Abstract
Intricately decorated Lapita pottery (3100–2700 BP) was made and deposited by the prehistoric colonizers of Pacific islands, east of the main Solomon's chain. For decades, analyses of this pottery have focused on the ancestor–descendant relationships of populations and the relative degree of interaction across the region to explain similarities in Lapita decoration. Cladistic analyses, increasingly used to examine the evolutionary relationships of material culture assemblages, have not been conducted on Lapita artefacts. Here, we present the first cladistic analysis of Lapita pottery and note the difficulties in using cladistics to investigate datasets where a high degree of horizontal transmission and non-branching evolution may explain observed variation. We additionally present NeighborNet and phenetic distance network analyses to generate hypotheses that may account for Lapita decorative similarity.
Keywords: networks, cladistics, Lapita, Oceania, pottery
1. Introduction
Cladistic techniques have demonstrated their potential to investigate patterns of human cultural, biological and linguistic relatedness in an elegant statistical manner (Mace & Pagel 1994; O'Brien et al. 2001; Tehrani & Collard 2002; McMahon & McMahon 2005; Kimbel et al. 2006; Lipo et al. 2006; Skelton 2008). However, particular historical circumstances may be difficult to investigate using cladistics (cf. Tëmkin & Eldridge 2007). For example, excessive rates of horizontal transmission may adversely affect the ability of cladistics to resolve an accurate population history (Nunn et al. 2010). Borgerhoff Mulder et al. (2006; see also Nunn et al. 2010) have examined this problem through simulations and determined that with increasing horizontal transmission relative to vertical transmission, geographical distance, not phylogenetic distance, is a better predictor of cultural trait variation. When it seems likely that horizontal transmission structures vary to a large degree, some scholars have relied upon phylogenetic techniques that allow for and attempt to identify reticulation (e.g. Hurles et al. 2003; Gray et al. 2007). Gray et al. (2007) note that horizontal transmission can be investigated using techniques such as NeighborNet (Bryant & Moulton 2004), and that incongruent transmission histories for different traits or suites of traits can be investigated using Bayesian multiple topology mixture models (Pagel & Meade 2004).
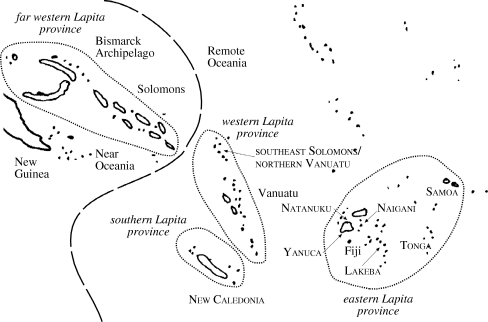
These potential problems may also be addressed by comparing results from cladistic analysis and from non-phylogenetic phenetic distance analysis. In this paper, we explain variation in archaeological materials by combining phenetic distance networks, cladistics and NeighborNet. We use these techniques to analyse variation in the decorative motifs of the Lapita pottery tradition, the earliest (3100–2700 BP) prehistoric pottery in Remote Oceania (figure 1). Our cladistic and NeighborNet analyses indicate that decorative variation across pottery assemblages may be largely explained by horizontal transmission between contemporary populations with several spatially overlapping local populations (demes) identified, some previously recognized through traditional archaeological approaches. Network analysis of phenetic distances in the same data identify similar local populations, but also several phenetic similarity-based links between assemblages that help define hypotheses of population structure in the southwest Pacific during the first several hundred years of human occupation (3100–2700 BP). The next section briefly compares the approaches of cladistics, NeighborNet and phenetic distance networks. Following this we present our analyses within the context of previous archaeological research and discuss methodological issues in the phylogenetic analysis of pottery assemblages.
Figure 1.
Some of the archipelagos of Near and Remote Oceania with Lapita provinces noted. Analysed assemblages are labelled using small capital letters.
2. Phenetic distance networks, cladograms and neighbornets
Our description of phenetic distance network methods draws largely on the work of quantitative sociologists and others including Carrington et al. (2005), Hage & Harary (1996), Lipo (2006), Scott (2000) and Wasserman & Faust (1994). Nodes represent taxa that are connected by edges describing the quantitative difference between taxa. To quantify taxon similarity, we calculate Hamming distances (Hamming 1980), the number of character state differences between taxa. For example, two taxa described by four binary characters as 0001 and 1011 differ by a Hamming distance of two. We call the resulting phenetic distance network a mini-max graph, because it displays only those edges with Hamming distances equal to, or less than, the highest value required to connect all nodes in the graph to at least one other node using a distance-minimizing algorithm. This graph, therefore, employs a parsimony criterion as it accounts for the greatest number of character similarities among all nodes in the simplest way within the rules of the method. Node position as depicted visually is a product of multi-dimensional scaling (MDS) performed on the data matrix. Edge thickness corresponds to the number of character states shared between nodes. Thicker edges indicate a greater number of character states shared and thus a lower Hamming distance. The matrix of pairwise Hamming distances is calculated using simple Microsoft Excel macros, and the graphical depiction of the resulting network is obtained using NetDraw, a program within UCINET 6 for Windows (Borgatti et al. 2010).
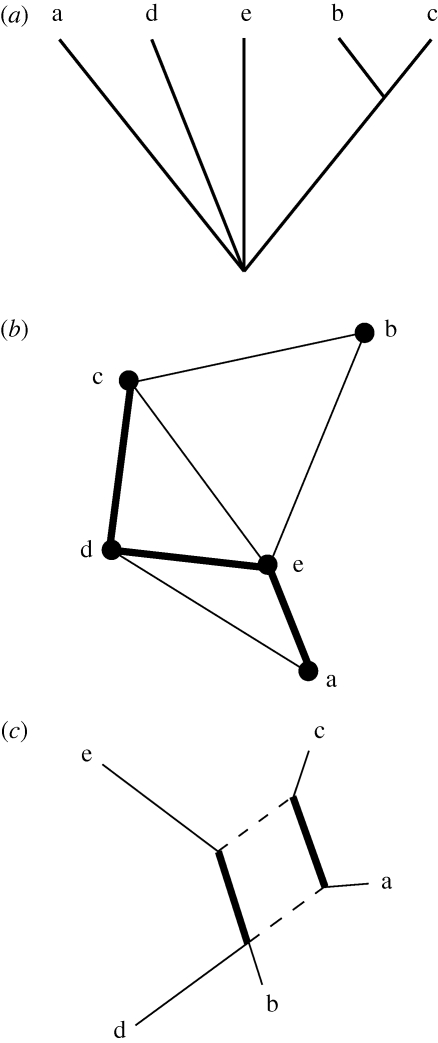
Table 1 gives a set of character states for a theoretical dataset with five taxa and seven characters. Figure 2 compares the different depictions of taxon similarity and assumed relatedness using cladistics, the mini-max graph method and NeighborNet. Figure 2a is a consensus cladogram (CI = 0.63, RI = 0.33) based on five trees generated from this character matrix using PAUP* 4.0. The relationships between classes a, d and e are apparently unresolved. Figure 2b is a mini-max graph of the same character matrix. Finally, for comparison, NeighborNet output obtained for the same data using Splits Tree4 (Huson & Bryant 2006) is shown in figure 2c and displays two conflicting splits (or clades). The split including taxa ‘c’ and ‘e’ (bold lines) conflicts with the split including taxa ‘a’ and ‘c’ (dashed lines).
