Abstract
Helping behaviour in cooperative breeding systems has been attributed to kin selection, but the relative roles of direct and indirect fitness benefits in the evolution of such systems remain a matter of debate. In theory, helpers could maximize the indirect fitness benefits of cooperation by investing more in broods with whom they are more closely related, but there is little evidence for such fine-scale adjustment in helper effort among cooperative vertebrates. In this study, we used the unusual cooperative breeding system of the long-tailed tit Aegithalos caudatus to test the hypothesis that the provisioning effort of helpers was positively correlated with their kinship to broods. We first use pedigrees and microsatellite genotypes to characterize the relatedness between helpers and breeders from a 14 year field study. We used both pedigree and genetic approaches because long-tailed tits have access to pedigree information acquired through social relationships, but any fitness consequences will be determined by genetic relatedness. We then show using both pedigrees and genetic relatedness estimates that alloparental investment by helpers increases as their relatedness to the recipients of their care increases. We conclude that kin selection has played a critical role in moulding the investment decisions of helpers in this cooperatively breeding species.
Keywords: kinship, kin selection, indirect fitness, helper, provisioning effort, long-tailed tits
1. Introduction
Cooperative breeding is a reproductive system where some individuals, referred to as helpers, assist with the breeding attempts of others (Brown 1987). In most cooperative breeding systems, helping occurs within groups of relatives, and kin selection (Hamilton 1964) is usually invoked as a key process in the evolution of this behaviour (Lehmann & Keller 2006). However, the importance of indirect fitness benefits for explaining helping behaviour is still debated for a number of reasons (Cockburn 1998; Clutton-Brock 2002; Griffin & West 2002). First, helpers in some cooperative breeders care for non-kin or distant kin and invest as heavily as close relatives do (e.g. Dunn et al. 1995; Wright et al. 1999; Legge 2000; Clutton-Brock et al. 2001; Dickinson 2004; Canestrari et al. 2005). Second, there are several sources of direct fitness benefits that helpers may gain by investing in non-descendant offspring, such as group augmentation and by-product mutualism (Kokko et al. 2001; Clutton-Brock 2002). Third, kin association might be a consequence of demographic viscosity rather than active choice. Finally, shared reproduction or extra-group paternity can result in lower levels of genetic relatedness within groups than is predicted from social relationships (e.g. Mulder et al. 1994; Richardson et al. 2002). Nevertheless, indirect fitness gains have been shown to play a key role in the evolution of helping behaviour in several species, measured using both pedigree (e.g. Emlen & Wrege 1988; Komdeur 1994) and genetic relatedness (e.g. Richardson et al. 2003; MacColl & Hatchwell 2004; Covas et al. 2006; Wright et al. 2010).
Kin selection theory proposes that cooperative investment should be positively correlated with a donor's kinship to the recipients (Hamilton 1964). Thus, one of the best ways to test for a role of kin selection in the evolution of cooperative breeding is to determine whether helpers adjust their helping behaviour according to their kinship to the assisted brood. Such kin discriminatory helping behaviour may be expressed in two ways. First, in species with facultative cooperation helpers may decide whether to help or whom to help on the basis of their perceived kinship to the potential recipients (Curry 1988; Emlen & Wrege 1988; Lessells 1990; Komdeur 1994; Dickinson et al. 1996; Baglione et al. 2003; Richardson et al. 2003; Covas et al. 2006). Secondly, in just a few species, helpers have been shown to fine-tune their cooperative investment by adjusting their provisioning effort according to their relatedness to the helped brood (Clarke 1984; Komdeur 1994; Richardson et al. 2003; Wright et al. 2010). Griffin & West (2003) showed that helpers do consistently discriminate in favour of kin although the degree of kin discrimination varies across species. This interspecific variability in the degree of kin discrimination has subsequently been shown to be a function of species' social systems, discrimination being greater when variation in the relatedness of potential recipients is high, so that indiscriminate helping would not be favoured (Cornwallis et al. 2009).
The long-tailed tit Aegithalos caudatus has a kin-selected cooperative breeding system that is ideal for investigation of kin discrimination in helping. Helpers are failed breeders who switch from breeding to helping at the end of a temporally constrained season (MacColl & Hatchwell 2002). Potential helpers usually have a choice of helping at nests belonging to kin or non-kin and Russell & Hatchwell (2001) showed through observation and experiment that helpers exhibit a strong kin preference in their decision of whether and who to help. Helpers increase the recruitment rate of nestlings (Hatchwell et al. 2004), thereby gaining substantial indirect fitness benefits (MacColl & Hatchwell 2004). It is also known that long-tailed tits use learned vocal cues in the absence of spatial cues to kinship to recognize their relatives (Hatchwell et al. 2001a; Sharp et al. 2005). However, it is not known whether the provisioning effort of helpers varies in relation to kinship.
The aim of this study was to determine whether long-tailed tits helpers make fine-scale adjustments in their provisioning effort according to how closely related they are to the recipient brood. We first analysed the relatedness of long-tailed tit helpers to the broods they help, using pedigree data from 14 years of field observations and genetic data from microsatellite genotypes to determine the coefficients of genetic relatedness. We then used these measures of relatedness to investigate whether helpers adjust their provisioning rate according to their kinship to the recipients of their care. Both measures of kinship are important and informative because although the fitness consequences of helping will be influenced by the genetic relatedness of helpers to recipients, helpers do not have access to this information. Instead, long-tailed tits use recognition cues learned during development (i.e. socially acquired cues—the equivalent of pedigree information) to discriminate kin from non-kin (Hatchwell et al. 2001a; Sharp et al. 2005).
