Abstract
Wherever individuals perform cooperative behaviours, each should be selected to adjust their own current contributions in relation to the likely future contributions of their collaborators. Here, we use the sentinel system of pied babblers (Turdoides bicolor) to show that individuals anticipate contributions by group mates, adjusting their own contribution in response to information about internal state broadcast by others. Specifically, we show that (i) short-term changes in state influence contributions to a cooperative behaviour, (ii) individuals communicate short-term changes in state, and (iii) individuals use information about the state of group mates to adjust their own investment in sentinel behaviour. Our results demonstrate that individual decisions about contributions to a cooperative effort can be influenced by information about the likely future contribution of others. We suggest that similar pre-emptive adjustments based on information obtained from collaborators will be a common feature of cooperative behaviour, and may play an important role in the development of complex communication in social species.
Keywords: contributions to cooperation, evolution of communication, sentinel behaviour, negotiation
1. Introduction
Conflict over individual contributions appears inevitable wherever individuals collaborate to perform cooperative activities (Hardin 1968; West et al. 2007). Such conflict forces each to make decisions about their own contributions in a way that balances the risk of exploitation by collaborators against the need to invest enough to ensure a fitness return (Trivers 1971), with variation in individual contributions generally selecting for adjustment by collaborators (Wright & Cuthill 1990; Hatchwell 1999; Hinde & Kilner 2007). Current analyses assume these adjustments must occur in response to the previous behaviour of a collaborator, expecting individuals to make sequential decisions, each adjusting to a partner's previous move (Houston & Davies 1985; McNamara et al. 1999; Barta et al. 2002; Johnstone & Hinde 2006). However, information about the likely future investment of collaborators may allow individuals to make pre-emptive adjustments to their own investment. While this may come from direct observations of a potential collaborator's previous behaviour (Nowak & Sigmund 2005), individuals should also be selected to use cues that predict behaviour in advance. Because contributions to cooperative activities are frequently state dependent (Wright & Cuthill 1990; Clutton-Brock et al. 1999; Russell et al. 2003), individuals who obtain information about the state of collaborators may be able to predict the likely future investment of collaborators, and adjust their own contribution accordingly.
From the opposite perspective, selection should favour mechanisms for manipulating the contributions of others. This need not involve coercion or deception, because it may often be possible for individuals to influence the contribution of others simply by providing information about their own likelihood of contributing. For example, if state influences contributions, then cues associated with state effectively signal likely contributions, and these cues could represent credible ‘promises’ (Barta et al. 2002; Johnstone & Hinde 2006). Dependent offspring do exactly this when begging, providing conspicuous information about internal state in order to influence investment by carers (Kilner & Johnstone 1997). As yet unexplored is the possibility that adults also actively signal current state in order to influence investment by collaborators.
We suggest that the exchange of information about changes in short-term state should be a common feature of cooperative systems, effectively allowing individuals to negotiate their contributions, in behavioural time, and we use sentinel behaviour in pied babblers (Turdoides bicolor) to investigate this suggestion. Pied babblers are group-living, cooperative passerines of semi-arid southern Africa. Groups forage on the ground, with a sentinel present ca 60 per cent of the time. All adult individuals contribute to sentinel behaviour, with extensive variation in both bout length and frequency (Ridley & Raihani 2007a; Hollen et al. 2008; Bell et al. 2009), which is likely to be influenced by individual state (Clutton-Brock et al. 1999; Wright et al. 2001a,b). Foraging individuals gain considerable benefits from the presence of a sentinel (Hollen et al. 2008), yet becoming a sentinel is likely to be costly to the sentinel, in particular via lost foraging time. There is continuous information exchange within foraging groups: both sentinels and foragers produce frequent quiet vocalizations, which group mates use as sources of information about predation risk (Bell et al. 2009), sentinel presence and position (Hollen et al. 2008; Radford et al. 2009) and forager spatial position (Radford & Ridley 2007). These calls may also convey information about individual internal state, since forager ‘close’ calls are affected by foraging success (Radford & Ridley 2008). If internal state influences call characteristics, and if there is a correlation between state and investment in sentinel behaviour, then changes in calling may effectively signal the probability and duration of sentinel bouts in advance. If so, we expect individuals to monitor the state of group mates, comparing their own state with that of others, and we expect individual contributions to sentinelling to be influenced by the call characteristics of their group mates. We first assess whether state influences contributions to sentinel behaviour, and then ask: (i) whether individuals communicate changes in state, (ii) whether any such communication provides information about future contributions to sentinel behaviour, and (iii) whether individuals use information about the state of others to adjust their own contributions to sentinel behaviour.