Table 1.
Example dataset with seven characters and five taxa.
| class | C1 | C2 | C3 | C4 | C5 | C6 | C7 |
|---|---|---|---|---|---|---|---|
| a | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| b | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| c | 1 | 0 | 1 | 1 | 1 | 0 | 1 |
| d | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| e | 1 | 0 | 0 | 1 | 0 | 1 | 0 |
Figure 2.
Three graphic representations of relatedness among the same set of taxa using different methodological assumptions: (a) consensus tree, (b) a mini-max graph with node position determined through metric MDS and line thickness indicating Hamming distance, (c) a neighbornet with conflicting splits indicated by bold and dashed lines, respectively.
Although the cladistic depiction of relatedness is based on the distribution of derived characters, the mini-max graph depiction is based on the minimum phenetic distances required to link all the taxa under consideration. This is not the same use of phenetic distance as in numerical taxonomy (e.g. Sokal & Sneath 1963) or other statistical clustering approaches, where all pairwise phenetic distances contribute to a final hierarchical arrangement of taxa or similarity matrix order. Instead, assuming that our taxa descriptions track similarity that mainly results from common cultural transmission pathways, the phenetic distance measures used in our mini-max graphs are the minimal transmission pathways necessary to connect a set of taxa, but without specifying ancestor–descendant relationships as part of the ordering algorithm.
3. What was the population structure of the lapita colonizers of remote oceania?
(a). Previous research on the cultural relatedness of Lapita colonists
Remote Oceania was colonized from Near Oceania approximately 3100 BP (figure 1). This is about 400 years after Lapita pottery is first deposited in Near Oceania, a region inhabited for over 40 000 years. The similarity of complex Lapita designs across Near and Remote Oceania suggests that design variation is a product of cultural relatedness.
Lapita pottery comprises diverse vessel forms and an intricate decorative system that has been the subject of intense archaeological study for the last few decades (summarized in Kirch 1997), including multiple classifications of the Lapita decorative system, all with the intent of measuring homologous similarity (Mead et al. 1973; Anson 1983; Poulsen 1987; Siorat 1990; Chiu 2003). Mead et al. (1973) observed that the decorations on Lapita pots were made using a limited number of individual dentate stamps and shaped tools to create ‘design elements’ (DEs). DEs were identified as the smallest or most exclusive components of decoration that frequently occurred on pottery sherds across archaeological sites (Mead et al. 1973, p. 20) and include, for example, a dashed crescent line placed vertically (DE 1) and a dashed oval with pointed ends (DE 4). DEs may appear by themselves or are combined in patterned ways to form motifs that are repeated across the surface of vessels. Hundreds of motifs are recognized, with new motifs still being identified (e.g. Chiu 2003).
Motifs are the primary analytical unit used in comparative research examining the cultural relatedness of Lapita communities. Green (1979), for example, suggested that transmission across the Lapita population in Remote Oceania was spatially structured, or at least became so soon after colonization. In support of this Green identified an inventory of early (ca 3100–3000 BP) motifs recorded from across Remote Oceania (figure 3), but soon thereafter the spatial distribution of many motifs was restricted to different regions: far western, western, southern and eastern (figure 1). These geographical groupings, except for the southern region added later, were mostly determined by comparing motif inventories at archaeological sites using Jaccard correlation coefficients (Green 1979). Archaeologists have largely confirmed these regional groupings in subsequent analyses using other correlation measures and ordination techniques (e.g. Best 1984; Kirch 1988; Summerhayes 2001).
Figure 3.
A sample of Lapita motifs from Green (1979) with the motif number from the Mead system noted.
Archaeologists have also analysed Lapita motif distributions to determine the pattern of colonization within Remote Oceania and the frequency of post-colonization interaction between island populations. This is relevant for research that attempts to explain the origins of distinct cultural lineages in Remote Oceania that probably evolved from the earlier, possibly more culturally homogeneous, colonizing population (Green 1995). Of particular importance to many scholars is the evolution of Ancestral Polynesian Society, conceived as a distinct cultural lineage with its origins in the Tonga–Samoa region that diverged from a common cultural ancestor shared with Fijian populations (Kirch 1984; Kirch & Green 1987, 2001; cf. Boyd et al. 1997; Terrell et al. 1997).
Addressing questions about the patterns of colonization, Burley & Dickinson (2001, 2009) have identified Nukuleka (site Tonga1 in table 2) as the likely earliest colonization site in Tonga. The oldest Lapita sherds at Nukuleka bear similar motifs to those found in the Western Lapita province, specifically northern Vanuatu. Burley & Dickinson argue that Nukuleka was the initial settlement of migrants from northern Vanuatu and that these migrants may have bypassed Fiji. Additionally, Nukuleka may have ‘served as the initial staging point for population expansion within western Polynesia … to other parts of Tonga and into Samoa’ (Burley & Dickinson 2001, p. 11 830). This suggests that cladogenesis may explain some material culture variation between archipelago populations in this part of Remote Oceania (see also Burley et al. 2002).
Table 2.
Presence–absence matrix of Lapita motifs at selected Remote Oceanic sites. Data from Best (1984, table 9.3). Numbers after islands indicate early (1), middle (2) or late (3) assemblages as describe in text.