2. Material and methods
(a). Study species and field observations
We studied a population of 17–72 pairs of long-tailed tits from 1994–2007 in the Rivelin Valley, Sheffield, UK (55°23′ N, 1°34′ W). Long-tailed tits spend the winter in flocks of about 6–30 birds, including overlapping generations of kin from one or more families and also unrelated male and female immigrants (Hatchwell et al. 2001b; Sharp et al. 2008b). All birds start the season by attempting to breed independently in monogamous pairs. They are single-brooded, raising a maximum of one brood per year, but they often have several breeding attempts because of nest failures, caused mainly by predators, which occur at all stages of the breeding cycle (Hatchwell et al. 1999). Breeders whose nests fail early in the season usually re-nest, but if failure occurs later in the season, breeders abandon breeding for that year, and some of these failed breeders become helpers at the nest of another pair, assisting that pair in provisioning nestlings and fledglings (MacColl & Hatchwell 2002). For further details of the study species and system, see Hatchwell & Sharp (2006).
Adults were captured in mist-nets and ringed with unique colour combinations prior to breeding (mean proportion of adult population ringed greater than 95%). Throughout the breeding season (March–June), the breeding attempts of all pairs were located and closely monitored until fledging or failure. We recorded breeding events, such as lay dates, clutch size, incubation period, hatching and fledging dates at each nest. Breeding events for the small proportion of nests that were inaccessible were determined by observing breeder behaviour. Long-tailed tit nests are closed, so brood size was determined when nestlings were removed from nests to be ringed with unique combinations of colour rings when 10–13 days old (86% of broods were ringed on day 11, day of hatching = day 0). During the nestling period, we observed the provisioning behaviour of carers using binoculars from hides 10–15 m from nests, or a telescope 30–50 m from nests. Most nests were observed every 2 days from day 2 to fledging (day 16 or 17) or until nest failure. Most observation periods lasted 1 h (mean ± s.d. = 66 ± 10 min, n = 784), during which we recorded the identities of all individuals that fed nestlings and the visit rate of all carers. For further details of provisioning observations, see below and MacColl & Hatchwell (2003a). Provisioning rates are a valid measure of individual effort/investment in long-tailed tits because nestling growth is positively related to provisioning rate (Hatchwell et al. 2004), individual provisioning rates are repeatable and heritable (MacColl & Hatchwell 2003b), female fitness is related to her provisioning rate (MacColl & Hatchwell 2004), and reduced provisioning by male breeders when helped results in higher survival through load-lightening (Meade et al. 2010).
(b). Genetic relatedness
We took blood samples from the brachial vein of all adults and nestlings (under UK Home Office Licence) to determine their gender and analyse genetic relatedness. Genomic DNA was extracted from blood and amplified as previously described (Simeoni et al. 2007). All sampled individuals were sexed (Griffiths et al. 1998) and genotyped at nine microsatellite loci. The following loci were selected from a recently characterized set of 20, taking into account their degree of polymorphism, deviations from Hardy–Weinberg equilibrium, null alleles, linkage disequilibrium and sex linkage: Ase18; Ase37; Ase64; Hru2; Hru6; LOX1; Pca3; Pma22 and Ppi2 (mean number of alleles = 17.8, range = 7–42; Simeoni et al. 2007). We used the software SPAGeDi v. 1.2 (Hardy & Vekemans 2002) to calculate an estimator of genetic relatedness, r (pairwise relationship coefficients (r); Queller & Goodnight 1989), among individuals in the study population. We determined specific reference allele frequencies based on all adult genotypes, which we used in all genetic analyses in the SPAGeDi programme. Mean relatedness (±s.e.) among individuals was estimated by jack-knifing over nests or loci. The accuracy of estimates of relatedness, r, was tested by calculating r for known categories of relatives. The mean relatedness r (±s.e.) between parents and their nestlings was 0.49 ± 0.01 (expected r = 0.5) in a random sample of 60 families, among full-siblings r = 0.50 ± 0.01 (expected r = 0.5) in a random sample of 149 broods, and among half-siblings r = 0.25 ± 0.01 (expected r = 0.25) in a random sample of 22 broods. To test whether helpers were more likely to care for kin when compared with the population as a whole, we compared helpers' mean relatedness to nestlings in helped nests with their mean relatedness to nestlings in other nests in the same breeding season at which they did not help (58 helpers at 63 nests). To test whether there was an effect of relatedness on the provisioning rate of helpers, we calculated the helper's mean relatedness to all the nestlings in a nest at which they helped (110 cases of helping by 87 individuals at 72 nests).
(c). Pedigrees
Pedigrees provide a reliable estimate of kinship in long-tailed tits because there is no intraspecific brood parasitism and extra-pair paternity is uncommon (Hatchwell et al. 2002). We used data from 14 years of field observations of individually marked birds to determine the relationship between helpers and the breeders that they helped. This pedigree was inevitably incomplete because of the substantial number of immigrants to our study population each year, so this dataset is smaller than that available for analysis of effort in relation to genetic relatedness. Pedigree relationships fell into several categories: helpers fed at nests belonging to both parents, one parent, a sibling, son, daughter, half-sibling, grandparent or aunt, and those belonging to apparently unrelated breeders. To investigate the effect of kinship on helper provisioning rates, relationships of helpers to breeders were categorized as being either ‘first-order relative’ (relatedness to nestlings: rpedigree ≥ 0.25), where a helper was a first-order relative (rpedigree = 0.5) of one or both breeders, or ‘non-relative’ (rpedigree = 0) when there was no known kinship between helper and breeder. A small number of helpers had intermediate relatedness to breeders (0 < rpedigree < 0.25) but for none of these did we have complete data for provisioning analyses, so that they could not be included in analyses.
