Abstract
Zooplankton feed in any of three ways: they generate a feeding current while hovering, cruise through the water or are ambush feeders. Each mode generates different hydrodynamic disturbances and hence exposes the grazers differently to mechanosensory predators. Ambush feeders sink slowly and therefore perform occasional upward repositioning jumps. We quantified the fluid disturbance generated by repositioning jumps in a millimetre-sized copepod (Re ∼ 40). The kick of the swimming legs generates a viscous vortex ring in the wake; another ring of similar intensity but opposite rotation is formed around the decelerating copepod. A simple analytical model, that of an impulsive point force, properly describes the observed flow field as a function of the momentum of the copepod, including the translation of the vortex and its spatial extension and temporal decay. We show that the time-averaged fluid signal and the consequent predation risk is much less for an ambush-feeding than a cruising or hovering copepod for small individuals, while the reverse is true for individuals larger than about 1 mm. This makes inefficient ambush feeding feasible in small copepods, and is consistent with the observation that ambush-feeding copepods in the ocean are all small, while larger species invariably use hovering or cruising feeding strategies.
Keywords: viscous vortex ring, copepod jump, Acartia tonsa, optimal foraging
1. Introduction
Zooplankton feed in one of three different principal modes: they can cruise through the water while searching for prey; they may generate a feeding current and capture prey arriving in this current; or they may be ambush feeders, sitting motionless in the water while waiting for prey to pass through their dining sphere, only occasionally performing upward jumps to compensate for their slow sinking (Kiørboe in press). Each of these feeding modes produces different hydrodynamical disturbances in the ambient water and thus causes different exposures to predators, because many zooplankton predators perceive their prey by the hydrodynamical disturbance that the prey produces (Feigenbaum & Reeve 1977; Jakobsen et al. 2006; Jiang & Paffenhöfer 2008). Hence, the advantages that a zooplankter achieves from a particular feeding behaviour should be traded off against the costs, including the predation risk that it entails.
The continuous fluid signals produced by cruising and feeding-current foragers are rather well understood in both unicellular (Langlois et al. 2009; Leptos et al. 2009) and larger metazoan zooplankters (Visser 2001; Jiang et al. 2002; Malkiel et al. 2003; Catton et al. 2007), whereas the short-lasting instantaneous fluid signals produced by jumps have not been well studied. Jumps are a common component of the motility repertoire of many plankters (Fenchel & Hansen 2006; Jakobsen et al. 2006). Planktonic copepods, the dominating group of mesozooplankton in the ocean, jump to escape predators (Fields & Yen 1997), to attack prey (Jiang & Paffenhöfer 2008; Kiørboe et al. 2009) or to reposition in the water column (Svensen & Kiørboe 2000). While the latter jumps are obviously less powerful than the former (Buskey et al. 2002), they may be much more frequent. Thus, ambush feeders typically reposition by jumping upwards every 1–10 s (Tiselius & Jonsson 1990; Titelman & Kiørboe 2003). As a result, the frequent weak repositioning jumps may create a hydrodynamic signal that may expose ambush feeders to a significant predation risk (Tiselius et al. 1997).
With the aim of evaluating the predation risk associated with the three principal feeding modes, we examined the fluid disturbances generated by repositioning jumps of the copepod Acartia tonsa. Ambush feeding is common among smaller pelagic copepods, mainly within the genus Oithona (e.g. Paffenhöfer 1993), while feeding-current feeding and cruising dominate among larger species.
It is well established that copepods performing strong escape jumps at Reynolds numbers (Re) >100 generate toroidal vortices in their wake (Yen & Strickler 1996; Duren & van Videler 2003). Vortex formation is indeed an inescapable consequence of any unsteady motion in water occurring at high Reynolds numbers (Dickinson 1996), and has consequently been much studied in the context of animal propulsion in fluid media at Re >100 and by applying inviscid theory (Dabiri 2009). We show here that even weak repositioning jumps (Re = 20–100) generate two viscous vortex rings: one vortex in the wake of the copepod and one vortex around the decelerating body. These viscous vortex rings are formed through the application of short-lasting localized momentum sources (e.g. Afanasyev 2004), which represents a different mechanism from flow separation such as the formation of a Kármán vortex street. We quantify the intensity of the fluid signal using particle image velocimetry (PIV), and use a simple analytical viscous vortex ring model and scaling arguments to demonstrate that ambush-feeding reposition jumps expose small copepods to a much lower predation risk than other feeding modes, whereas the reverse is true for copepods larger than about 1 mm.
2. Material and methods
Copepods, A. tonsa (prosome length 0.7–1.1 mm), were collected in November and December in Woods Hole, MA, USA, at sea temperatures of 6–10°C, and allowed to acclimate to room temperature overnight (20°C). Observations for flow visualization (PIV) were made in small aquaria (approx. 100 ml) with about 20 copepods and sufficient 5 µm tracer particles to make the water slightly cloudy. A vertical laser sheet was oriented through the aquarium. We used either a 1 W red continuous laser (200 µs shutter time) or a 45 W pulsed green laser with a 150 ns pulse duration (Photonics Industries DM30-527). High-speed, high-resolution (1024 × 1024 pixels) video recordings (1000 Hz) were made through a horizontally oriented dissecting microscope fitted with a Photron Fastcam 1024 PCI camera. The field of view was approximately 1 × 1 cm2.
