site in DNA from mutant animals at codon 86 and the presence or absence of a Dde I site at codon 175. The loss of the Ava II site can be used for rapid, economical, nonradioactive detection of heterozygotes for bovine citrullinemia.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atweh G. F., Brickner H. E., Zhu X. X., Kazazian H. H., Jr, Forget B. G. New amber mutation in a beta-thalassemic gene with nonmeasurable levels of mutant messenger RNA in vivo. J Clin Invest. 1988 Aug;82(2):557–561. doi: 10.1172/JCI113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudet A. L., O'Brien W. E., Bock H. G., Freytag S. O., Su T. S. The human argininosuccinate synthetase locus and citrullinemia. Adv Hum Genet. 1986;15:161-96, 291-2. doi: 10.1007/978-1-4615-8356-1_3. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock H. G., Su T. S., O'Brien W. E., Beaudet A. L. Sequence for human argininosuccinate synthetase cDNA. Nucleic Acids Res. 1983 Sep 24;11(18):6505–6512. doi: 10.1093/nar/11.18.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Daar I. O., Maquat L. E. Premature translation termination mediates triosephosphate isomerase mRNA degradation. Mol Cell Biol. 1988 Feb;8(2):802–813. doi: 10.1128/mcb.8.2.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freytag S. O., Beaudet A. L., Bock H. G., O'Brien W. E. Molecular structure of the human argininosuccinate synthetase gene: occurrence of alternative mRNA splicing. Mol Cell Biol. 1984 Oct;4(10):1978–1984. doi: 10.1128/mcb.4.10.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper P. A., Healy P. J., Dennis J. A., O'Brien J. J., Rayward D. H. Citrullinaemia as a cause of neurological disease in neonatal Friesian calves. Aust Vet J. 1986 Nov;63(11):378–379. doi: 10.1111/j.1751-0813.1986.tb02907.x. [DOI] [PubMed] [Google Scholar]
- Healy P. J., Babidge P. J., Embury D. H., Harrison M. A., Judson G. J., Mason R. W., Petterson D. S., Sinclair A. J. Control of alpha-mannosidosis in Angus cattle. Aust Vet J. 1983 May;60(5):135–137. doi: 10.1111/j.1751-0813.1983.tb05925.x. [DOI] [PubMed] [Google Scholar]
- Healy P. J. Diagnosis of genotypes for generalized glycogenosis in cattle. Biochem Med. 1982 Oct;28(2):224–228. doi: 10.1016/0006-2944(82)90073-4. [DOI] [PubMed] [Google Scholar]
- Healy P. J. Lysosomal hydrolase activity in leucocytes from cattle, sheep, goats, horses and pigs. Res Vet Sci. 1982 Nov;33(3):275–279. [PubMed] [Google Scholar]
- Hocking J. D., Jolly R. D., Batt R. D. Deficiency of alpha-mannosidase in Angus cattle. An inherited lysosomal storage disease. Biochem J. 1972 Jun;128(1):69–78. doi: 10.1042/bj1280069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper M., Hardy K., Handyside A., Hunter S., Monk M. HPRT-deficient (Lesch-Nyhan) mouse embryos derived from germline colonization by cultured cells. Nature. 1987 Mar 19;326(6110):292–295. doi: 10.1038/326292a0. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., Beaudet A. L., O'Brien W. E. Mammalian urea cycle enzymes. Annu Rev Genet. 1986;20:431–464. doi: 10.1146/annurev.ge.20.120186.002243. [DOI] [PubMed] [Google Scholar]
- Kuehn M. R., Bradley A., Robertson E. J., Evans M. J. A potential animal model for Lesch-Nyhan syndrome through introduction of HPRT mutations into mice. Nature. 1987 Mar 19;326(6110):295–298. doi: 10.1038/326295a0. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., Barr R. H. Argininosuccinate lyase: purification and characterization from human liver. Biochemistry. 1981 Mar 31;20(7):2056–2060. doi: 10.1021/bi00510a049. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E. Isolation and characterization of argininosuccinate synthetase from human liver. Biochemistry. 1979 Nov 27;18(24):5353–5356. doi: 10.1021/bi00591a015. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., McInnes R., Kalumuck K., Adcock M. Cloning and sequence analysis of cDNA for human argininosuccinate lyase. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7211–7215. doi: 10.1073/pnas.83.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan B. M., Healy P. J., Fraser I. R., Nieper R. E., Whittle R. J., Sewell C. A. Generalised glycogenosis in Brahman cattle. Aust Vet J. 1981 May;57(5):227–229. doi: 10.1111/j.1751-0813.1981.tb02666.x. [DOI] [PubMed] [Google Scholar]