| motifa | Tonga1 | Tonga2 | Tonga3 | Lakeba1 | Lakeba2 | Lakeba3 | Yanuca1 | Yanuca2 | Yanuca3 | Natunuku1 | Natunuku2 | Natanuku3 | Naigani1 | Naigani2 | Samoa | SE Solomons | New Caledonia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DE1 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| M99 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| DE2 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 |
| P25, P26 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DE2.4 (m) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M1.5 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| A25, A26 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| DE3 (m) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| DE4 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 |
| DE4.2 (m) | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 393 (a) | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| DE9 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| DE10 (m) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M1 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 9 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| B11, B12, B16 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| B13, B14, B15 (p) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| M2 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| M2.5 (m) | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| M3 (m) | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M85 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M5 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 |
| M5.6 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M6 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 |
| M7 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| M8 (m) | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| M10 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 |
| M11 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| L3,L4 (b) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M13 (m) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M15 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| M16 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 |
| K8, K9 (p) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M16.1a (m) | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| D10, D11, D12, D14 (p) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| 169 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| P30 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M17 (m) | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 |
| M18 (m) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| M19 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| M20 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K6 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 209 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M21.1a (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M22 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M23 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M24 (m) | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| M34 (m) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| M34.2 (m) | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M25 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| M26 (m) | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| N4 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 313 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 |
| M37 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| M44 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M39 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| N6 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N7 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M27 (m) | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M28 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| M29 (m) | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| M30 (m) | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M45 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P13, P14 (p) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| P15, P16 (p) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 283 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P36 (p) | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 |
| P1-6 (p) | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| P32 (p) | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L14 (b) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M31 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M32 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M35 (m) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M1-4 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| M42 (m) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| E1-4 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M46 (m) | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| M12.2 (m) | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| M50, M51 (m) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| K17 (p) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K5 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K4 (p) | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| M14(2).10 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| K15 (p) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| K16 (p) | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| L1-6 (p) | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N8 (p) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D15 (p) | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| D16 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P7-10 (p) | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P23 (p) | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P17-19 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A31,A32 (p) | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| M76 (m) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| E5 (p) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| 272, 273 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| M65.3 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 100 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 345 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 41 (k) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| M33 (m) | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| M84 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| M74 (d) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 102 (k) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 104 (b) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 |
| 327 (a) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
aSources for motif descriptions in brackets: m = Mead et al. 1973; p = Poulsen 1987; a = Anson 1983, d = Donovan 1973; k = Kay 1984.
Clark & Murray (2006) also examined the pattern of colonization in Fiji and Tonga, and post-colonization interaction, through the distribution of Lapita motifs. Using the assumptions of ‘distance-decay’ (Green 1979) and unbiased transmission (Boyd & Richerson 1985; Neiman 1995; Bentley & Shennan 2003) to understand motif distribution, Clark & Murray argue that in any particular area, the oldest motifs will be the most abundant and the youngest will be least abundant. When a population colonizes a new area, primarily only the oldest, most abundant motifs, will enter the archaeological record of the colonized area, with the newer, less-established motifs dropping out of the decorative system. Clark & Murray (2006, p. 114–115) argue that their analysis of motif ranks suggests that east Fiji was colonized by populations from both Tonga and west Fiji and that there was no significant transmission of motif variants between newly arrived Fiji–Tonga populations and populations to the west in Vanuatu and New Caledonia.
These analyses and others (e.g. Kirch 1988; Sand 2007) have come to different, though not necessarily mutually exclusive, conclusions about colonization and transmission history in western Remote Oceania. One probable reason for these different conclusions is the different methods used by researchers to assess cultural relatedness. The new analyses presented here build upon this earlier work and improve our understanding of Remote Oceanic Lapita population structure and colonization history through methods designed to tease out ancestor–descendant relationships and horizontal transmission (see also O'Brien et al. 2001; Cochrane 2009, ch. 2).
(b). Methodological concerns in phylogenetic analyses of Lapita motifs in Remote Oceania
Even though phylogenetic models have been used to investigate variation in Pacific Island material culture for several decades (e.g. Kirch 1984; Kirch & Green 1987, 2001), it is only in the last few years that quantitative cladistic analyses have been applied to artefact datasets in the Pacific (e.g. Cochrane 2004, 2008, 2009; Shennan & Collard 2005; Tolstoy 2008). At least two methodological issues arise in the application of cladistics, NeighborNet and phenetic distance network analysis to Lapita decorative variation in Remote Oceania.
First, when taxa comprise artefact assemblages or types what does a cladogram, rooted tree, neighbornet or phenetic distance network represent in terms of human population history? Setting aside the depositional and taphonomic processes that may structure artefact assemblages, assemblage-based cladograms, neighbornets and phenetic distance networks may depict the homologies shared by assemblages and thus the population structure of the potters who transmitted these traditions, while a rooted assemblage tree, as argued by Tolstoy (2008), might also represent more general ancestor–descendant relationships among local populations (Collard & Shennan 2000). General ancestor–descendant relationships among human populations have of course been hypothesized from language trees (e.g. Gray & Jordan 2000; Rexova et al. 2003; cf. Borgerhoff-Mulder 2001; Terrell 2001). A problem with using artefact assemblage trees to reconstruct population history is that it is unlikely that variation in one, or even a few, specialized artefact types will correspond closely to the general pattern of genetic or linguistic relatedness (see Guglielmino et al. 1995; Jordan & Shennan 2003), although many would argue that Lapita pottery is a special case where just this correspondence would have occurred (Spriggs 1984; Kirch 1997; Green 2003).
A second methodological problem concerns classification. Whereas the similarity of Lapita motifs across Remote Oceania is certainly a result of cultural transmission, there have been no attempts to estimate the degree to which motifs are shared between assemblages because of common cultural descent, as opposed to chance convergence or mechanical constraints on motif application. Specht (2004) has discussed similar issues, noting that simple comparisons of Lapita assemblages based on various measures of motif similarity might not accurately estimate transmission history (see also Best 2002, p. 94). One possible solution is to weight characters in Lapita motif datasets. Alternatively, Pocklington (2006, p. 25–27) argues that if similarities in an analytical unit such as a design motif indicate shared cultural histories, then two or more subunits within the motif should generate assemblage dissimilarity matrices that are correlated when evaluated by a Mantel test. Such an analysis requires motif definitions to be formed from combinations of similarly scaled subunits, such as in a paradigmatic classification (Dunnell 1971), but this has not been done for Lapita motifs. As a work around, if Lapita motifs are shared owing to shared cultural transmission histories, then we can expect some general spatial patterns of motif similarity when comparing early and late assemblages. Abundant research (see Kirch 1997) indicates that the frequency of transmission between local populations in Remote Oceania declined during the first 500 years. Thus all else being equal, correlations between pairwise distance and motif similarity matrices for early sites should be greater than for late sites, because as transmission between local populations declines, these populations will probably diverge in their production and the use of stylistic motifs owing to drift (Dunnell 1978; Rogers & Ehrlich 2008).