(d). Statistical analysis
We conducted two analyses of the effect of kinship on the provisioning rate of helpers, one using pedigree data and the other genotype data. We separated the different kinds of kinship information because the datasets differed, even though there is considerable overlap between them. First, we investigated whether helper provisioning rates varied with respect to their relatedness to breeders based on pedigree data using a linear mixed effects model with a normal error structure and an identity link function that was fitted using the function lmer in the R package lme4 (Bates et al. 2008). Of the helped nests with pedigree information, we had complete data on provisioning rates and other variables from 233 observation periods of 53 individual birds at 45 nests over 14 years (mean duration of observation ± s.e. = 325.6 min ± 40.2 per nest, range = 1–18 h, mean feeding rate (visit per hour) ± s.e. = 5.8 ± 0.5 per helper, range = 0.7–12.0). Bird identity and nest identity were included as random effects to control for non-independence of repeated observations of feeding rates by the same birds, and repeated observations of feeding rates at the same nest. We used the provisioning rate of helpers (number of visits/hour) as our response variable. The fixed effects were nestling age, brood size, number of helpers at the nest (all of which influenced provisioning rates of parents and male helpers in the analysis of MacColl & Hatchwell 2003a) and relatedness between helpers and breeders based on pedigree data. ‘Nestling age’ was measured in days from hatching (day 0); long-tailed tit broods hatch synchronously. ‘Brood size’ was the number of chicks present in the nest on day 11; this is a good indicator of brood size from hatching because nestling starvation is rare (Hatchwell et al. 2004). ‘Number of helpers’ was treated as a continuous variable. For relatedness, we used a binary variable set to 1 if helpers were first-order relatives of one or both breeders that they helped, otherwise set to 0 if helpers were not related to either breeder; this explanatory variable is subsequently referred to as ‘relative?’. We have not included helper age in these statistical models because previous analyses have found no significant effect of helper or breeder age on provisioning behaviour (MacColl & Hatchwell 2003a), reproductive parameters or likelihood of becoming a helper (Hatchwell et al. 2004).
To examine whether helpers adjusted their provisioning rate in relation to their genetic relatedness to nestlings, we used a linear mixed effects model, with normal error structure including random (bird identity and nest identity) and fixed effects (nestling age, brood size, number of helpers and relatedness). ‘Relatedness’ was estimated genetic relatedness, r, between helpers and the nestlings that they helped. We used data on provisioning rates from 404 observation periods of 87 individual birds at 72 nests with complete data over 14 years (mean duration of observation ± s.e. = 369.8 min ± 37.0 per nest, range = 1–18 h, mean feeding rate (visit per hour) ± s.e. = 5.8 ± 0.4 per helper, range = 0.7–17.1).
Sample sizes in the two models differed because all provisioning data had to be characterized for all explanatory variables (e.g. broods that failed before day 11 were excluded even though provisioning and kinship data were available because brood size was unknown). In models, we used Akaike information criteria (AIC) to refine the model by backwards stepwise deletion, removing terms in the order of increasing χ2 value only if dropping them resulted in a model with a lower AIC value, and used restricted maximum likelihood to estimate the parameters of the minimal adequate model. All biologically meaningful two-way interaction terms were also tested. All statistical analyses were performed in the R environment, v. 2.7.0 (R Development Core Team 2008). Means are reported as ±s.e.
3. Results
(a). Kin-biased helping
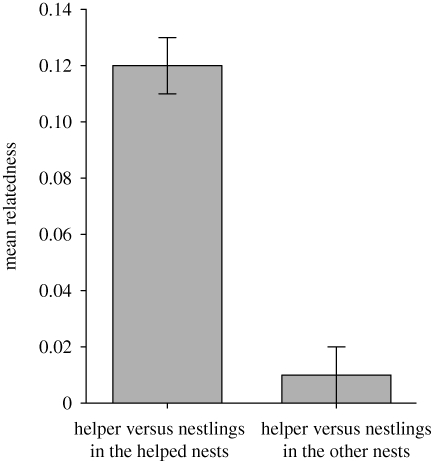
For the sample of helpers used in the analysis of provisioning versus genetic relatedness, the mean relatedness estimate, r, of helpers to the nestlings they cared for was 0.15 ± 0.02 (110 cases, 87 helpers, range = −0.23 to 0.52, 95% CI = 0.12–0.18). The mean relatedness of helpers to the nestlings in broods that they provisioned was significantly higher than their relatedness to other broods in the population that they did not provision (paired t-test, t = 5.391, d.f. = 62, p < 0.001; figure 1). This result is consistent with the finding of Russell & Hatchwell (2001) that helpers exhibit a kin preference in their choice of which brood to help.
Figure 1.
Mean genetic relatedness estimate (±s.e.) between helpers and nestlings in the helped nest and in other nests in the study population that they did not help.
Table 1 describes the relationship of helpers to breeders and nestlings using pedigree data. Helpers rarely assisted at a nest belonging to both parents, but in 73 per cent of cases, helpers cared for broods belonging to at least one first-order relative, the most frequent relationship being that the helper was a sibling of one breeder (42% of all helpers, 58% of first-order relatives). Very few helpers (less than 5% of cases) helped at the nest of a second-order relative. In 23 per cent of cases, helpers were apparently unrelated to the breeders. In order to verify these cases of help for non-kin, we calculated the genetic relatedness of helpers to breeders and nestlings using genotype data and found that they were indeed unrelated to either male breeders (r = −0.02 ± 0.02, n = 17, 95% CI = −0.06 to 0.02), female breeders (r = −0.06 ± 0.03, n = 18, 95% CI = −0.12 to 0) or nestlings (r = −0.07 ± 0.02, n = 15, 95% CI = −0.12 to −0.02).
Table 1.