2. Methods
We conducted observations and experiments between 16 March and 3 June 2009, on the Kuruman River Reserve, southern Kalahari desert, South Africa (26°58′ S, 21°49′ E) (see Ridley & Raihani 2007a for ecological details). We observed eight colour-ringed groups of pied babblers habituated to close (less than 5 m) observation on foot (median group size = 5, range 3–12). We defined birds as sentinels when perched 1 m or more above ground for 30 s or more, actively scanning and giving sentinel calls. Sentinel bouts ended when birds flew down from the perch, started to preen or started to receive preening from another individual. For details of routine sentinel calling, see Hollen et al. (2008) and Bell et al. (2009). All sound recordings were taken using a Sennheiser MKH416T microphone and Marantz PMD670 hard-drive sound recorder.
(a). Supplementary feeding experiments: effect on interval between sentinel bouts and sentinel bout duration
Supplementary feeding experiments were conducted on 21 adults (10 females, 11 males). Each was fed once with a single mealworm and once with 10 mealworms (order alternated between individuals; trials on the same bird 2 or more days apart). For each trial, we identified a focal bird, and then timed the duration of a natural sentinel bout (mean bout length 4.7 min ± 0.81 s.e.). Immediately after the end of that bout, we fed the focal bird, before timing the interval until its next sentinel bout, and timing the duration of that second bout. Records for the interval between sentinel bouts are only available for 16 trials owing to equipment failure.
(b). Supplementary feeding experiments: effect on sentinel and forager call rate
We fed each bird twice (foragers: one mealworm or six; sentinels one mealworm or 10), alternating the order of trials between individuals and conducting trials on the same bird 2 or more days apart. Foragers were fed fewer mealworms because pilot experiments showed that they frequently stopped foraging when fed seven or more. For foragers, we commenced recording after the focal bird had been foraging continuously for 2 min or more, with a sentinel present. We recorded call rate for 1 min, then fed the bird and then recorded call rate for another minute. For sentinels, we recorded call rate during the first minute of the experimental sentinel bouts described above (before and after sentinels were fed one or 10 mealworms).
(c). Relationship between sentinel call rate during first minute of a bout and bout duration
We recorded calls from 94 sentinel bouts by 25 adults (12 females, 13 males; mean bout length = 3.87 min ± 0.28 s.e.). To investigate variables predicting bout length, we constructed a linear mixed model (LMM), with duration of the bout (in minutes) as the response variable, and call rate during the first minute as a covariate (see electronic supplementary material, table S1, for other variables tested). Because we took multiple recordings from the same individuals, we included individual identity as a random term. To investigate whether call rate changed across sentinel bouts, for each individual, we calculated average call rate during the first and the last minute for all their sentinel bouts, and then carried out a paired comparison.
(d). Effect of information about the state of collaborators on individual contributions to sentinel behaviour
(i). Sentinels responding to foragers
We exposed each sentinel (n = 19) to two playbacks: low-rate close calls simulating a satiated forager (5 calls min−1) and higher rate close calls simulating an average forager (15 calls min−1), with call rates simulating the two forager states based on the results of the experimental feeding trials. We commenced playbacks when the focal bird started a natural sentinel bout, from speakers concealed on the ground, 5–8 m from the base of the sentinel's tree. Each focal sentinel was exposed to a pair of playbacks recorded from the same individual, no individual was used to provide more than one pair of recordings and recordings always came from an individual in the same group as the focal sentinel. We alternated the playback order between subjects, and never played subjects their own calls. There is currently no evidence for individual vocal recognition in this species, and we never observed birds reacting to their own calls or group mates reacting to playbacks of an individual clearly visible in a different location to the speaker.
To construct the playback tracks, we recorded an adult foraging in the presence of a sentinel, and then extracted 20 calls (chosen at random) and pasted these into 5 min recordings of background noise (previously recorded in the centre of the relevant group's territory). For tracks simulating a satiated forager, we pasted calls at 12 s intervals; for tracks simulating a hungry forager, we pasted calls at 4 s intervals—so both tracks consisted of identical calls and identical background noise, the only difference being call rate. Sound files edited using Cool Edit 2000 (Syntrillium Software Corp., Phoenix, AZ, USA), stored as WAVE audio files on an Apple iPod and played back on Sony SRS-A35 speakers (playback amplitude standardized at 51.7 dB, the natural amplitude of close calls).