4. Material and methods
(a). Lapita pottery dataset
The dataset of Lapita motifs used here (table 2) is generated from Best's (1984) archaeological research on Lakeba, an island in eastern Fiji (figure 1). To compare the Lakeba Lapita assemblage with others in the region, Best compiled the abundance of 106 motifs in 17 assemblages through direct analysis of pottery assemblages, examination of motif drawings, and previously published datasets. These assemblages are either from single-component sites with relatively homogeneous cultural deposits such as Mulifanua in Samoa, or from grouped single-component sites in the cases of two sites from New Caledonia (Ile des Pins and Site 13) and four sites from the southeast Solomons (northern Vanuatu, sites SE-RF 2 and 6, SE-SZ 8 and 45). Best placed the single-component sites in an early period based on radiocarbon dates and motif inventories (see also Sand 1997; Green et al. 2008; Rieth & Hunt 2008). Four sites from Tonga are grouped into three temporal assemblages: early, middle and late time periods (see also Burley 1998; Burley & Dickinson 2001; Burley & Connaughton 2007). Three sites from Fiji (Lakeba, Yanuca and Natunuku) are each considered separate assemblages and are divided into early, middle and late periods by Best (see also Clark & Anderson 2009). One site from Fiji, Naigani, is considered a separate assemblage divided into early and middle periods by Best. Precise provenience information (e.g. excavation squares and layers) for each assemblage is given by Best (1984, p. 619, table 9.2). Best's raw abundance data for motifs are transformed into presence/absence data in table 2. Oceanic archaeologists will recognize the weakness of this dataset: much archaeological work in Remote Oceania over the last 25 years has produced larger ceramic assemblages with many more recognized motifs, although this recent work lacks any detailed concordance data for the different motif classification systems used. If the research presented here proves worthwhile, the next step is to use a single, comprehensive classificatory system (e.g. Chiu 2003) to quantify all recovered Lapita assemblages in Remote Oceania for phylogenetic analysis.
(b). Methods: Mantel matrix test
To assess the ability of Lapita motifs to measure transmission, a Euclidean distance dissimilarity matrix was constructed from presence–absence data on the early Lapita motif assemblages at the archaeological sites of Tonga1, Lakeba1, Yanuca1 and Natunuku1 (table 2) and a second dissimilarity matrix was constructed for the late assemblages (Tonga3, Lakeba3, etc.) from these same sites. These are the only sites or areas in the dataset analysed here that contain both early and late Lapita assemblages. The geographical distances between sites were placed in matrices for both straight-line distance and estimated sailing distance (also straight-line, but not over land) and converted to Euclidean dissimilarities. Separate Mantel tests of the correlations between the early and late Lapita motif matrices and both geographical distance matrices were carried out using XLSTAT, a statistical analysis add-in for Microsoft Excel.
(c). Methods: cladistic, NeighbourNet and phenetic distance network analyses
To examine how cultural transmission may structure Lapita assemblage similarity, the dataset in table 2 was initially analysed using PAUP* 4.0 (Swofford 2001). To investigate the importance of reticulation events, the same dataset was also analysed using both NeighborNet, as implemented in Splits Tree4 (Huson & Bryant 2006), and our phenetic distance network procedures (see above).
5. Results
Separate Mantel tests of the correlations between the early Lapita motif matrices and both geographical distance matrices indicate that these matrices are correlated (straight-line distance: r = 0.845, two-tailed p value = 0.043; sailing distance: r = 0.855, two-tailed p value = 0.04). Mantel tests of the correlation between the late Lapita motif matrix and both geographical distance matrices indicate that these matrices are not correlated (straight-line distance: r = 0.409, two-tailed p value = 0.425; sailing distance: r = 0.428, two-tailed p value = 0.386). The tests support previous research indicating a decline over time in the frequency of transmission between local populations in Remote Oceania and therefore suggest Lapita motifs measure (through presence–absence) the degree to which assemblage similarity is a product of cultural transmission.
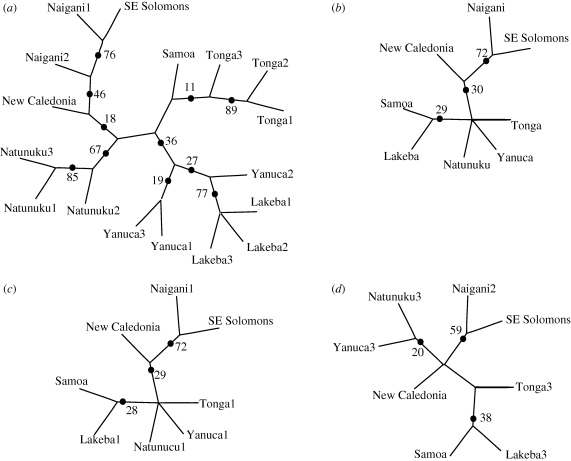
Cladistic parsimony analysis of all assemblages (taxa) described by Lapita motifs (characters, unordered) and divided into two time periods produced two unrooted cladograms (length = 225, CI = 0.45, RI = 0.49) that differ only in their resolution of the relationships between the three Lakeba assemblages. The 50 per cent majority-rule consensus cladogram (figure 4a) depicts several relationships between assemblages also identified through other archaeological research. These relationships include the high relative similarity and putative cultural relatedness of the Naigani assemblages and assemblages from archipelagos to the west (Best 1987), and the high similarity shared between Tonga and Samoa (particularly the late Tongan assemblage), and between Lakeba and Yanuca, respectively. However, regardless of outgroup choice, eastern Fiji (represented by Lakeba) is more closely related to Yanuca, a western Fijian assemblage, than to Tonga. This is not expected from research suggesting east Fijian populations are most closely related to Tongan populations (Burley & Dickinson 2001; Burley et al. 2002). Tree support statistics (CI, RI) for this cladogram are, however, not strong and a bootstrap analysis of 10 000 replicate matrices performed using PAUP* 4.0 default settings shows almost no support for the Samoa–Tonga clade, as it appears in less than 5 per cent of the replicate matrices. Additionally, the bootstrap analysis only weakly supports clades that contain both Yanuca and Lakeba assemblages, and in general, the higher bootstrap percentages towards the tips more strongly support clades comprised of temporally divided assemblages from the same site or island than clades combining assemblages from different regions of Remote Oceania.
Figure 4.
Consensus cladograms (50% majority rule) produced through cladistic analysis of the matrix in table 2. Numbers indicate the frequency of clades defined by the dot appearing in 10 000 replicate matrices. Clades without dots appear at less than 5% frequency in replicate matrices. (a) All assemblages divided by time periods, (b) all assemblages collapsed into single time period, (c) early assemblages and those that are not divided into periods, (d) late assemblages and those that are not divided into periods.
When temporally divided assemblages are collapsed into a single assemblage, and the presence–absence of motifs re-tabulated, the resulting analysis produces a consensus cladogram from 11 equal length cladograms (length = 152, CI = 0.59, RI = 0.35) that again does not strongly support a set of branching relationships for Lapita assemblages in Remote Oceania, except for the sister-taxa status of the Naigani and southeast Solomons assemblages (figure 4b). Additionally, the relationship of Tonga to other Remote Oceania Lapita assemblages is still ambiguous. Finally, consensus cladograms generated from matrices including either predominantly early Lapita assemblages (figure 4c; length = 152, CI = 0.59, RI = 0.35) or late Lapita assemblages (figure 4d; length = 101, CI = 0.71, RI = 0.38) suggest different geographical patterns of relatedness in different time periods. Resolved clades in the early Lapita period cladogram (figure 4c) include New Caledonia, the southeast Solomons and Naigani, along with a Samoa–Lakeba clade. Resolved clades in the late Lapita cladogram (figure 4d) include Naigani and the southeast Solomons, Natunuku and Yanuca, and again Samoa and Lakeba. In the late Lapita assemblages, Natunuku and Yanuca in western Fiji are separated from Lakeba in eastern Fiji, as well as Tonga and Samoa. Bootstrap analyses, however, do not strongly support the early and late Lapita cladograms. The poorly resolved consensus cladograms and generally low bootstrap values in these analyses suggest that horizontal transmission may explain similarities between assemblages.