Of the many jumps observed, we selected 16 jumps that occurred in the plane of the laser sheet and perpendicular to the view direction, and with the copepod oriented with its side towards the camera; no jumps were perfect in this respect. In some cases (four), the copepod jumped out of the field of view, allowing us to analyse only the jump wake. The jumps analysed were only the relatively weak reposition jumps described by Kiørboe et al. (in press). Unavoidable advection in the aquarium rarely allowed us to analyse induced flow velocities less than 1 mm s−1. Copepod prosome lengths were measured on the video, and copepod masses were estimated from their volumes, assuming the shape of a prolate spheroid with an aspect ratio 0.38. We assumed the mass density of both the copepod and the water to be 1 mg mm−3. We estimated the maximum linear momentum of the copepod, M, as the product of its peak velocity and mass and its (maximum) Reynolds number from its peak velocity and body width.
Video sequences were analysed using standard PIV software (LaVision, DaVis 7) to get instantaneous velocity and vorticity fields, as well as movies thereof. Time-integrated velocity and vorticity fields were computed in Matlab. Jump kinematics were analysed using ImageJ that was also used to compute the circulation of vortex rings. The circulation of a vortex ring is equal to the vorticity integrated in a meridional plane over the extension of the ring,
| 2.1 |
where ω(x,y,t) is the vorticity at position x, y at time t. In ImageJ, the selected ring was cropped and the picture thresholded at various vorticities (1, 3, 5, 10, 15, …, 50 s−1) and the extension areas (Aω(i,t)) within each threshold vorticity estimated. The circulation at each time step was then estimated as
| 2.2 |
Areas with flow velocity magnitude exceeding U* threshold velocities were estimated in a similar manner after subtraction of background flow magnitudes. Finally, we quantified the temporal change in the position of the maximum vorticity as the centre of mass of the 5 s−1 contour line (identified automatically by ImageJ).
3. Theory
The jumping copepod leaves a vortex in its wake, generated by the backward power stroke of the swimming legs. In order to analyse this vortex and allow us to extrapolate our observations to copepods of other sizes and jumps of other intensities, we apply a simple analytical model of a viscous vortex ring generated by an impulsive point force, an idealized description of the near-instantaneous power kick of the swimming legs. Here, we provide enough detail for the reader to follow the arguments, but refer the main derivations to electronic supplementary material, appendix A1.
The point force acts impulsively and imparts locally a finite momentum ρI to the fluid, where ρ is the mass density of the fluid and I the hydrodynamic impulse (length4time−1). The resulting backward jet forms a vortex ring, and the circulation of this ring subsequent to a virtual time origin t0 decays as
| 3.1 |
where υ is the kinematic viscosity of the fluid (electronic supplementary material, appendix A, equation (A 5)).
Rheotactic predators perceive prey from the fluid velocity that the prey generates and respond to velocities that exceed a critical magnitude, U* (Kiørboe & Visser 1999). We are thus interested in quantifying the extension of the region within which the induced flow velocity magnitude exceeds this critical value (U*). Right after the jump is initiated, it follows from electronic supplementary material, equations (A 11) and (A 12), that the maximum area of the vortex in the meridional plane with velocity magnitudes exceeding U* scales as
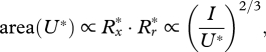 |
3.2 |
and from electronic supplementary material, equation (A 13), it follows that the time t* after which the whole flow field is below the threshold velocity is
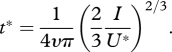 |
3.3 |
The vortex ring will expand radially owing to diffusion and translate downstream owing to advection, and the combined drift (electronic supplementary material, equation (A 17), Δx(t)) and diffusion (electronic supplementary material, equation (A 14), rω(t)) of the vorticity maximum leads to a total time-dependent distance of the vorticity maximum to the point of origin subsequent to a virtual time origin t0,
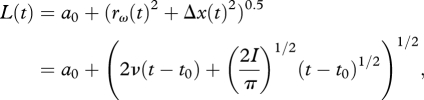 |
3.4 |
where a0 is the distance travelled from t0 until the vortex ring can be identified in the flow field.
4. Results
(a). Jump kinematics, flow and vorticity fields
A repositioning jump is initiated by the copepod sequentially striking backwards each of the four pairs of swimming legs while accelerating the body forward to a peak velocity of 100–200 mm s−1, reached after 10–25 ms at the end of the power stroke (figure 1a). During the subsequent leg recovery, the copepod coasts at a decelerating velocity and comes to an almost complete stop after another 10–25 ms. In some jumps, this beat cycle may be repeated several times (applies mainly to escape jumps). All jumps follow this scheme, although the detailed characteristics of the individual jumps with respect to the duration of the power stroke and peak velocity vary (table 1). The Reynolds numbers of the examined repositioning jumps varied between 20 and 100, and the momentum of the copepod at its peak velocity ranged from 5 to 30 mg mm s−1 (table 1).
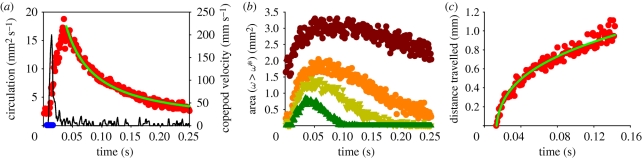
Figure 1.