Number and percentage of helpers falling into different categories of pedigree relationship with breeders and coefficients of relatedness (rpedigree) between helpers and the brood they care for calculated from pedigrees.
| helper assisting | categories | rpedigree | number of cases | % |
|---|---|---|---|---|
| both parents | first-order relative | 0.5 | 5 | 5.7 |
| father or mother only | 0.25 | 12 | 13.6 | |
| son or daughter | 0.25 | 10 | 11.4 | |
| sibling | 0.25 | 37 | 42.0 | |
| half-sibling | second-order relative | 0.125 | 1 | 1.1 |
| grandfather | 0.125 | 1 | 1.1 | |
| aunt | 0.125 | 2 | 2.3 | |
| non-relative | non-relative | 0 | 20 | 22.7 |
| total | — | — | 88 | 100 |
(b). The effect of kinship on provisioning rate
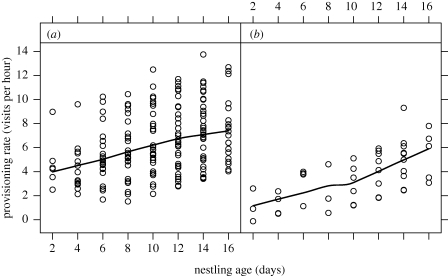
We first examined the effect of kinship between helpers and breeders on the provisioning rate of helpers using pedigree data (table 2). In a linear mixed effects model, the provisioning rates of helpers increased significantly with nestling age (p < 0.001; figure 2) and brood size (p = 0.020) and decreased as the number of helpers increased (p = 0.025). Most importantly, there was a significant difference in the provisioning rate of helpers depending on whether they fed at a nest belonging to at least one first-order relative or a non-relative (p = 0.004): helpers worked harder when assisting relatives (figure 2). There was no significant effect of any interaction terms.
Table 2.
Analysis of the effect of kinship (first-order relative versus non-relative) between breeders and helpers on the provisioning rate of helpers, using a linear mixed effects model (number of observation periods = 233, number of individuals = 53, number of nests = 45). All biologically meaningful two-way interaction terms were also tested, and none were significant (p > 0.05). Significant p-values are shown in italic.
| random effects | variance | s.d. | |||
|---|---|---|---|---|---|
| bird identity | 1.636 | 1.279 | |||
| nest identity | 5.901 | 2.429 | |||
| residual | 6.864 | 2.620 | |||
| fixed effects | estimate | s.e. | χ2 | d.f. | p |
| (intercept) | 0.108 | 6.347 | — | — | — |
| year | — | — | 7.938 | 11 | 0.719 |
| date | 0.012 | 0.055 | 0 | 1 | 1 |
| time | −0 | 0 | 0.150 | 1 | 0.699 |
| nestling age | 0.302 | 0.076 | 19.905 | 1 | <0.001 |
| brood size | 0.637 | 0.298 | 5.449 | 1 | 0.020 |
| number of helpers | −0.910 | 0.390 | 5.033 | 1 | 0.025 |
| relative? | −2.782 | 1.022 | 8.091 | 1 | 0.004 |
Figure 2.
The relationship between provisioning rate of helpers and nestling age: (a) first-order relatives, (b) non-relatives, the relatedness between breeders and helpers determined from pedigree data (table 2). The lines show means from the data.
In the analysis for an effect of the genetic relatedness of helpers to nestlings on their provisioning rate (table 3), helper effort was again positively related to nestling age (p < 0.001) and brood size (p < 0.001), and negatively to the number of helpers (p < 0.001). There was no effect of any of the interaction terms. As we found using categorical pedigree data, the provisioning rate of helpers increased as their genetic relatedness to nestlings increased (p = 0.002; figure 3).
Table 3.
Analysis of the effect of the genetic relatedness between helpers and nestlings on the provisioning rate of helpers, using a linear mixed effects model (number of observation periods = 404, number of individuals = 87, number of nests = 72). All biologically meaningful two-way interaction terms were also tested, and none were significant (p > 0.05). Significant p-values are shown in italic.
| random effects | variance | s.d. | |||
|---|---|---|---|---|---|
| bird identity | 4.926 | 2.219 | |||
| nest identity | 2.418 | 1.555 | |||
| residual | 7.284 | 2.699 | |||
| fixed effects | estimate | s.e. | χ2 | d.f. | p |
| (intercept) | −4.392 | 4.501 | — | — | — |
| year | — | — | 6.996 | 11 | 0.799 |
| date | 0.042 | 0.041 | 0.862 | 1 | 0.353 |
| time | −0 | 0.001 | 0.023 | 1 | 0.881 |
| nestling age | 0.258 | 0.059 | 21.249 | 1 | <0.001 |
| brood size | 0.584 | 0.169 | 13.469 | 1 | <0.001 |
| number of helpers | −1.226 | 0.293 | 18.398 | 1 | <0.001 |
| relatedness | 6.138 | 2.024 | 9.715 | 1 | 0.002 |
Figure 3.
The provisioning rate of helpers in relation to their mean genetic relatedness to the nestlings they provisioned. The line show predicted values from the model (table 3) with other parameters set to their mean values.
4. Discussion
(a). Kin-biased helping
In this study, we have shown that most long-tailed tit helpers fed broods to which they were related, typically via first-order kinship to one of the parents (table 1). This result is consistent with the results of an experimental study by Russell & Hatchwell (2001) that showed active kin-biased helping behaviour when controlling for spatial effects. Helpers in most cooperatively breeding species provision kin (Hatchwell 2009), but several studies have suggested that because helpers are generally offspring from a previous breeding event that assist their parents after delaying dispersal, kin-directed helping might simply be a consequence of demographic viscosity rather than active choice (Clutton-Brock et al. 2001; Clutton-Brock 2002; Canestrari et al. 2005). In long-tailed tits, however, helping is not a consequence of delayed dispersal. Instead, all individuals attempt to breed independently each year and become a helper only if they fail to breed successfully themselves, when the expected indirect fitness gain from helping exceeds the expected direct fitness gain from breeding (MacColl & Hatchwell 2002). Thus, individuals can switch back and forth between breeding and helping throughout their life (Hatchwell et al. 2004). Furthermore, each year approximately half of all failed breeders do not become helpers (Hatchwell et al. 2004). Therefore, a key feature of their cooperative system is that helpers are not constrained by membership of discrete family groups, but must make a decision about whether and whom to help; kinship clearly plays a key role in this decision (Russell & Hatchwell 2001; this study). Several other studies of cooperatively breeding birds have provided similar evidence for active kin-bias in the decision of whether to help or whom to help (Emlen & Wrege 1988; Lessells 1990; Komdeur 1994; Dickinson et al. 1996; Baglione et al. 2003; Richardson et al. 2003; Covas et al. 2006).