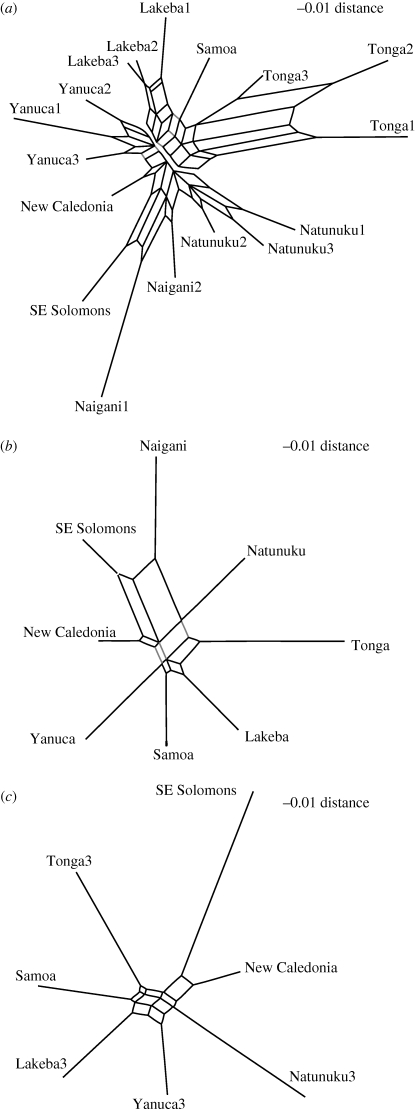
To investigate this horizontal transmission hypothesis, network analyses using both NeighborNet, as implemented in Splits Tree4 (Huson & Bryant 2006), and our phenetic distance network procedures were conducted. A neighbornet of all assemblages divided by time periods (figure 5a) places the Lakeba assemblages (east Fiji), Samoa, and the Tonga assemblages into a split separate from west Fijian assemblages, New Caledonia, and the southeast Solomons. A conflicting split groups the Lakeba and Yanuca (west Fiji) assemblages. Samoa can also be added to the Lakeba–Yanuca group, but this increases the phenetic distance linking all taxa in a Lakeba–Yanuca–Samoa group. The neighbornet in figure 5b shows temporally divided assemblages collapsed into single assemblages, and here any split containing Samoa and Tonga must also contain Lakeba and, importantly, Yanuca, suggesting no simple eastern Fiji/western Fiji population structure when examining the relatedness of Fijian, Tongan and Samoan populations (as seen through their ceramics). An almost exactly similar neighbornet (not shown) is produced when examining the early Lapita assemblages. A Neighbornet of predominantly late Lapita assemblages (figure 5c) produces several conflicting splits including Lakeba–Samoa–Tonga or Samoa–Lakeba–Yanuca, although late Lapita Tonga and Samoa share the greatest similarity. These neighbornets demonstrate that when looking at assemblages across Remote Oceania, and irrespective of focusing on early, late or combined Lapita assemblages, conflicting pictures of population structure may be obtained. However, divisions separating east Fiji–Tonga–Samoa from west Fiji (and New Caledonia) are certainly identifiable, particularly in the late Lapita assemblages.
Figure 5.
Neigbornets of assemblages described by the presence/absence of Lapita motifs. In (a) all assemblages are divided by time periods and a split containing the Lakeba and Tonga assemblages along with Samoa is highlighted by removing the edges that connect this split to the rest of the neighbornet. A conflicting split grouping Lakeba, Samoa and Yanuca is highlighted by light grey edges. In (b) all assemblages are collapsed into a single time period and to group Tonga and Samoa in the same split, Yanuca must be included (indicated by grey edges). (c) Late assemblages and those that are not divided into periods.
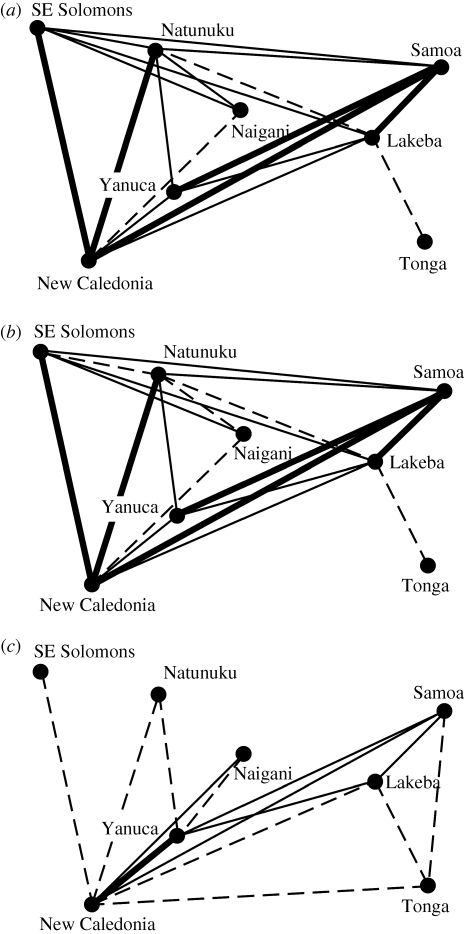
Combined with the cladograms and neighbornets, can the mini-max graph method help us generate clear hypotheses about evolutionary relationships in Remote Oceania? The mini-max graph with assemblages collapsed into a single time period (figure 6a) indicates that Tonga is the most weakly connected assemblage in the network, whereas Samoa is similarly connected to eastern (Lakeba) and western (Yanuca) Fijian assemblages. Density in network analysis is the ratio of edges present to the maximum possible edges in a network or some defined group within a network (Wasserman & Faust 1994), and a cohesion index measures the extent to which edges are concentrated within a group relative to between groups (Bock & Husain 1950; Wasserman & Faust 1994). A cohesion index value of 1 indicates no difference in the relative concentration of edges, whereas values above 1 indicate greater within-group concentration of edges and values less than 1 indicate greater between-group concentration of edges. The density of edges in both a Tonga–Lakeba–Samoa group and a Nagani–Yanuca–Natunuku (west Fiji) group is 0.67 while the cohesion indices for these groups are 0.25 and 0.22, respectively. These low cohesion indices indicate greater between-group connections than within groups and suggest that, considered as a whole (figure 6a), transmission during the Lapita period in Remote Oceania was relatively unstructured, at least relative to often recognized groups such as west Fiji and east Fiji–Tonga–Samoa. Figure 6b presents the early assemblages in a mini-max graph that is largely unchanged from the temporally collapsed mini-max graph in figure 6a. The only difference is the relatively weaker connections between Natunuku and both the southeast Solomons and Naigani. The mini-max graph in figure 6c consists of only the late Lapita assemblages and those not divided by time periods. In this graph, the density of edges in the Tonga–Lakeba–Samoa group is 1.0 (each is connected to the other) and the cohesion index is 0.5. In the Nagani–Yanuca–Natunuku group (west Fiji) density is 0.67 and cohesion is 0.3. The greater density index for the east Fiji–Tonga–Samoa group compared with the west Fiji group is owing to the edge connecting Tonga and Samoa and suggests a relative greater frequency of transmission within this group in the late Lapita period. The cohesion indices for both groups still indicate greater between-group than within-group transmission and that population structure during the late Lapita period may be more complicated than a division between west Fiji and east Fiji–Tonga–Samoa. The generally higher Hamming distances in the late Lapita period mini-max graph mirrors expectations of lower levels of transmission between local populations towards the end of Lapita.