Example of formation, decay, extent and translation of the wake vortex. (a) Temporal variation in the velocity of the copepod, the duration of the active stroke phase and the observed circulation of the eddy together with a least-square fit of equation (3.1) to the decaying phase of the vortex circulation. (b) Area of a wake eddy with vorticities exceeding threshold magnitudes. (c) Translation of the vorticity maximum position with a fit of equation (3.4). (a,b) jump 20-2, (c) jump 78 (table 1). (a) Red circles, observed circulation; black line, copepod velocity; blue circles, beat phase; green line, fitted decay. (b) Brown circles, ω > 1 s−1; orange circles, ω > 3 s−1; light green triangles, ω > 5 s−1; dark green triangles, ω > 10 s−1. (c) Red circles, observed; green line, regression.
Table 1.
Summary statistics for 16 analysed copepod jumps. Copepod length is prosome length; Umax is the maximum speed of copepod; Re is the Reynolds number of the copepod (= Umax × 0.38 × length/viscosity), where 0.38 is the aspect ratio of the copepod prosome; τ is the duration of the power stroke(s); copepod momentum was Umax × copepod volume, where the volume was computed by assuming the shape of a prolate sphere with length equal to prosome length and short axes equal to 0.38 × length.
| jump no. | copepod length, mm | Umax, mm s−1 | Re | τ, ms | peak vortex circulation, mm2 s−1 | momentum of copepod, mg mm s−1 | I, hydrodynamic impulse, mm4 s−1 | number beat cycles | comment |
|---|---|---|---|---|---|---|---|---|---|
| 12 | 0.97 | 173 | 64 | 7 | 17 | 10.3 | 7.7 | 1 | |
| 17 | 1.08 | 170 | 70 | 9 | 22.3 | 14.0 | 19.2 | 1 | |
| 20-1 | 1.04 | 208 | 82 | 10 | 14 | 15.3 | 8.8 | 1 | |
| 20-2 | 1.04 | 200 | 79 | 7 | 15 | 14.7 | 8.2 | 1 | |
| 26 | 0.93 | 89 | 31 | 16 | 5.4 | 4.6 | 4.2 | 1 | |
| 29 | 1.13 | 131 | 56 | 15 | 5.7 | 12.4 | 5.0 | 1 | |
| 34 | 0.99 | 82 | 31 | 12 | 6.5 | 5.2 | 3.6 | 1 | |
| 39-1 | 0.85 | 326 | 105 | 6 | 13 | 13.1 | 4.6 | 2 | jump out of view |
| 49 | 0.7 | 80 | 21 | 16 | 3.2 | 1.8 | 3.6 | 1 | |
| 58 | 1.11 | 130 | 55 | 16 | 13 | 11.6 | 11.9 | 1 | |
| 69 | 0.72 | 160 | 44 | 15 | 5.7 | 3.9 | 4.0 | 2 | |
| 73-2 | 1.12 | 172 | 73 | 23 | 31 | 15.8 | 18.6 | 3 | |
| 78 | 1.04 | 383 | 151 | 15 | 51.5 | 28.2 | 24.6 | 2 | jump out of view |
| 83 | 1.03 | 168 | 66 | 11 | 36.2 | 12.0 | 10.4 | 1 | |
| 88 | 0.97 | 273 | 101 | 22 | 15.6 | 16.3 | 6.2 | 3 | jump out of view |
| 93 | 0.97 | 188 | 69 | 8 | 23 | 11.2 | 9.22 | 1+ | jump out of view |
The power stroke of the swimming legs sends a jet of water backwards, which forms a vortex ring, evident in the two-dimensional plane as two counter-rotating eddies (figure 2 and electronic supplementary material, appendix movie S1). Another counter-rotating ring forms around the body of the decelerating copepod.
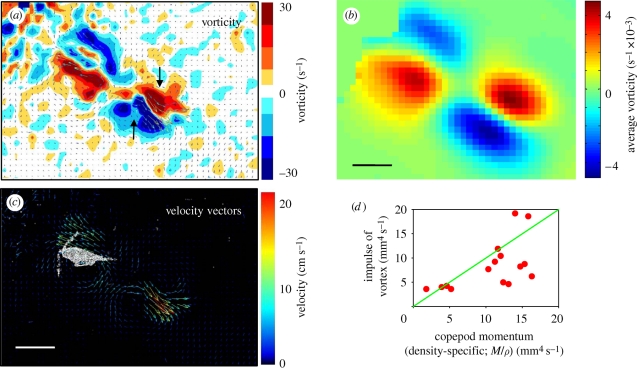
Figure 2.
Instantaneous flow and vorticity fields (a,c) around the jumping copepod 29 ms after initiation of the jump, and the vorticity field averaged over 250 ms (b). The black arrows in (a) show the approximate position around which the water circulates (vortex centre) (jump 20-2, table 1). (d) Hydrodynamic impulse of the wake vortex estimated from the temporal decay of the circulation (equation (3.1), figure 1a) as a function of the density-specific momentum of the copepod. (b,c) Scale bar, 1 mm.