(ii). Foragers responding to sentinels
We exposed each group (n = 8) to two playbacks: low-rate sentinel calls simulating a satiated sentinel (5 calls min−1), and higher rate sentinel calls simulating an average sentinel (20 calls min−1), with call rates simulating the two sentinel states based on the results of the experimental feeding trials. We commenced playbacks when a natural sentinel bout ended, from speakers concealed 2 m high in standing vegetation, 5–8 m from the centre of the group. We alternated the playback order between groups. For each group, both playbacks were constructed from recordings of the dominant male of that group: we recorded him while guarding, at least 5 min after the last disturbance, and only if he was undisturbed during the bout. We extracted 20 calls (chosen at random) and pasted these into 5 min recordings of background noise (previously recorded in the centre of the relevant group's territory). For tracks simulating a satiated sentinel, we pasted calls at 12 second intervals; for tracks simulating an average sentinel, we pasted calls at 3 second intervals.
(iii). Foragers responding to other foragers
We exposed each group (n = 8) to two playbacks: low-rate forager calls simulating the presence of a satiated forager (5 min−1) and higher rate forager calls simulating the presence of an average forager (15 min−1), and alternated playback order between groups. We commenced playbacks when a natural sentinel bout ended, from speakers concealed on the ground, 5–8 m from the centre of the group. Each group was exposed to a pair of recordings taken from the same individual, and we constructed the playback tracks as for playbacks to sentinels (above). Recording were taken from individuals previously used during the playbacks to sentinels—so to minimize any habituation effects, we used different tracks and ensured that playbacks of the same bird occurred a minimum of four weeks apart. For a full summary of the treatment structure, see the electronic supplementary material.
3. Results
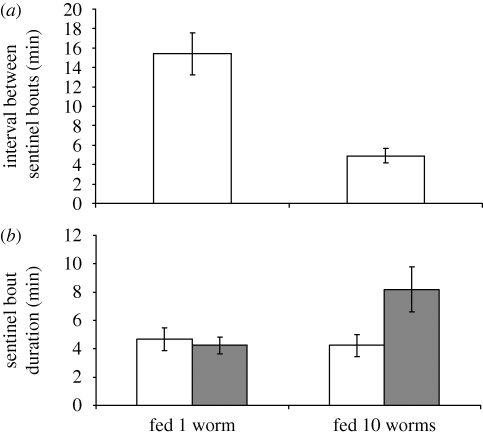
Supplementary feeding experiments on sentinels (n = 21) confirmed that state influences contributions to sentinel behaviour: after receiving 10 mealworms (Tenebrio spp. larva), retiring sentinels started a new sentinel bout sooner (paired t-test, t16 = 4.72, p < 0.0001; figure 1a) and stayed on guard longer (two-way repeated-measures ANOVA, interaction between treatment and experimental stage: F1,21 = 6.17, p = 0.016; figure 1b), compared with when they received one mealworm. This supports previous studies indicating that contributions to sentinel behaviour should be strongly state dependent (Bednekoff 1997; Clutton-Brock et al. 1999; Wright et al. 2001a,b), which means that individuals should be selected to monitor the state of group mates, and that individuals who signal their current state effectively signal their probability of guarding in the near future.
Figure 1.
Contributions to sentinel behaviour are state dependent: the effect of experimentally feeding one or 10 meal worms on individual contributions to sentinel behaviour: (a) interval between sentinel bout (n = 16) and (b) duration of sentinel bouts (n = 21; means ± s.e.). (b) White bars, before feed; grey bars, after feed.