The most frequent pedigree relationship between breeders and helpers in this study was that they were siblings (42% of all relationships; table 1). This preponderance of helping within cohorts also differs from typical cooperative breeders where parents are helped by offspring (Brown 1987). There are several reasons why that pattern of helping is uncommon in long-tailed tits. First, offspring do not delay dispersal and local recruitment rates are high when compared with many other small passerines, so that siblings often become breeders in close proximity to each other (Sharp et al. 2008b). Furthermore, those birds that do disperse often do so in sibling coalitions, so they may have the opportunity to help at a sibling's nest in subsequent breeding seasons (Sharp et al. 2008a). Second, in contrast to most cooperative species, long-tailed tits have a low annual survival rate of ca 50 per cent (McGowan et al. 2003), so many parents die before their offspring have an opportunity to help them. Third, surviving pairs of long-tailed tits have a divorce rate of 63 per cent, and among successful pairs this is even higher (81%; Hatchwell et al. 2000), so very few birds have the chance to feed full siblings in a nest belonging to both parents. Finally, the stochastic nature of nest predation plays a key role in determining the identities of potential helpers and recipients, so there is little reason why offspring should help their parents any more frequently than parents help their offspring, as observed (table 1). Together, these demographic traits mean that failed breeders have more chance of finding a sibling to help than any other relative, so helping a sibling is likely to be the most frequent route to gain indirect fitness benefits, even though the indirect fitness return from assisting full-siblings of their parents would be greater.
Long-tailed tit helpers gain substantial indirect fitness benefits by enhancing the recruitment rate of fledglings from helped broods (Hatchwell et al. 2004; MacColl & Hatchwell 2004). Helpers also reduce the reproductive costs of breeders by allowing them to work less hard when provisioning their brood (Hatchwell & Russell 1996; MacColl & Hatchwell 2003a; Meade et al. 2010). By contrast, we have no evidence that helpers gain any substantial direct fitness benefit from their cooperative behaviour (J. Meade & B. J. Hatchwell 2010, unpublished data), so it is surprising that 23 per cent of helpers were found to assist at the nests of non-kin (table 1). Interestingly, we have some evidence that help by non-kin was the consequence of a previous social relationship. There were 20 unrelated helpers in our sample, and in eight cases the helper had been a partner of one of the breeders or one of the other helpers at the same nest. It is possible that there is some direct benefit of helping that we have overlooked or been unable to detect in analyses to date, perhaps driven by the maintenance of social relationships among individuals in non-breeding flocks or during the following breeding season. Alternatively, help for non-kin may be a consequence of the kin recognition mechanism used by long-tailed tits, a possibility we discuss further below.
(b). The effect of kinship on provisioning rate
The provisioning rate of long-tailed tit helpers varied significantly with nestling age, brood size and number of helpers (tables 2 and 3). The effects of nestling age and brood size on helper provisioning are similar to the results of MacColl & Hatchwell (2003a), but that study found no effect of the number of helpers on helper provisioning. Using a much larger sample, we found that helpers did reduce their provisioning rate significantly as the number of helpers increased, as found in several other cooperative species (Hatchwell 1999). Most importantly, we found a strongly significant positive effect of relatedness, whether measured using pedigrees (figure 2) or genetically (figure 3), on helper provisioning rates. It is this result that we focus on in the remainder of the discussion.
In kin-selected cooperative breeding systems, helpers can maximize their indirect fitness gains by adjusting investment according to their relatedness to a brood (Hamilton 1964). Given that cooperation in long-tailed tits is driven by kin selection, kin-biased helping and adjustments in care according to kinship would be expected, provided that there is a mechanism for effective kin discrimination. A few previous studies have also provided evidence for an effect of relatedness on the provisioning rate of helpers in cooperatively breeding species (Clarke 1984; Komdeur 1994; Richardson et al. 2003; Wright et al. 2010), but these findings are far from universal (Dunn et al. 1995; Wright et al. 1999; Legge 2000; Dickinson 2004; Canestrari et al. 2005; Komdeur et al. 2008). Kin discrimination would not be expected if indirect fitness benefits were unimportant in the evolution and/or maintenance of helping behaviour. However, even if helping is kin-selected, there are several reasons why helpers may still not adjust their helping effort in relation to kinship. First, the degree of kin discrimination is likely to depend on the variation in relatedness between group members. When helpers live in social groups that always contain predominantly close relatives, it may not be necessary to recognize kin in order to gain indirect fitness benefits, so no adjustment in helping effort would be expected (Cornwallis et al. 2009). Second, an individual's optimal effort will depend in part on the trade-off between the benefits of current investment against the costs for future reproductive investment. In some cases, the current costs of caring or the opportunities for future reproduction may be low, so high current investment will be selected for, analogous to terminal investment in senescent breeders (Clutton-Brock 1984; Canestrari et al. 2008). Long-tailed tits helpers have an approximately 50 per cent chance of surviving to breed in the following year, so future reproductive opportunities are substantial and should influence current investment. Such considerations emphasize the importance of taking a life-history perspective on investment decisions (Heinsohn 2004; Covas & Griesser 2007; Canestrari et al. 2008). Third, helper care may be enforced by breeders so that they are not able to optimize their own investment. This could apply when helpers have to ‘pay rent’ to stay on a territory (e.g. Mulder & Langmore 1993), but evidence for this idea is scant (Russell 2004). Finally, the degree of kin discrimination in caring will depend on the mechanism of recognition used in any particular system (Komdeur & Hatchwell 1999; Komdeur et al. 2008). In long-tailed tits, individuals recognize their relatives using vocal cues learned early in life (Hatchwell et al. 2001a; Sharp et al. 2005) allowing helpers to preferentially assist close kin in the absence of spatial cues to kinship (Russell & Hatchwell 2001). But, this raises two questions: why do some helpers help non-kin? And how do helpers discriminate between kin of varying relatedness?