(b). The wake vortex: formation, extent, translation, decay
During and subsequent to the jump, the vortex in the wake of the copepod forms and grows (figure 1). It reaches its largest extension about 50 ms after initiation of the jump, whereupon it shrinks and decays. In the example shown (figure 1b), the area in the meridional plane within which the vorticity exceeds 1 s−1 peaks at about 3 mm2, corresponding to an equivalent circular radius of the vortex of about 1 mm and, hence, a diameter of the doughnut-shaped vortex ring of approximately 4 mm. The circulation of the vortex ring reaches its peak value simultaneous with the maximum spatial extension of the eddy, whereupon it declines hyperbolically (figure 1a).
The point momentum source model (equation (3.1)) provides a good fit to the decay phase of the observed circulation (figure 1a) and thus allows us to use the model to estimate the hydrodynamic impulse (I) of the vortex ring (table 1). Because the momentum of the copepod has to equal the momentum of the backward jet of water, the magnitude of the estimated hydrodynamic impulse of the vortex impulse should be of similar magnitude as the density-specific momentum (M/ρ) of the copepod. The observations are consistent with this, although the vortex impulse in several cases appears to be underestimated (figure 2d).
After the wake vortex ring has formed, it grows radially owing to diffusion and at the same time translates downstream. As a result of these two processes, the position of the vorticity maximum moves and the movement is generally well described by the point momentum source model (equation (3.4); figure 1c). For a vortex generated by an instantaneous point force, the centre of the vortex and the position of the vorticity maximum separate over time (electronic supplementary material, appendix A). This separation is rather obvious from visual inspections of vorticity and flow fields (figure 2a) and can also be verified quantitatively (not shown), even though it is difficult to accurately define the centre of the vortex.
(c). The body-bound vortex
The stopping vortex ring developing around the decelerating copepod rotates opposite the wake vortex, but is of similar magnitude (figure 2b). In the two cases (jumps 20-2 and 58) where we analysed all 2 × 2 meridional plane sections of the vortex rings, the time evolution of the body-bound ring was very similar to that of the wake ring, and the estimated peak circulations of the two vortices were within 5 per cent of one another.
(d). Extension of the velocity field
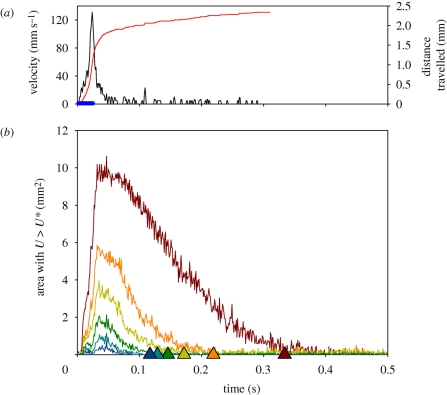
The cross-sectional area enveloping fluid velocities exceeding preset threshold magnitudes (U > U*) increases rapidly to a maximum simultaneously with the copepod reaching its peak velocity, and then declines (figure 3). Depending on the threshold magnitude, the decline is slower than the deceleration of the copepod. High velocities last for only a very short time, whereas velocities greater than 1 mm s−1 are evident ca 300 ms after jump initiation for the example shown. The duration of a velocity signal varies approximately in proportion to its maximum areal extension. Signal durations predicted using the point momentum source model (equation (3.3)) correspond well with the observed durations (figure 3b). The peak area with velocities exceeding 1 mm s−1 is approximately 10 mm2, about 50 times the cross-sectional area of the copepod itself for the example shown.
Figure 3.
(a) Duration of the power stroke, temporal variation in the velocity of the copepod and the net distance moved as a function of time. (b) Temporal variation in the extension of the cross-sectional area within which the induced flow velocity exceeds certain threshold magnitudes, U*. Triangles show the duration of fluid velocities exceeding the threshold as predicted from the momentum of the copepod (equation (3.3); jump 58, table 1); (a) Black lines, copepod velocity; red line, distance travelled; blue circles, active beat phase. (b) Brown lines, U* = 1 mm s−1; orange lines, U* = 2 mm s−1; light green lines, U* = 3 mm s−1; dark green lines, U* = 4 mm s−1; light blue lines, U* = 5 mm s−1; dark blue lines, U* = 6 mm s−1.
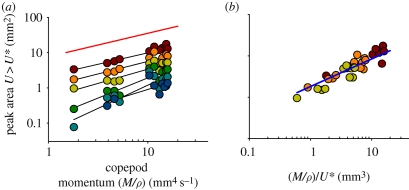
The extension of the area with U > U* depends on the size and velocity of the copepod, with the latter expressed as the density-specific momentum of the copepod (figure 4). For the lower threshold velocities, this area scales with the momentum to a power of near 2/3; for the higher threshold velocities, where velocity contour lines come closer to the copepod, the relations become noisier (figure 4a). If the momentum of the copepod is normalized by the threshold velocity, the observations for threshold velocities between 1 and 3 mm s−1 collapse into one relationship (figure 4b), with the area of influence proportional to (M/ρU*)2/3 (figure 4b).
Figure 4.
Peak areas with induced flow velocities exceeding threshold velocities (U*) as a function of (a) the density-specific momentum of the copepod or (b) the specific momentum normalized by the threshold velocity. Individual regression lines have been plotted in (a), and a regression including only threshold velocities of 1–3 mm s−1 and forced with a slope of 2/3 have been computed in (b). This regression is log (area, mm2) = log (1.815) + (2/3) × log (M/ρU*, mm3); R2 = 0.83. Only jumps where the entire imposed flow field was within the field of view are included (n = 12). Orange line, slope 2/3; blue line, regression. Colours of circles represent U* values as defined in the legend to figure 3 above.