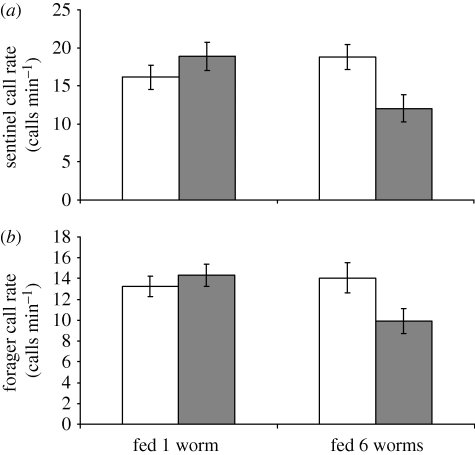
The same supplementary feeding experiments on sentinels and further feeding experiments on foragers (n = 29) demonstrated that individuals actively signal changes in state: sentinels called at lower rates during the first minute of sentinel bouts immediately after being fed 10 mealworms compared with the first minute of their previous bouts, but showed no change after being fed a single mealworm (two-way repeated-measures ANOVA, interaction between treatment and stage, F1,21 = 17.56, p < 0.0001; figure 2a). Foraging birds gave close calls at lower rates after receiving six mealworms, but not after receiving one mealworm (two-way repeated-measures ANOVA, interaction between treatment and stage: F1,29 = 14.71, p < 0.0001; figure 2b). Previous observations on Florida scrub jays (Aphelocoma coerulescens; Bednekoff et al. 2008) suggest that similar calls given by scrub-jay sentinels may also signal hunger, indicating that state-dependent signals may indeed be widespread.
Figure 2.
Call rate indicates state: the effect of experimental feeding on individual call rate for (a) sentinels (fed 10 mealworms; n = 21) and (b) foragers (fed 6 mealworms; means ± s.e.; n = 29). White bars, before feed; grey bars, after feed.
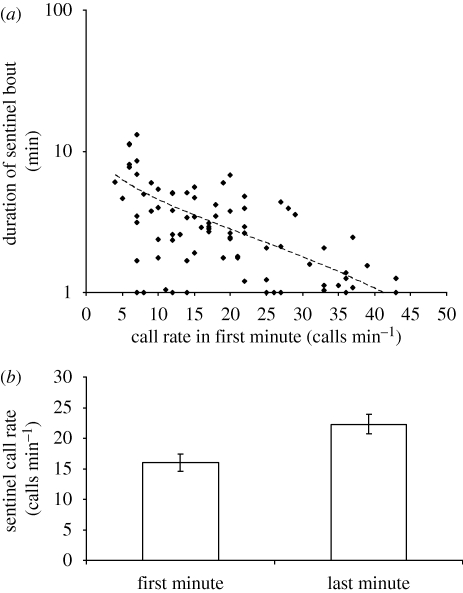
If call rate indicates state, and if state influences bout duration, then we would expect call rate at the start of a bout to signal likely bout duration, and we would expect call rate to increase towards the end of sentinel bouts, as sentinels become hungrier. Recordings of 94 entire, natural sentinel bouts by 25 birds revealed a negative correlation between sentinel call rate in the first minute of a sentinel bout and the duration of that bout (LMM χ2 = 24.75, p < 0.001; figure 3a). The analysis also revealed that older birds (χ2 = 12.75, p < 0.001) and dominant birds (χ2 = 12.01, p < 0.001) spent longer on guard, consistent with sentinel bout duration being state dependent. In addition, these recordings confirmed that sentinels called at higher rates during the last minute of sentinel bouts than during the first minute (paired t-test, t25 = 5.02, p < 0.0001; figure 3b).
Figure 3.
The information carried by sentinel call rate: (a) the relationship between call rate in the first minute of a sentinel bout and the eventual duration of that bout (n = 94 sentinel bouts recorded from 25 individuals); (b) comparison of average call rate in the first and last minutes of the same sentinel bouts (means ± s.e.; n = 25).