It has been suggested that help for non-kin might result from recognition errors caused by the extensive mixing of families that occurs during the non-breeding season (Hatchwell et al. 2001b; Sharp et al. 2005). Alternatively, helpers may have a threshold for acceptance of kin that means they are liable to make recognition errors (Reeve 1989). For long-tailed tits, the potential fitness gains of helping kin are high (Hatchwell et al. 2004; MacColl & Hatchwell 2004). Conversely, the costs may be low because they help for a short period (Hatchwell et al. 2004), which is not at the expense of independent breeding (MacColl & Hatchwell 2002) or future survival (McGowan et al. 2003). Therefore, it may pay to have a recognition system prone to acceptance errors rather than rejection errors (Reeve 1989). Such a strategy may be particularly effective if combined with a recognition system that perceives a relatedness gradient. Sharp et al. (2005) suggested that learned recognition cues allow long-tailed tits to distinguish those individuals in the population with whom and by whom they were reared from all other members of the population, and a simple rule of thumb based on this dichotomy explained a very high proportion of helper–breeder relationships. However, our finding that helpers also adjust their effort according to the degree of relatedness, indicates that they perceive a gradient rather than a simple threshold of kinship. The mechanism that allows such discrimination remains to be determined, but could involve assessment of the degree of similarity of their own calls to those of the breeders whose brood they care for.
In conclusion, from this study and from that of Russell & Hatchwell (2001) we have demonstrated two levels of kin-biased helping behaviour in long-tailed tits. First, the decision of whether to help and who to help is driven largely by kinship of potential helpers to available recipients. Second, helpers adjust their level of investment according to their degree of relatedness to the broods they have chosen to help. Therefore, this study provides further evidence for a positive effect of kinship on helper investment, and further support for the conclusion that kin selection has played a critical role in the evolution of cooperative behaviour in this species.
Acknowledgements
All research was conducted under appropriate licences for taking blood samples from wild birds (UK Home Office project licence 4003214 and personal licence PIL 80/623) and for capture and ringing of wild birds (British Trust for Ornithology ringing permit (3770).
We thank A. Bamford, M. K. Fowlie, N. Green, J.-W. Lee, A. MacColl, A. McGowan, J. Meade, D. Richardson, D. J. Ross and A. F. Russell for their invaluable assistance with data collection in the field. We also thank the Sheffield Molecular Genetics Facility for their assistance with genetic analysis and Sheffield City Council, Yorkshire Water and Hallamshire Golf Club for permission to research on their land. We are grateful to A. P. Beckerman, D. Gillespie, J.-W. Lee, J. Meade, A. F. Russell and two anonymous reviewers for their useful discussions and comments on the manuscript and for statistical advice. This work was partly funded by the University of Sheffield and the Natural Environment Research Council, for which we are most grateful. B.J.H. held a Leverhulme Research Fellowship during the preparation of this manuscript.
References
- Baglione V., Canestrari D., Marcos J. M., Ekman J.2003Kin selection in cooperative alliances of carrion crows. Science 300, 1947–1949 (doi:10.1126/science.1082429) [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Dai B.2008lme4: linear mixed-effect models using S4 classes. R package version 0.9999375-28. See http://lme4.r-forge.r-project.org/ [Google Scholar]
- Brown J. L.1987Helping and communal breeding in birds. Princeton, NJ: Princeton University Press [Google Scholar]
- Canestrari D., Marcos J. M., Baglione V.2005Effect of parentage and relatedness on the individual contributions to cooperative chick care in the carrion crow Corvus corone corone. Behav. Ecol. Sociobiol. 57, 422–428 (doi:10.1007/s00265-004-0879-1) [Google Scholar]
- Canestrari D., Chiarati E., Marcos J. M., Ekman J., Baglione V.2008Helpers but not breeders adjust provisioning effort to year-round territory resource availability in carrion crows. Anim. Behav. 76, 943–949 (doi:10.1016/j.anbehav.2008.05.013) [Google Scholar]
- Clarke M. F.1984Co-operative breeding by the Australian bell miner Manorina melanophrys Latham: a test of kin selection theory. Behav. Ecol. Sociobiol. 14, 137–146 (doi:10.1007/BF00291904) [Google Scholar]
- Clutton-Brock T. H.1984Reproductive effort and terminal investment in iteroparous animal. Am. Nat. 123, 212–229 (doi:10.1086/284198) [Google Scholar]
- Clutton-Brock T. H.2002Breeding together: kin selection and mutualism in cooperative vertebrates. Science 296, 69–72 (doi:10.1126/science.296.5565.69) [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T. H., Brotherton P. N. M., O'Riain M. J., Griffin A. S., Gaynor D., Kansky R., Sharpe L. L., McIlrath G. M.2001Contributions to cooperative rearing in meerkats, Suricata suricatta. Anim. Behav. 61, 672–683 (doi:10.1006/anbe.2000.1631) [Google Scholar]
- Cockburn A.1998Evolution of helping behavior in cooperatively breeding birds. Annu. Rev. Ecol. Syst. 29, 141–177 (doi:10.1146/annurev.ecolsys.29.1.141) [Google Scholar]