- PETRACK B., RATNER S. Biosynthesis of urea. VII. Reversible formation of argininosuccinic acid. J Biol Chem. 1958 Dec;233(6):1494–1500. [PubMed] [Google Scholar]
- RATNER S., PETRACK B. Biosynthesis of urea. IV. Further studies on condensation in arginine synthesis from citrulline. J Biol Chem. 1953 Jan;200(1):161–174. [PubMed] [Google Scholar]
- Ratner S. Argininosuccinate synthetase of bovine liver: chemical and physical properties. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5197–5199. doi: 10.1073/pnas.79.17.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner S. Enzymes of arginine and urea synthesis. Adv Enzymol Relat Areas Mol Biol. 1973;39:1–90. doi: 10.1002/9780470122846.ch1. [DOI] [PubMed] [Google Scholar]
- Rochovansky O., Kodowaki H., Ratner S. Biosynthesis of urea. Molecular and regulatory properties of crystalline argininosuccinate synthetase. J Biol Chem. 1977 Aug 10;252(15):5287–5294. [PubMed] [Google Scholar]
- Saheki T., Kusumi T., Takada S., Katsunuma T. Studies of rat liver argininosucciante synthetase. I. Physicochemical, catalytic, and immunochemical properties. J Biochem. 1977 Mar;81(3):687–696. doi: 10.1093/oxfordjournals.jbchem.a131505. [DOI] [PubMed] [Google Scholar]
- Saheki T., Nakano K., Kobayashi K., Imamura Y., Itakura Y., Sase M., Hagihara S., Matuo S. Analysis of the enzyme abnormality in eight cases of neonatal and infantile citrullinaemia in Japan. J Inherit Metab Dis. 1985;8(3):155–156. doi: 10.1007/BF01819306. [DOI] [PubMed] [Google Scholar]
- Sanger F. Determination of nucleotide sequences in DNA. Science. 1981 Dec 11;214(4526):1205–1210. doi: 10.1126/science.7302589. [DOI] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Abnormal mRNA for argininosuccinate synthetase in citrullinaemia. Nature. 1983 Feb 10;301(5900):533–534. doi: 10.1038/301533a0. [DOI] [PubMed] [Google Scholar]
- Su T. S., Beaudet A. L., O'Brien W. E. Increased translatable messenger ribonucleic acid for argininosuccinate synthetase in canavanine-resistant human cells. Biochemistry. 1981 May 12;20(10):2956–2960. doi: 10.1021/bi00513a037. [DOI] [PubMed] [Google Scholar]
- Su T. S., Bock H. G., Beaudet A. L., O'Brien W. E. Molecular analysis of argininosuccinate synthetase deficiency in human fibroblasts. J Clin Invest. 1982 Dec;70(6):1334–1339. doi: 10.1172/JCI110736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh L. C., Morris S. M., O'Brien W. E., Beaudet A. L. Nucleotide sequence of the cDNA encoding the rat argininosuccinate synthetase. Nucleic Acids Res. 1988 Oct 11;16(19):9352–9352. doi: 10.1093/nar/16.19.9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd J. A., Bell J. I., McDevitt H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987 Oct 15;329(6140):599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- Wood P. A., Partridge C. A., O'Brien W. E., Beaudet A. L. Expression of human argininosuccinate synthetase after retroviral-mediated gene transfer. Somat Cell Mol Genet. 1986 Sep;12(5):493–500. doi: 10.1007/BF01539920. [DOI] [PubMed] [Google Scholar]
- Zoghbi H. Y., Daiger S. P., McCall A., O'Brien W. E., Beaudet A. L. Extensive DNA polymorphism at the factor XIIIa (F13A) locus and linkage to HLA. Am J Hum Genet. 1988 Jun;42(6):877–883. [PMC free article] [PubMed] [Google Scholar]