Figure 6.
Mini-max graphs showing assemblages and edges of the highest Hamming distance or less required to connect all assemblages to the graph. Node positions are a schematic of geographical positions. Edges are grouped into three categories: dashed for highest Hamming distance (i.e. least similar), solid-thin for medium Hamming distance and solid-thick for lowest Hamming distance (i.e. most similar). Bin ranges for these categories were determined by generating a histogram of all Hamming distance scores with three bins and testing the fit of this distribution to a normal distribution (via Kolmogorov–Smirnov test). (a) All assemblages are collapsed into a single time period; (b) early assemblages and those that are not divided into periods; (c) late assemblages and those that are not divided into periods.
The mini-max graphs identify New Caledonia as consistently connected to the early and late Lapita assemblages throughout Fiji and Samoa, and to Tonga during the late Lapita period. These similarities are not readily apparent in the cladograms and neighbornets and suggest that New Caledonian populations may be more closely related to Fijian and west Polynesian populations throughout the Lapita period than is generally recognized (Sand 2001, 2007; Clark & Murray 2006; cf. Matisoo-Smith & Robins 2004). Interestingly, post-Lapita ceramic surface treatments in New Caledonia and Fiji are also similar, consisting of carved-paddle impressed designs. Best (2002, pp. 29–30) argues that these post-Lapita similarities are explained by cultural transmission. The mini-max graph analysis leads us to hypothesize that New Caledonia shares a relatively high degree of similarity with both west Fijian and east Fijian populations throughout the Lapita period and that this similarity is explained by relatively high levels of horizontal transmission.
6. Conclusion
Cladistic analyses of Remote Oceanic assemblages indicate that a nested hierarchy based on ancestral and derived traits, and therefore possibly a branching mode of evolutionary change, does not account for variation in the presence or absence of Lapita motifs. Lapita motif variation is more probably explained by the rapid colonization of this region and post-colonization transmission between local populations for 200 or more years. NeighborNet analyses also indicate no unambiguous grouping or population structure in the motif data, although groups, such as east Fiji–Tonga–Samoa, identified through other research are visible.
This analysis confirms that horizontal transmission is the best explanation for variation in Lapita motifs in Remote Oceania (cf. Summerhayes 2001). The mini-max graph method, like NeighborNet, explores phenetic similarity and identifies New Caledonia as sharing relatively high similarity with other Remote Oceanic Lapita populations. These same graphs indicate a low level of similarity between Tonga and other populations, a finding also supported by a high bootstrap frequency (72%) for two clades, one including the Tongan assemblages, and another clade containing all others. We propose that variation within Remote Oceanic Lapita material culture may be profitably analysed by including New Caledonian datasets to a greater degree, especially when investigating the putative cultural differences between prehistoric west Fiji, and east Fiji–Tonga–Samoa, as well as the Lapita origins of Polynesian society. Finally, a dimensional or paradigmatic (Dunnell 1971) classification of Lapita motif variation across Remote Oceania is required to carry these analyses forward.
Acknowledgements
This paper has benefited from comments provided by the participants at the AHRC CECD Theme B conference at Missenden Abbey, December 2008. The AHRC CECD provided funding for conference participation and software used in this analysis. The advice and editorial suggestions of Roger Green, John Terrell and two journal reviewers greatly improved the paper. We dedicate this paper to the memory of Roger Green.
Footnotes
One contribution of 14 to a Theme Issue ‘Cultural and linguistic diversity: evolutionary approaches’.
References
- Anson D.1983Lapita pottery of the Bismarck archipelago and its affinities. Sydney, Australia: University of Sydney [Google Scholar]
- Bentley R. A., Shennan S. J.2003Cultural transmission and stochastic network growth. Am. Antiquity 68, 459–485 (doi:10.2307/3557104) [Google Scholar]
- Best S. B.1984Lakeba: the prehistory of a Fijian island. PhD thesis, University of Auckland, New Zealand [Google Scholar]
- Best S.1987Long-distance obsidian travel and possible implications for the settlement of Fiji. Archaeol. Oceania 22, 31–32 [Google Scholar]
- Best S.2002Lapita: a view from the east. Auckland, New Zealand: Archaeological Association [Google Scholar]
- Bock R. D., Husain S. Z.1950An adaptation of Holzinger's B-coefficients for the analysis of sociometric data. Sociometry 13, 146 (doi:10.2307/2784941) [Google Scholar]
- Borgatti S., Everett M., Freeman L.2010UCINET 6 for Windows. Lexington, KY: Analytic Technologies [Google Scholar]
- Borgerhoff-Mulder M.2001Using phylogenetically based comparative methods in anthropology: more questions than answers. Evol. Anthropol. 10, 99–111 (doi:10.1002/evan.1020) [Google Scholar]
- Borgerhoff Mulder M., Nunn C. N., Towner M. C.2006Cultural macroevolution and the transmission of traits. Evol. Anthropol. 15, 52–64 (doi:10.1002/evan.20088) [Google Scholar]
- Boyd R., Richerson P. J.1985Culture and the evolutionary process. Chicago, IL: University of Chicago Press [Google Scholar]
- Boyd R., Borgerhoff-Mulder M., Durham W. H., Richerson P. J.1997Are cultural phylogenies possible? In Human by nature (eds Weingart P., Mitchell S. D., Richerson P. J., Maasen S.). Mahwah, NJ: Lawrence Erlbaum [Google Scholar]
- Bryant D., Moulton V.2004NeighborNet: an agglomerative algorithm for the constrution of planar phylogenetic networks. Mol. Biol. Evol. 21, 255–265 (doi:10.1093/molbev/msh018) [DOI] [PubMed] [Google Scholar]