- Cornwallis C. K., West S. A., Griffin A. S.2009Routes to indirect fitness in cooperatively breeding vertebrates: kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2467 (doi:10.1111/j.1420-9101.2009.01853.x) [DOI] [PubMed] [Google Scholar]
- Covas R., Griesser M.2007Life history and the evolution of family living in birds. Proc. R. Soc. B 274, 1349–1357 (doi:10.1098/rspb.2007.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covas R., Dalecky A., Caizergues A., Doutrelant C.2006Kin associations and direct vs. indirect fitness benefits in colonial cooperatively breeding sociable weavers Philetairus socius. Behav. Ecol. Sociobiol. 60, 323–331 (doi:10.1007/s00265-006-0168-2) [Google Scholar]
- Curry R. L.1988Influence of kinship on helping behaviour in Galapagos mockingbirds. Behav. Ecol. Sociobiol. 22, 141–152 (doi:10.1007/s00265-006-0168-2) [Google Scholar]
- Dickinson J. L.2004A test of the importance of direct and indirect fitness benefits for helping decisions in western bluebirds. Behav. Ecol. 15, 233–238 (doi:10.1093/beheco/arh001) [Google Scholar]
- Dickinson J. L., Koenig W. D., Pitelka F. A.1996The fitness consequences of helping behavior in the western bluebird. Behav. Ecol. 7, 168–177 (doi:10.1093/beheco/7.2.168) [Google Scholar]
- Dunn P. O., Cockburn A., Mulder R. A.1995Fairy-wren helpers often care for young to which they are unrelated. Proc. R. Soc. Lond. B 259, 339–343 (doi:10.1098/rspb.1995.0050) [Google Scholar]
- Emlen S. T., Wrege P. H.1988The role of kinship in helping decisions among white-fronted bee-eaters. Behav. Ecol. Sociobiol. 23, 305–315 (doi:10.1007/BF00300577) [Google Scholar]
- Griffin A. S., West S. A.2002Kin selection: fact and fiction. Trends Ecol. Evol. 17, 15–21 (doi:10.1016/S0169-5347(01)02355-2) [Google Scholar]
- Griffin A. S., West S. A.2003Kin discrimination and the benefit of helping in cooperatively breeding vertebrates. Science 302, 634–636 (doi:10.1126/science.1089402) [DOI] [PubMed] [Google Scholar]
- Griffiths R., Double M. C., Orr K., Dawson R. J. G.1998A DNA test to sex most birds. Mol. Ecol. 7, 1071–1075 (doi:10.1046/j.1365-294x.1998.00389.x) [DOI] [PubMed] [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour I, II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Hardy O. J., Vekemans X.2002SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes 2, 618–620 (doi:10.1046/j.1471-8286.2002.00305.x) [Google Scholar]
- Hatchwell B. J.1999Investment strategies of breeders in avian cooperative breeding systems. Am. Nat. 154, 205–219 (doi:10.1086/303227) [DOI] [PubMed] [Google Scholar]
- Hatchwell B. J.2009The evolution of cooperative breeding in birds: kinship, dispersal and life history. Phil. Trans. R. Soc. B 364, 3217–3227 (doi:10.1098/rstb.2009.0109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell B. J., Russell A. F.1996Provisioning rules in cooperatively breeding long-tailed tits Aegithalos caudatus: an experimental study. Proc. R. Soc. Lond. B 263, 83–88 (doi:10.1098/rspb.1996.0014) [Google Scholar]
- Hatchwell B. J., Sharp S. P.2006Kin selection, constraints, and the evolution of cooperative breeding in long-tailed tits. Adv. Study Behav. 36, 355–395 (doi:10.1016/S0065-3454(06)36008-1) [Google Scholar]
- Hatchwell B. J., Russell A. F., Fowlie M. K., Ross D. J.1999Reproductive success and nest-site selection in a cooperative breeder: effect of experience and a direct benefit of helping. Auk 116, 355–363 [Google Scholar]
- Hatchwell B. J., Russell A. F., Ross D. J., Fowlie M. K.2000Divorce in cooperatively breeding long-tailed tits: a consequence of inbreeding avoidance? Proc. R. Soc. Lond. B 267, 813–819 (doi:10.1098/rspb.2000.1076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell B. J., Ross D. J., Fowlie M. K., McGowan A.2001aKin discrimination in cooperatively breeding long-tailed tits. Proc. R. Soc. Lond. B 268, 885–890 (doi:10.1098/rspb.2001.1598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatchwell B. J., Anderson C., Ross D. J., Fowlie M. K., Blackwell P. G.2001bSocial organisation in cooperatively breeding long-tailed tits: kinship and spatial dynamics. J. Anim. Ecol. 70, 820–830 (doi:10.1046/j.0021-8790.2001.00541.x) [Google Scholar]
- Hatchwell B. J., Ross D. J., Chaline N., Fowile M. K., Burke T.2002Parentage in the cooperative breeding system of long-tailed tits, Aegithalos caudatus. Anim. Behav. 64, 55–63 (doi:10.1006/anbe.2002.3033) [Google Scholar]
- Hatchwell B. J., Russell A. F., MacColl A. D. C., Ross D. J., Fowlie M. K., McGowan A.2004Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10 (doi:10.1093/beheco/arg091) [Google Scholar]
- Heinsohn R.2004Parental care, load-lightening, and costs. In Ecology and evolution of cooperative breeding in birds (eds Koenig W. D., Dickinson J.), pp. 67–80 Cambridge, UK: Cambridge University Press [Google Scholar]
- Kokko H., Johnstone R. A., Clutton-Brock T. H.2001The evolution of cooperative breeding through group augmentation. Proc. R. Soc. Lond. B 268, 187–196 (doi:10.1098/rspb.2000.1349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komdeur J.1994The effect of kinship on helping in the cooperative breeding Seychelles warbler Acrocephalus sechellensis. Proc. R. Soc. Lond. B 256, 47–52 (doi:10.1098/rspb.1994.0047) [Google Scholar]
- Komdeur J., Hatchwell B. J.1999Kin recognition: function and mechanism in avian societies. Trends Ecol. Evol. 14, 237–241 (doi:10.1016/S0169-5347(98)01573-0) [DOI] [PubMed] [Google Scholar]