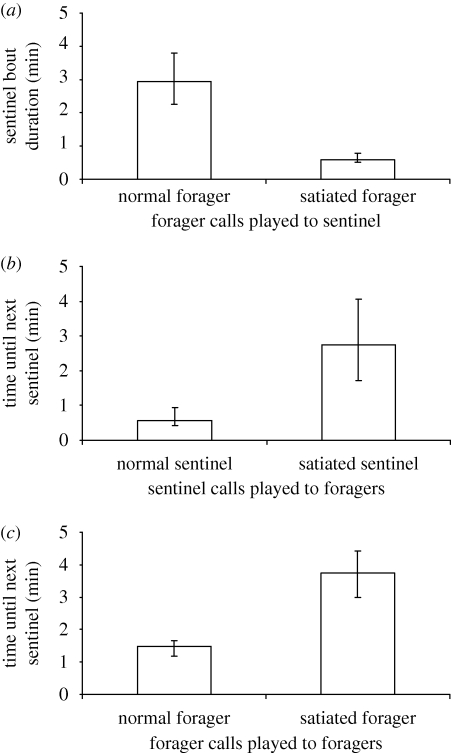
These results lead to the key question: do individuals use the information produced by group mates to adjust their own contributions? If sentinels use the calls of foragers to monitor forager state, then we would expect sentinels to shorten their sentinel bouts when exposed to playbacks simulating the presence of satiated foragers. Playback experiments on 19 birds in eight groups demonstrated that sentinels exposed to playbacks of low-rate (5 calls min−1) forager calls had shorter sentinel bouts than those exposed to higher rate (15 calls min−1) forager call playbacks (Wilcoxon: n = 19, W = 189, p < 0.0001; figure 4a). If foragers use the calls of sentinels to monitor sentinel state—and hence the likelihood of the sentinel ending its bout—we would expect the gap between sentinels to be extended when groups are exposed to playbacks simulating the presence of satiated sentinels. Playbacks to eight groups demonstrated that groups exposed to playbacks of higher rate sentinel calls had shorter intervals with no sentinel than when exposed to low-rate sentinel call playbacks (Wilcoxon: n = 8, W = 36, p = 0.014; figure 4b). If foragers use calls to monitor each other's state (and hence their own state relative to every other forager), we would expect the gap between sentinels to be extended when groups are exposed to playbacks simulating the presence of a satiated forager: we would expect individuals to be unwilling to start guarding when another individual is more satiated. Playbacks to eight groups demonstrated that groups exposed to playbacks of low-rate forager calls had longer intervals with no sentinel than when exposed to higher rate forager call playbacks (Wilcoxon: n = 8, W = 36, p = 0.014; figure 4c).
Figure 4.
The use of information about the state of group mates: (a) the effect of normal and satiated forager call playbacks on sentinel bout duration (n = 19); (b) the effect of normal and satiated sentinel call playbacks on forager behaviour (n = 8) and (c) the effect of normal and satiated forager call playbacks on other forager behaviour (medians ± I.Q.R.) (n = 8).
4. Discussion
We have shown that pied babblers both adjust their own investment in response to information about future contributions by collaborators and actively signal their own state in a way that influences contributions by others. This is the first study to demonstrate that individuals make anticipatory adjustments to personal investment based on information about the likely future contributions of collaborators, and we suggest that pre-emptive adjustments may be an important source of variation in individual contributions to cooperative behaviours: for instance, cooperative breeders adjust offspring characteristics in relation to the number or type of helpers they expect to care at the nest (Griffin et al. 2005; Russell et al. 2007) and female birds adjust egg investment in response to information about male quality (Cunningham & Russell 2000). Because cues associated with state or condition—and therefore probably levels of investment—are probably common, we expect the use of such information to be a major component of individual decisions about contributions to cooperative behaviours in all social species.
This raises two questions: why individuals are selected to actively signal such information, and how the honesty of these signals is maintained? Here, call rate signals the cost of guarding, which is higher for hungry than for satiated birds: in a sense, they are signalling their need to forage rather than guard. Wherever individuals have an interest in the longer term fitness of collaborators, they should be selected to increase their own investment when the cost of investment to collaborators increases, providing an incentive for collaborators to advertise the current cost of investment. Collaborator fitness will be important when they are kin (Hamilton 1964), and when collaborations are extended (or permanent), so individuals stand to benefit from future contributions by their collaborators (Kokko et al. 2001). Pied babbler group members are close relatives (Nelson-Flower 2010), and they gain considerable benefits from the presence (Ridley & Raihani 2007b; Ridley et al. 2008) and state of group mates (this study), in associations that can last for up to 6 years. This indicates why selection for cheating may be limited: free-loaders not only impose indirect fitness costs on themselves, but by over-exploiting others, risk being forced to forage without a sentinel when group mates become exhausted. In contexts where the interests of collaborators diverge further, individuals may indeed be selected to falsify information about state, for instance, by exaggerating need. Honesty may then be imposed by the cost of signalling, creating a situation where conflict between collaborators is mediated by a costly signal, analogous to the way begging is thought to mediate conflict between parents and offspring (Kilner & Johnstone 1997).