- Burley D. V.1998Tongan archaeology and the Tongan past. J. World Prehist. 12, 337–392 (doi:10.1023/A:1022322303769) [Google Scholar]
- Burley D. V., Connaughton S.2007First Lapita settlement and its chronology in Vava'u, Kingdom of Tonga. Radiocarbon 49, 131–137 [Google Scholar]
- Burley D. V., Dickinson W. R.2001Origin and significance of a founding settlement in Polynesia. Proc. Natl Acad. Sci. USA 98, 11 829–11 831 (doi:10.1073/pnas.181335398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burley D. V., Dickinson W. R.2009Among Polynesia's first pots. J. Archaeol. Sci. 37, 1020–1026. (doi:10.1016/j.jas.2009.12.002) [Google Scholar]
- Burley D. V., Storey A., Witt J.2002On the definition and implication of eastern Lapita ceramics in Tonga. In Fifty years in the field. Essays in honour and celebration of Richard Shutler Jr's archaeological career (eds Bedford S., Sand C., Burley D. V.). Auckland, New Zealand: Archaeological Association [Google Scholar]
- Carrington P. J., Scott J., Wasserman S.(eds)2005Models and methods in social network analysis. Cambridge, UK: Cambridge University Press [Google Scholar]
- Chiu S.2003The socio-economic functions of Lapita ceramic production and exchange: a case study from Site WKO013A, Koné, New Caledonia. PhD thesis,University of California, Berkeley, CA [Google Scholar]
- Clark G. R., Anderson A.2009Site chronology and review of radiocarbon dates from Fiji. In The early prehistory of Fiji (eds Clark G., Anderson A.). Canberra, Australia: Australian National University E Press [Google Scholar]
- Clark G. R., Murray T.2006Decay characteristics of the eastern Lapita design system. Archaeol. Oceania 41, 107–117 [Google Scholar]
- Cochrane E. E.2004Explaining cultural diversity in ancient Fiji: the transmission of ceramic variability. DPhil. dissertation: University of Hawaii, Honolulu, HI [Google Scholar]
- Cochrane E. E.2008Migration and cultural transmission: investigating human movement as an explanation for Fijian ceramic change. In Cultural transmission in archaeology: issues and case studies (ed. O'Brien M. J.). Washington, DC: Society for American Archaeology [Google Scholar]
- Cochrane E. E.2009The evolutionary archaeology of ceramic diversity in ancient Fiji. Oxford, UK: Archaeopress [Google Scholar]
- Collard M., Shennan S.2000Processes of culture change in prehistory: a case study from the European Neolithic. In Archaeogenetics: DNA and the population prehistory of Europe (eds Renfrew C., Boyle K.). Oxford, UK: McDonald Institute for Archaeological Research [Google Scholar]
- Donovan L. J. 1973. A study of the decorative system of the Lapita potters in reefs and Santa Cruz islands. Masters essay, University of Auckland, New Zealand. [Google Scholar]
- Dunnell R. C.1971Systematics in prehistory. New York, NY: The Free Press [Google Scholar]
- Dunnell R. C.1978Style and function: a fundamental dichotomy. Am. Antiquity 43, 192–202 (doi:10.2307/279244) [Google Scholar]
- Gray R. D., Jordan F. M.2000Language trees support the express-train sequence of Austronesian expansion. Nature 405, 1052–1055 (doi:10.1038/35016575) [DOI] [PubMed] [Google Scholar]
- Gray R. D., Greenhill S. J., Ross R. M.2007The pleasures and perils of Darwinizing culture (with phylogenies). Biol. Theory 2, 360–375 (doi:10.1162/biot.2007.2.4.360) [Google Scholar]
- Green R. C.1979Lapita. In The prehistory of Polynesia (ed. Jennings J. D.). Cambridge, MA: Harvard University Press [Google Scholar]
- Green R. C.1995Linguistic, biological, and cultural origins of the original inhabitants of Remote Oceania. N. Z. J. Archaeol. 17, 5–27 [Google Scholar]
- Green R. C.2003The Lapita horizon and traditions–signature for one set of Oceanic migrations. In Pacific archaeology: assessments and prospects (ed. Sand C.). Nouméa, New Caledonia: Service des Musées et du Patrimonie de Nouvelle-Calédonie [Google Scholar]
- Green R. C., Jones M., Sheppard P.2008The reconstructed environment and absolute dating of SE-SZ-8 Lapita site on Nendö, Santa Cruz, Solomon Islands. Archaeol. Oceania 43, 49–61 [Google Scholar]
- Guglielmino C. R., Viganotti C., Cavalli-Sforza L. L.1995Cultural variation in Africa: role of mechanisms of transmission and adaptation. Proc. Natl Acad. Sci. USA 92, 7585–7589 (doi:10.1073/pnas.92.16.7585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hage P., Harary F.1996Island networks: communication, kinship, and classification structures in Oceania. Cambridge, UK: Cambridge University Press [Google Scholar]
- Hamming R.1980Coding and information theory. Upper Saddle River, NJ: Prentice-Hall [Google Scholar]
- Hurles M. E., Elizabeth M.-S., Gray R. D., Penny D.2003Untangling Oceanic settlement: the edge of the knowable. Trends Ecol. Evol. 18, 531–540 (doi:10.1016/S0169-5347(03)00245-3) [Google Scholar]
- Huson D. H., Bryant D.2006Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (doi:10.1093/molbev/msj030) [DOI] [PubMed] [Google Scholar]
- Jordan P., Shennan S.2003Cultural transmission, language, and basketry traditions amongst the California Indians. J. Anthropol. Archaeol. 22, 42–74 (doi:10.1016/S0278-4165(03)00004-7) [Google Scholar]
- Kay R. 1984. Analysis of archaeological material from Naigani. Masters thesis, University of Auckland, New Zealand. [Google Scholar]
- Kimbel W. H., Lockwood C. A., Ward C. V., Leakey M. G., Rak Y., Johanson D. C.2006Was Australopithecus anamensis ancestral to A. afarensis? A case of anagenesis in the hominin fossil record. J. Hum. Evol. 51, 134–152 (doi:10.1016/j.jhevol.2006.02.003) [DOI] [PubMed] [Google Scholar]
- Kirch P.1997The Lapita peoples. Oxford, UK: Blackwell [Google Scholar]
- Kirch P. V.1984The evolution of the Polynesian chiefdoms. Cambridge, UK: Cambridge University Press [Google Scholar]
- Kirch P. V.1988Niuatoputapu, the prehistory of a Polynesian chiefdom. Seattle, WA: Thomas Burke Memorial Washington State Museum [Google Scholar]
- Kirch P. V., Green R. C.1987History, phylogeny, and evolution in Polynesia. Curr. Anthropol. 28, 431–456 (doi:10.1086/203547) [Google Scholar]