- Komdeur J., Richardson D. S., Hatchwell B. J.2008Kin-recognition mechanisms in cooperative breeding systems: ecological causes and behavioural consequences of variation. In Ecology of social evolution (eds Korb J., Heinze J.), pp. 175–193 Berlin, Germany: Springer [Google Scholar]
- Legge S.2000Helper contributions in the cooperatively breeding laughing kookaburra: feeding young is no laughing matter. Anim. Behav. 59, 1009–1018 (doi:10.1006/anbe.2000.1382) [DOI] [PubMed] [Google Scholar]
- Lehmann L., Keller L.2006The evolution of cooperation and altruism—a general framework and a classification of models. J. Evol. Biol. 19, 1365–1376 (doi:10.1111/j.1420-9101.2006.01119.x) [DOI] [PubMed] [Google Scholar]
- Lessells C. M.1990Helping at the nest in European bee-eaters: who helps and why? In Population biology of passerine birds: an integrated approach (eds Blondel J., Gosler A., Lebreton J.-D., McLeery R. H.), pp. 357–368 Berlin, Germany: Springer [Google Scholar]
- MacColl A. D. C., Hatchwell B. J.2002Temporal variation in fitness pay-offs promotes cooperative breeding in long-tailed tits Aegithalos caudatus. Am. Nat. 160, 186–194 (doi:10.1086/341013) [DOI] [PubMed] [Google Scholar]
- MacColl A. D. C., Hatchwell B. J.2003aSharing of caring: nestling provisioning behaviour of long-tailed tit, Aegithalos caudatus, parents and helpers. Anim. Behav. 66, 955–964 (doi:10.1006/anbe.2003.2268) [Google Scholar]
- MacColl A. D. C., Hatchwell B. J.2003bHeritability of parental care in a passerine bird. Evolution 57, 2191–2195 (doi:10.1111/j.0014-3820.2003.tb00398.x) [DOI] [PubMed] [Google Scholar]
- MacColl A. D. C., Hatchwell B. J.2004Determinants of lifetime fitness in a cooperative breeder, the long-tailed tit Aegithalos caudatus. J. Anim. Ecol. 73, 1137–1148 (doi:10.1111/j.0021-8790.2004.00887.x) [Google Scholar]
- Meade J., Nam K.-B., Beckerman A. P., Hatchwell B. J.2010Consequences of ‘load-lightening’ for future indirect fitness gains by helpers in a cooperatively breeding bird. J. Anim. Ecol. 79, 529–537 (doi:10.1111/j.1365-2656.2009.01656.x) [DOI] [PubMed] [Google Scholar]
- McGowan A., Hatchwell B. J., Woodburn R. J. W.2003The effect of helping behaviour on the survival of juvenile and adult long-tailed tits Aegithalos caudatus. J. Anim. Ecol. 72, 491–499 (doi:10.1046/j.1365-2656.2003.00719.x) [Google Scholar]
- Mulder R. A., Langmore N. E.1993Dominant males punish helpers for temporary defection in superb fairy-wrens. Anim. Behav. 45, 830–833 (doi:10.1006/anbe.1993.1100) [Google Scholar]
- Mulder R. A., Dunn P. O., Cockburn A., Lazenby-Cohen K. A., Howell M. J.1994Helpers liberate female fairy-wrens from constraints on extra-pair mate choice. Proc. R. Soc. Lond. B 255, 223–229 (doi:10.1098/rspb.1994.0032) [Google Scholar]
- Queller D. C., Goodnight K. F.1989Estimating relatedness using genetic markers. Evolution 43, 258–275 (doi:10.2307/2409206) [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2008R: A language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing; ISBN 3-900051-07-0. See http://www.R-project.org [Google Scholar]
- Reeve H. K.1989The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435 (doi:10.1086/284926) [Google Scholar]
- Richardson D. S., Burke T., Komdeur J.2002Direct benefits explain the evolution of female biased cooperative breeding in the Seychelles warbler. Evolution 56, 2313–2321 (doi:10.1111/j.0014-3820.2002.tb00154.x) [DOI] [PubMed] [Google Scholar]
- Richardson D. S., Burke T., Komdeur J.2003Sex-specific associative learning cues and inclusive fitness benefits in the Seychelles warbler. J. Evol. Biol. 16, 854–861 (doi:10.1046/j.1420-9101.2003.00592.x) [DOI] [PubMed] [Google Scholar]
- Russell A. F.2004Mammals: comparisons and contrasts. In Ecology and evolution of cooperative breeding in birds (eds Koenig W. D., Dickinson J. L.), pp. 210–227 Cambridge, UK: Cambridge University Press [Google Scholar]
- Russell A. F., Hatchwell B. J.2001Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. R. Soc. Lond. B 268, 2169–2174 (doi:10.1098/rspb.2001.1790) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S. P., McGowan A., Wood M. J., Hatchwell B. J.2005Learned kin recognition cues in a social bird. Nature 434, 1127–1130 (doi:10.1038/nature03522) [DOI] [PubMed] [Google Scholar]
- Sharp S. P., Simeoni M., Hatchwell B. J.2008aDispersal of sibling coalitions promotes helping among immigrants in a cooperatively breeding bird. Proc. R. Soc. B 275, 2125–2130 (doi:10.1098/rspb.2008.0398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp S. P., Baker M. B., Hadfield J. D., Simeoni M., Hatchwell B. J.2008bNatal dispersal and recruitment in a cooperatively breeding bird. Oikos 117, 1371–1379 (doi:10.1111/j.2008.0030-1299.16392.x) [Google Scholar]
- Simeoni M., Dawson D. A., Ross D. J., Chaline N., Burke T., Hatchwell B. J.2007Characterisation of 20 microsatellite loci in the long-tailed tit Aegithalos caudatus (Aegithalidae, AVES). Mol. Ecol. Notes 7, 1319–1322 (doi:10.1111/j.1471-8286.2007.01868.x) [Google Scholar]
- Wright J., Parker P. G., Lundy K. J.1999Relatedness and chick feeding effort in the cooperatively breeding Arabian babbler. Anim. Behav. 58, 779–785 (doi:10.1006/anbe.1999.1204) [DOI] [PubMed] [Google Scholar]
- Wright J., McDonald P. G., te Marvelde L., Kazem A. J. N., Bishop C. M.2010Helping effort increases with relatedness in bell miners, but ‘unrelated’ helpers of both sexes still provide substantial care. Proc. R. Soc. B 277, 437–445 (doi:10.1098/rspb.2009.1360) [DOI] [PMC free article] [PubMed] [Google Scholar]