In pied babblers, adjustment to cooperative behaviour based on information exchanged about individual state may not just be restricted to sentinel behaviour: individuals may similarly negotiate nest visits when feeding chicks, although this remains to be investigated. In broader terms, the selection pressures acting on collaborators will be similar wherever cooperation occurs. Whether or not communication systems develop to coordinate and negotiate individual contributions will depend on the extent to which the fitness interests of collaborators converge; on the extent to which the benefits of investing depend on coordinated contributions; and on the extent to which individuals obtain direct benefits from contributing. The need to monitor the likely contributions of collaborators, and the benefits to be gained from manipulating them, will have exerted considerable selection on the development of complex communication in all social species: the human ability to negotiate contracts is rooted in a similar exchange of information about relative need to that occurring within groups of pied babblers.
Acknowledgements
The Pied Babbler Project is maintained by the Percy Fitzpatrick Institute of African Ornithology, University of Cape Town. Lexy Russell and Fraser Niven helped record sentinel calls. Nikki Raihani and Maple Nelson-Flower helped establish or maintain the study population. Tom Flower provided advice and assistance. Tim Clutton-Brock and Marta Manser provided logistic support and access to the Kuruman River Reserve. The Northern Cape Conservation Authority provided permission to work in South Africa. M.B.V.B. was supported by Magdalene College, Cambridge, and the Association for the Study of Animal Behaviour; A.N.R. was funded by a Biotechnology and Biological Sciences Research Council David Phillips Fellowship; A.R.R. was funded by CoE at the Fitzpatrick Institute. Alex Thornton, Tim Clutton-Brock, Marta Manser, Stu Sharp, Sinead English, Becky Kilner, Peter Bednekoff and an anonymous reviewer provided valuable comments.
References
- Barta Z., Houston A. I., McNamara J. M., Szekely T.2002Sexual conflict about parental care: the role of reserves. Am. Nat. 159, 687–705 (doi:10.1086/339995) [DOI] [PubMed] [Google Scholar]
- Bednekoff P. A.1997Mutualism among safe selfish sentinels: a dynamic game. Am. Nat. 150, 373–392 (doi:10.1086/286070) [DOI] [PubMed] [Google Scholar]
- Bednekoff P. A., Bowman R., Woolfenden G. E.2008Do conversational gutturals help Florida scrub jays coordinate their sentinel behaviour? Ethology 114, 313–317 (doi:10.1111/j.1439-0310.2008.01467.x) [Google Scholar]
- Bell M. B. V., Radford A. N., Rose R., Wade H., Ridley A. R.2009The value of constant surveillance in a risky environment. Proc. R. Soc. B 276, 2997–3005 (doi:10.1098/rspb.2009.0276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock T. H., O'Riain M. J., Brotherton P. N. M., Gaynor D., Kansky R., Griffin A. S., Manser M.1999Selfish sentinels in cooperative mammals. Science 284, 1640–1644 (doi:10.1126/science.284.5420.1640) [DOI] [PubMed] [Google Scholar]
- Cunningham E. J. A., Russell A. F.2000Egg investment is influenced by male attractiveness in the mallard. Nature 404, 74–77 (doi:10.1038/35003565) [DOI] [PubMed] [Google Scholar]
- Griffin A. S., Sheldon B. C., West S. A.2005Cooperative breeders adjust offspring sex ratios to produce helpful helpers. Am. Nat. 166, 628–632 (doi:10.1086/491662) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour. I, II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Hardin G.1968The tragedy of the commons. Science 162, 1243–1248 [PubMed] [Google Scholar]
- Hatchwell B. J.1999Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 154, 205–219 (doi:10.1086/303227) [DOI] [PubMed] [Google Scholar]
- Hinde C. A., Kilner R. M.2007Negotiations within the family over the supply of parental care. Proc. R. Soc. B 274, 53–60 (doi:10.1098/rspb.2006.3692) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollen L. I., Bell M. B. V., Radford A. N.2008Cooperative sentinel calling? Foragers gain increased biomass intake. Curr. Biol. 18, 576–579 (doi:10.1016/j.cub.2008.02.078) [DOI] [PubMed] [Google Scholar]