5. Discussion
Vortices are created almost universally when animals move through fluids at Reynolds numbers (Re) exceeding 100, and vortex formation and dynamics are widely studied for flying and swimming organisms in this Reynolds number regime (see review by Dabiri 2009). In contrast, the vortex ring formed in the wake of copepods performing weak repositioning jumps occurs at lower Reynolds numbers (table 1). The spatial and temporal dynamics of vortices in viscous-dominated fluid differ from those formed at higher Reynolds numbers and are much less studied.
The wake vortex results from viscous diffusion of vorticity in the shear layer of the jet produced by the backward kicking legs. This is a different formation mechanism from the vortices generated owing to vortex sheet roll-up in a piston–cylinder apparatus, the classical model system for studies of vortex ring formation (Gharib et al. 1998). Consequently, while inviscid theory has proved useful in the analysis of animal propulsion at higher Re (Dickinson 1996), it does not work in this much lower Re range. For example, there is no formation process, and it becomes irrelevant to define a formation number, often used to characterize vortex ring formation both in piston/cylinder arrangements and in flying and swimming organisms at high Re (Dabiri 2009).
As an alternative, we proposed here a simple model—an impulsive point force—to characterize the viscous toroidal vortex formed in the wake of the jumping copepod. Overall, our observations are consistent with the predictions of this simple and highly idealized model with respect to spatial extension, temporal decay and translation of the wake vortex, which are all different than would be predicted using inviscid theory. For example, the temporal separation of the position of the vorticity maximum and the vortex centre (figure 2) is a characteristic specific to these viscous vortices. This observation confirms that the model is relevant to lower Re copepod jumps. Also, the axial travel speed and distance of the wake vortex are much less than inviscid theory would predict. In fact, according to inviscid theory, vortex rings with similar spatial dimensions and impulse would travel axially at speeds calculated in tens of millimetres per second (Saffman 1992), while in a typical copepod relocating jump, the wake vortex would last less than 1–2 s and the total axial travel distance would measure less than 1 mm. Finally, we found the circulation of the wake vortex to decay after formation owing to viscosity, while inviscid rings may grow and slow down owing to entrainment (i.e. by an entirely different dynamics).
Given the inherent difficulty of filming a non-tethered copepod, the low likelihood of jumps being directed exactly perpendicular to the view direction and within the laser sheet, and the consequent imperfection of this assumption for any particular jump, we find the quantitative correspondence between the model and observations satisfying. It is also reassuring that the estimates of the magnitudes and decays of the circulation of the two vortex rings are similar but of opposite orientation and, hence, that the net circulation is zero throughout (Kelvin's Law). Thus, the simple model provides a good description of our case, allowing us to use it to extrapolate our observations and to evaluate much more generally the fluid dynamic signal and the predation risk that ambush-feeding copepods experience.
(a). Spatial extension and temporal duration of the fluid velocity signal
The scaling of the extension of the velocity field observed agrees well with that proposed by the simple vortex model (equation (3.2)), at least in the far field (i.e. for small U*; figure 4). Strictly speaking, the model applies only to the wake vortex, but as the following loose argument suggests, the predicted scaling may be extended to the entire imposed flow field. The momentum of the forward-jumping copepod must be countered by an oppositely directed momentum of the water in the wake. The decelerating copepod, in turn, must ‘pay’ momentum back to the ambient water, and this would again be of the same magnitude. Because the momentum of the water scales with volume × velocity, it follows that the cross-sectional area—over the entire imposed flow field—with velocity magnitudes exceeding U* must scale with the momentum of the copepod to a power of 2/3 and with the threshold velocity to power −2/3, as observed (figure 4b).
The fluid disturbance generated by the jumping copepod does not last long, and by about a one-quarter of a second after the jump the fluid disturbance generated by the jump has vanished. The time scale is much shorter than the duration of the fluid disturbance generated by a copepod that performs a powerful escape jump. Duren & van Videler (2003) observed a significant fluid signal for more than 2 s after a powerful escape jump of a millimetre-sized copepod. This size dependency of the signal duration is to be expected, and is governed by the viscous time scale, L2/ν, where L is the linear extension of the fluid disturbance. Bigger eddies last longer. The duration of the fluid signal can be explicitly predicted for the wake vortex (equation (3.3)), and, because the durations of the two vortex rings are similar, we can in fact use equation (3.3) to estimate the duration of the entire flow field. These estimates accord well with observations, particularly for the lower threshold velocities (figure 3b).
(b). Predation risk and optimal foraging behaviour
Feeding exposes a zooplankton to predation risk (Tiselius et al. 1997), and the magnitude of the risk depends on the feeding behaviour. We can now use the model and our description of the imposed flow field to estimate the distance at which a mechanoreceptory predator may detect an ambush-feeding copepod, and compare that with estimates for the two other feeding modes (cruising and feeding-current feeding). The strength of the fluid signal to a mechanosensory predator is simply the imposed fluid velocity magnitude (Kiørboe & Visser 1999). The empirical relation (figure 4b) describes the areal extension of the velocity field (A) as a function of the momentum of the copepod and the critical flow velocity magnitude:
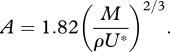 |
5.1 |
Because all pelagic copepods are of similar shape and perform jumps in a manner similar to that described here, we can take this theoretically founded relation to apply more generally.