- Kirch P. V., Green R. C.2001Hawaiki, ancestral Polynesia: an essay in historical anthropology. Cambridge, UK: Cambridge University Press [Google Scholar]
- Lipo C. P.2006The resolution of cultural phylogenies using graphs. In Mapping our ancestors: phylogenetic methods in anthropology and prehistory (eds Lipo C. P., O'Brien M. J., Collard M., Shennan S.). New York, NY: Aldine de Gruyter [Google Scholar]
- Lipo C. P., O'Brien M. J., Collard M., Shennan S.(eds)2006. In Mapping our ancestors: phylogenetic methods in anthropology and prehistory. New York, NY: Aldine de Gruyter [Google Scholar]
- Mace R., Pagel M.1994The comparative method in anthropology. Curr. Anthropol. 35, 549–564 (doi:10.1086/204317) [Google Scholar]
- Matisoo-Smith E., Robins J. H.2004Origins and dispersals of Pacific peoples: evidence from mtDNA phylogenies of the Pacific rat. Proc. Natl Acad. Sci. USA 101, 9167–9172 (doi:10.1073/pnas.0403120101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon A., McMahon R.2005Language classification by numbers. Oxford, UK: Oxford University Press [Google Scholar]
- Mead S. M., Birks L., Birks H., Shaw E. The Lapita pottery style of Fiji and its associations. Auckland, New Zealand: The Polynesian Society; 1973. [Google Scholar]
- Neiman F.1995Stylistic variation in evolutionary perspective: inferences from decorative diversity and interassemblage distance in Illinois woodland ceramic assemblages. Am. Antiquity 60, 7–36 (doi:10.2307/282074) [Google Scholar]
- Nunn C. L., Arnold C., Matthews L., Mulder M. B.2010Simulating trait evolution for cross-cultural comparison. Phil. Trans. R. Soc. B 365, 3807–3819 (doi:10.1098/rstb.2010.0009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien M. J., Darwent J., Lyman R. L.2001Cladistics is useful for reconstructing archaeological phylogenies: palaeoIndian points from the southeastern United States. J. Archaeol. Sci. 28, 1115–1136 (doi:10.1006/jasc.2001.0681) [Google Scholar]
- Pagel M., Meade A.2004A phylogenetic mixture model for detecting pattern-heterogeneity in gene sequence or character-state data. Syst. Biol. 53, 571–581 (doi:10.1080/10635150490468675) [DOI] [PubMed] [Google Scholar]
- Pocklington R.2006What is a culturally transmitted unit, and how can we find one? In Mapping our ancestors: phylogenetic methods in anthropology and prehistory (eds Lipo C. P., O'Brien M. J., Collard M., Shennan S.). New York, NY: Aldine de Gruyter [Google Scholar]
- Poulsen J.1987Early Tongan prehistory: the Laptia period on Tongatapu and its relationships. Canberra, Australia: Australian National University [Google Scholar]
- Rexova K., Frynta D., Zrzavy J.2003Cladistic analysis of languages: Indo-European classification based on lexicostatistical data. Cladistics 19, 120 (doi:10.1111/j.1096-0031.2003.tb00299.x) [Google Scholar]
- Rieth T. M., Hunt T. H.2008A radiocarbon chronology for Sāmoan prehistory. J. Archaeol. Sci. 35, 1901–1927 (doi:10.1016/j.jas.2007.12.001) [Google Scholar]
- Rogers D. S., Ehrlich P. R.2008Natural selection and cultural rates of change. Proc. Natl Acad. Sci. 105, 3416–3420 (doi:10.1073/pnas.0711802105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand C.1997The chronology of Lapita ware in New Caledonia. Antiquity 71, 539–547 [Google Scholar]
- Sand C.2001Evolutions in the Lapita cultural complex: a view from the southern Lapita province. Archaeol. Oceania 36, 65–76 [Google Scholar]
- Sand C.2007Looking at the big motifs: a typology of the central band decorations of the Lapita ceramic tradition of New Caledonia (Southern Melanesia) and preliminary regional comparisons. In Oceanic explorations: Lapita and western Pacific settlement (eds Bedford S., Sand C., Connaughton S. P.). Canberra, Australia: Australian National University E Press [Google Scholar]
- Scott J. Social network analysis: a handbook. London, UK:: Sage.; 2000. [Google Scholar]
- Shennan S., Collard M.2005Investigating processes of cultural evolution on the north coast of New Guinea with multivariate and cladistic analyses. In The evolution of cultural diversity: a phylogenetic approach (eds Mace R., Holden C. J., Shennan S.). London, UK: UCL Press [Google Scholar]
- Siorat J. P.1990A technological analysis of Lapita pottery decoration. In Lapita design, form, and composition. (ed. Spriggs M.). Canberra, Australia: Australia National University [Google Scholar]
- Skelton C.2008Methods of using phylogenetic systematics to reconstruct the history of the linear B script. Archaeometry 50, 158–177 [Google Scholar]
- Sokal R. R., Sneath P. H. A.1963Principles of numerical taxonomy. London, UK: W. H. Freeman [Google Scholar]
- Specht J.2004Lapita, the Solomons, and similarity measures. J. Polynesian Soc. 113, 369–376 [Google Scholar]
- Spriggs M.1984The Lapita cultural complex: origins, distribution, contemporaries, and successors. J. Pac. Hist. 19, 202–223 [Google Scholar]
- Summerhayes G. R.2001Far western, western, and eastern Lapita: a re-evaluation. Asian Perspect. 39, 109–138 (doi:10.1353/asi.2000.0013) [Google Scholar]
- Swofford D. L.2001PAUP*: Phylogenetic analysis using parsimony and other methods, 4.0 edn.Sunderland, MA: Sinauer Associates [Google Scholar]
- Tehrani J., Collard M.2002Investigating cultural evolution through biological phylogenetic analyses of Turkmen textiles. J. Anthropol. Archaeol. 21, 443–463 (doi:10.1016/S0278-4165(02)00002-8) [Google Scholar]
- Tëmkin I., Eldridge N.2007Phylogenetics and material culture evolution. Curr. Anthropol. 48, 146–153 (doi:10.1086/510463) [Google Scholar]
- Terrell J. E.(ed.)2001Archaeology, language, and history. Westport, CT: Bergin and Garvey [Google Scholar]
- Terrell J., Hunt T. L., Gosden C.1997The dimensions of social life in the Pacific: human diversity and the myth of the primitive isolate. Curr. Anthropol. 38, 155–195 (doi:10.1086/204604) [Google Scholar]
- Tolstoy P.2008Barkcloth, Polynesia and cladistics: an update. J. Polynesian Soc. 117, 15–57 [Google Scholar]
- Wasserman S., Faust K.1994Social network analysis: methods and applications. Cambridge, UK: Cambridge University Press [Google Scholar]