- Houston D. J., Davies N. B.1985The evolution of cooperation and life history in the dunnock (Prunella modularis). In British Ecological Society, vol. 25. Behavioural ecology: ecological consequences of adaptive behaviour. (eds Sibly R. M., Smith R. H.), pp. 471–487 Palo Alto, CA: Blackwell Scientific Publications [Google Scholar]
- Johnstone R. A., Hinde C. A.2006Negotiation over offspring care—how should parents respond to each others efforts? Behav. Ecol. 17, 818–827 (doi:10.1093/beheco/arl009) [Google Scholar]
- Kilner R. M., Johnstone R. A.1997Begging the question: are offspring solicitation behaviours signals of need? Trends Ecol. Evol. 12, 11–15 (doi:10.1016/S0169-5347(96)10061-6) [DOI] [PubMed] [Google Scholar]
- Kokko H., Johnstone R. A., Clutton-Brock T. H.2001The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J. M., Gasson C. E., Houston A. I.1999Incorporating rules for responding into evolutionary games. Nature 401, 368–371 (doi:10.1038/43872) [DOI] [PubMed] [Google Scholar]
- Nelson-Flower M. J.2010Kinship and its consequences in the cooperatively breeding pied babbler (Turdoides bicolor). PhD Thesis, University of Cape Town [Google Scholar]
- Nowak M. A., Sigmund K.2005Evolution of indirect reciprocity. Nature 437, 1291–1298 (doi:10.1038/nature04131) [DOI] [PubMed] [Google Scholar]
- Radford A. N., Ridley A. R.2007Individuals in foraging groups may use vocal cues when assessing their need for antipredator vigilance. Biol. Lett. 3, 249–252 (doi:10.1098/rsbl.2007.0110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford A. N., Ridley A. R.2008Close calling regulates spacing between foraging competitors in the group living pied babbler. Anim. Behav. 75, 519–527 (doi:10.1016/j.anbehav.2007.05.016) [Google Scholar]
- Radford A. N., Hollen L. I., Bell M. B. V.2009The higher the better: sentinel height influences foraging success in a social bird. Proc. R. Soc. B 276, 2437–2442 (doi:10.1098/rspb.2009.0187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A. R., Raihani N. J.2007aFacultative response to a kleptoparasite by the cooperatively breeding pied babbler. Behav. Ecol. 18, 324–330 (doi:10.1093/beheco/arl092) [Google Scholar]
- Ridley A. R., Raihani N. J.2007bVariable post-fledging care in a cooperative bird: causes and consequences. Behav. Ecol. 18, 994–1000 (doi:10.1093/beheco/arm074) [Google Scholar]
- Ridley A. R., Raihaini N. J., Nelson-Flower M. J.2008The cost of being alone: the fate of floaters in a population of cooperatively breeding pied babblers (Turdoides bicolor). J. Avian. Biol. 39, 389–392 [Google Scholar]
- Russell A. F., Sharpe L. L., Brotherton P. N. M., Clutton-Brock T. H.2003Cost minimisation by helpers in cooperative vertebrates. Proc. Natl Acad. Sci. USA 100, 3333–3338 (doi:10.1073/pnas.0636503100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell A. F., Langmore N. E., Cockburn A., Astheimer L. B., Kilner R. M.2007Reduced egg investment can conceal helper effects in cooperatively breeding birds. Science 317, 941–944 (doi:10.1126/science.1146037) [DOI] [PubMed] [Google Scholar]
- Trivers R. L.1971The evolution of reciprocal altruism. Q. Rev. Biol. 46, 35–57 (doi:10.1086/406755) [Google Scholar]
- West S. A., Griffin A. S., Gardner A.2007Evolutionary explanations for cooperation. Curr. Biol. 17, 661–672 [DOI] [PubMed] [Google Scholar]
- Wright J., Cuthill I. C.1990Biparental care: short term manipulation of partner contribution and brood size in the starling Sturnus vulgaris. Behav. Ecol. 1, 116–124 (doi:10.1093/beheco/1.2.116) [Google Scholar]
- Wright J., Maklakov A. A., Khazin V.2001aState-dependent sentinels: an experimental study in the Arabian babbler. Proc. R. Soc. Lond. B 268, 821–826 (doi:10.1098/rspb.2000.1574) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J., Berg E., de Kort S. R., Khazin V., Maklakov A. A.2001bSafe selfish sentinels in a cooperative bird. J. Anim. Ecol. 70, 1070–1079 [Google Scholar]