We may approximate the maximum detection distance to an ambush feeder (RA) by the equivalent circular radius of this area. Replacing the copepod momentum in equation (5.1) with the product of its mass (4/3πa2ρ) and jump velocity (vj), we get
| 5.2 |
where a is the equivalent radius of the copepod (approx. one-quarter of its length) and U* can be interpreted as the threshold signal strength required for detection. As a first-order approximation, similar expressions for hovering and cruising grazers, modelled as a stokeslet and a dipole, respectively, are (Kiørboe in press)
| 5.3 |
and
| 5.4 |
The reaction distance to the jumping copepod refers only to the peak signal immediately following the jump. For comparison with the more continuous signals of the two other feeding modes, it may be more relevant to consider the time-averaged detection distance. To estimate that, we need to know the duration of the signal, t* (equation (3.3)), and the jump frequency (f); the time-averaged signal then scales with t*fRA.
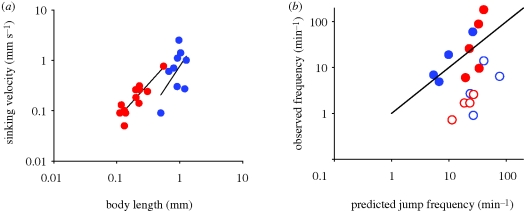
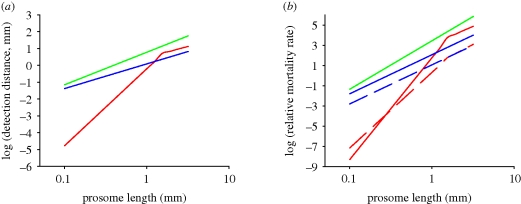
We may estimate the jump frequency of an ambush-feeding copepod by assuming that jumping should exactly counter sinking; hence f = sinking velocity/jump distance. One-beat repositioning jumps invariably bring the copepod roughly two body lengths forward, independent of the size of the copepod and the duration of the power stroke (Kiørboe et al. in press). Sinking velocity should increase with copepod length squared (Stokes Law) and, hence, jump frequency with length. Observed sinking velocities indeed show approximately this scaling, and predicted jump frequencies of ambush-feeding copepods and copepod nauplii conform largely with the expectation, while cruisers and feeding-current feeders jump much less frequently (figure 5). Combining the scaling properties of reaction distance (equation (5.2)), jump frequency and signal duration (equation (3.3)), the time-averaged detection distance increases with a3(vj/U*); that is, dramatically with the size of the copepod, and much faster than is the case for cruising and hovering copepods (figure 6a). Using a simple ballistic predator–prey encounter model, these considerations further demonstrate that ambush feeding becomes increasingly risky for larger copepods (figure 6b).
Figure 5.
(a) Sinking velocities and (b) jump frequencies of copepods and copepod nauplii reported in the literature. In (b), the predicted jump frequency is sinking velocity divided by two body lengths per jump. The lines in (a) are regression lines relating sinking velocity (vsink, mm s−1) to body length (L, mm); for nauplii, log (vsink) = 0.21 + 1.4 log(L); for copepods, log (vsink) = −0.12 + 1.9 log(L). Adapted from Tiselius & Jonsson (1990); Jonsson & Tiselius (1990); Svensen & Kiørboe (2000); Paffenhöfer & Mazzocchi (2002); and Titelman & Kiørboe (2003). (a) Red circles, nauplii; blue circles, copepodites. (b) Blue filled circles, ambushing copepodites; blue open circles, swimming copepodites; red filled circles, ambushing nauplii; red open circles, swimming nauplii. Black line, 1 : 1.
Figure 6.
(a) Predator detection distances and (b) relative mortalities of copepods with different feeding strategies towards cruise (full lines) or ambush predators (dotted lines) that perceive their copepod prey from the hydrodynamic disturbance that it generates. Detection distances computed from equations (5.2)–(5.4); for ambush-feeding copepods, the time-averaged detection distance is 0.5·jump frequency·t*RA. Jump frequency (f = vsink/2L; L = prosome length) was computed assuming a size-dependent sinking velocity (figure 6). Jump velocity (vj) was assumed to scale with L0.67 (Lenz et al. 2004) and equal 100 mm s−1 for a 1-mm-sized copepod. Cruise and feeding-current velocities, v, were assumed identical and estimated from the data compilation in Kiørboe et al. (in press), log (v, mm s−1) = 0.38 + 0.93 log (L, mm). The distinct bend on the curve for ambush-feeding copepods occurs when the signal duration exceeds the time between jumps (i.e. t*·f > 1). Relative predation mortality was computed as πR2(v2 + u2)0.5, where u is the predator velocity, taken to be 10v for a cruise predator. Copepod prey velocities (v) were assumed zero for ambush-feeding and hovering copepods. Relative predation mortality of ambush-feeding copepods to ambush-feeding predators was computed, assuming diffusive encounters, as 4πDR, where D=2/3 L2f (Berg 1993). A critical signal strength for the prey detection was assumed, U* = 0.1 mm s−1. Red line, ambush; blue line, cruise; green line, hovering.
Optimal foraging behaviour is the result of a compromise between gains and risks associated with a particular foraging mode (Lima & Dill 1990; McNamara & Houston 1992). Ambush feeding is inherently less efficient than the more active feeding modes, simply because the encounter velocity is governed by the swimming velocity of the prey for the former and by the (higher) velocity of the grazer for the latter (Kiørboe in press). While the above considerations of predation risk associated with the different feeding behaviours are correct only in an order of magnitude sense, they do suggest that the lower predation risk experienced by small ambush-feeding copepods makes this relatively inefficient feeding strategy feasible. Lower predation risks of ambush feeders have been assumed in models of optimal foraging in zooplankton (e.g. Visser 2007), and do conform with field observations, suggesting that mortality rates of ambush-feeding copepods are much lower than mortalities of similarly sized copepods with more active feeding strategies (Eiane & Ohman 2004). However, the relative advantage of ambush feeders in terms of low predation risk applies only to small copepods. Depending on the predator landscape, ambush feeding in larger copepods becomes similar to or more risky than other feeding modes (figure 6). The primary group of obligate ambush-feeding copepods in the ocean belong to the genus Oithona (Paffenhöfer 1993), and these are all small, typically less than 1 mm. Intermediate-sized copepods, such as A. tonsa, may switch between ambush and feeding-current feeding (Jonsson & Tiselius 1990), while copepods larger than 1–1.5 mm all appear to be cruise or feeding-current feeders (e.g. Calanus spp.).
In conclusion, our quantitative description of the fluid dynamics of repositioning jumps by copepods has enabled us to predict the hydrodynamic signature of ambush feeding, and has demonstrated the significance of hydrodynamics in understanding the ecology and behaviour of small plankton operating at low Re. Previous work has focused on inviscid vortices in the context of animal propulsion, which is irrelevant in the viscous world of the plankton. Many ecologically important marine organisms, including most zooplankton, small fish larvae and even krill, operate in the low Reynolds number regime (Re = 0.1–200), and their propulsion and hydrodynamic signalling may similarly be governed by the viscous vortices that may form as a result of their motion.
Acknowledgements
We enjoyed conversations with Tim Pedley. T.K. was supported by a grant from the Danish Research Council and by a Niels Bohr Fellowship. H.J. was supported by National Science Foundation grants NSF OCE-0352284 and IOS-0718506, and S.P.C. by NSF OCE-0351398 and OCE-0623534.
References
- Afanasyev Y. D.2004Wakes behind towed and self-propelled bodies: asymptotic theory. Phys. Fluids 16, 3235–3238 (doi:10.1063/1.1768071) [Google Scholar]
- Berg H. C.1993Random walks in biology, 2nd edn.Princeton, NJ: Princeton University Press [Google Scholar]
- Buskey E. J., Lenz P. H., Hartline D. K.2002Escape behaviour of planktonic copepods in response to hydrodynamic disturbances: high speed video analysis. Mar. Ecol. Prog. Ser. 235, 135–146 (doi:10.3354/meps235135) [Google Scholar]
- Catton K. B., Webster D. R., Brown J., Yen J.2007Quantitative analysis of tethered and free-swimming copepodid flow fields. J. Exp. Biol. 210, 299–310 (doi:10.1242/jeb.02633) [DOI] [PubMed] [Google Scholar]
- Dabiri J. O.2009Optimal vortex formation as a unifying principle in biological propulsion. Ann. Rev. Fluid. Mech. 41, 17–33 (doi:10.1146/annurev.fluid.010908.165232) [Google Scholar]
- Dickinson M. H.1996Unsteady mechanisms of force generation in aquatic and aerial locomotion. Am. Zool. 36, 537–554 [Google Scholar]
- Duren L. A., van Videler J. J.2003Escape from viscosity: the kinematics and hydrodynamics of copepod foraging and escape swimming. J. Exp. Biol. 206, 269–279 [DOI] [PubMed] [Google Scholar]
- Eiane K., Ohman M. D.2004Stage-specific mortality of Calanus finmarchicus, Pseudocalanus elongatus and Oithona similis on Fladen Ground, North Sea, during a spring bloom. Mar. Ecol. Prog. Ser. 268, 183–193 (doi:10.3354/meps268183) [Google Scholar]
- Feigenbaum D., Reeve M. R.1977Prey detection in the Chaetognatha: response to a vibrating probe and experimental determination of attack distance in large aquaria. Limnol. Oceanogr. 22, 1052–1058 (doi:10.4319/lo.1977.22.6.1052) [Google Scholar]
- Fenchel T., Hansen P. J.2006Motile behaviour of the bloom-forming ciliate Mesodinium rubrum. Mar. Biol. Res. 2, 33–40 (doi:10.1080/17451000600571044) [Google Scholar]
- Fields D. M., Yen J.1997The escape behavior of marine copepods in response to a quantifiable fluid mechanical disturbance. J. Plankton Res. 19, 1289–1304 (doi:10.1093/plankt/19.9.1289) [Google Scholar]
- Gharib M., Rambod E., Shariff K.1998A universal time scale for vortex ring formation. J. Fluid Mech. 360, 121–140 (doi:10.1017/S0022112097008410) [Google Scholar]
- Jakobsen H. H., Everett L. M., Strom S. L.2006Hydromechanical signaling between the ciliate Mesodinium pulex and motile protist prey. Aquat. Microb. Biol. 44, 197–206 (doi:10.3354/ame044197) [Google Scholar]
- Jiang H. S., Paffenhöfer G.-A.2008Hydrodynamic signal perception by the copepod Oithona plumifera. Mar. Ecol. Prog. Ser. 373, 37–52 (doi:10.3354/meps07749) [Google Scholar]
- Jiang H. S., Meneveau C., Osborn T. R.2002The flow field around a freely swimming copepod in steady motion. Part II: Numerical simulation. J. Plankton Res. 24, 191–213 (doi:10.1093/plankt/24.3.191) [Google Scholar]
- Jonsson P. R., Tiselius P.1990Feeding behavior, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar. Ecol. Prog. Ser. 60, 35–44 (doi:10.3354/meps060035) [Google Scholar]
- Kiørboe T.In press How zooplankton feed: mechanisms, traits and tradeoffs. Biol. Rev. [DOI] [PubMed] [Google Scholar]
- Kiørboe T., Visser A. W.1999Predator and prey perception in copepods due to hydromechanical signals. Mar. Ecol. Prog. Ser. 179, 81–95 (doi:10.3354/meps179081) [Google Scholar]
- Kiørboe T., Andersen A., Langlois V., Jakobsen H. H., Bohr T.2009Mechanisms and feasibility of prey capture in ambush feeding zooplankton. Proc. Natl Acad. Sci. USA 106, 12 394–12 399 (doi:10.1073/pnas.0903350106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiørboe T., Andersen A., Langlois V. J., Jakobsen H. J.In press Unsteady motion: escape jumps in copepods, their kinematics and energetics. J. R. Soc. Interface (doi:10.1098/rsif.2010.0176). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois V., Andersen A., Bohr T., Visser A., Kiørboe T.2009Significance of swimming and feeding currents for nutrient uptake in osmotrophic and interception feeding flagellates. Aquat. Microbiol. 54, 35–44 (doi:10.3354/ame01253) [Google Scholar]
- Lenz P. H., Hower A. E., Hartline D. K.2004Force production during pereiopod power strokes in Calanus finmarchicus. J. Mar. Syst. 49, 133–144 (doi:10.1016/j.jmarsys.2003.05.006) [Google Scholar]
- Leptos K. C., Guasto J. S., Gollub J. P., Pesci A. I., Goldstein R. E.2009Dynamics of enhanced tracer diffusion in suspensions of swimming eukaryotic microorganisms. Phys. Rev. Lett. 103, 198103 (doi:10.1103/PhysRevLett.103.198103) [DOI] [PubMed] [Google Scholar]
- Lima S. L., Dill L. M.1990Behavioral decisions made under the risk of predation: a review and prospectus. Can. J. Zool. 68, 619–640 (doi:10.1139/z90-092) [Google Scholar]
- Malkiel E., Sheng I., Katz J., Strickler J. R.2003The three-dimensional flow field generated by a feeding calanoid copepod measured using digital holography. J. Exp. Biol. 206, 3657–3666 (doi:10.1242/jeb.00586) [DOI] [PubMed] [Google Scholar]
- McNamara J. M., Houston A. I.1992Risk-sensitive foraging: a review of the theory. Bull. Math. Biol. 54, 255–378 [Google Scholar]
- Paffenhöfer G.-A.1993On the ecology of marine cyclopoid copepods (Crustacea, Copepoda). J. Plankton Res. 15, 37–55 [Google Scholar]
- Paffenhöfer G.-A., Mazzocchi M. G.2002On some aspects of the behaviour of Oithona plumifera (Copepoda: Cyclopoida). J. Plankton Res. 24, 129–135 (doi:10.1093/plankt/24.2.129) [Google Scholar]
- Saffman P. G.1992Vortex dynamics. Cambridge, UK: Cambridge University Press [Google Scholar]
- Svensen C., Kiørboe T.2000Remote prey detection in Oithona similis: hydromechanical versus chemical cues. J. Plankton Res. 22, 1155–1166 (doi:10.1093/plankt/22.6.1155) [Google Scholar]
- Tiselius P., Jonsson P. R.1990Foraging behaviour of six calanoid copepods: observations and hydrodynamic analysis. Mar. Ecol. Prog. Ser. 66, 23–33 (doi:10.3354/meps066023) [Google Scholar]
- Tiselius P., Jonsson P. R., Kaartvedt S., Olsen M. E., Jarstad T.1997Effects of copepod foraging behavior on predation risk: an experimental study of the predatory copepod Pareuchaeta norvegica feeding on Acartia clausi and A. tonsa (Copepoda). Limnol. Oceanogr. 42, 164–170 [Google Scholar]
- Titelman J., Kiørboe T.2003Motility of copepod nauplii and implications for food encounter. Mar. Ecol. Prog. Ser. 247, 123–135 (doi:10.3354/meps247123) [Google Scholar]
- Visser A. W.2001Hydromechanical signals in the plankton. Mar. Ecol. Prog. Ser. 222, 1–24 (doi:10.3354/meps222001) [Google Scholar]
- Visser A. W.2007Motility of zooplankton: fitness, foraging and predation. J. Plankton Res. 29, 447–461 (doi:10.1093/plankt/fbm029) [Google Scholar]
- Yen J., Strickler J. R.1996Advertisement and concealment in the plankton: what makes a copepod hydrodynamically conspicuous? Inv. Biol. 115, 191–205 (doi:10.2307/3226930) [Google Scholar]