Abstract
Songbird males learn to sing their songs from an adult ‘tutor’ early in life, much like human infants learn to speak. Similar to humans, in the songbird brain there are separate neural substrates for vocal production and for auditory memory. In adult songbirds, the caudal pallium, the avian equivalent of the auditory association cortex, has been proposed to contain the neural substrate of tutor song memory, while the song system is involved in song production as well as sensorimotor learning. If this hypothesis is correct, there should be neuronal activation in the caudal pallium, and not in the song system, while the young bird is hearing the tutor song. We found increased song-induced molecular neuronal activation, measured as the expression of an immediate early gene, in the caudal pallium of juvenile zebra finch males that were in the process of learning to sing their songs. No such activation was found in the song system. Molecular neuronal activation was significantly greater in response to tutor song than to novel song or silence in the medial part of the caudomedial nidopallium (NCM). In the caudomedial mesopallium, there was significantly greater molecular neuronal activation in response to tutor song than to silence. In addition, in the NCM there was a significant positive correlation between spontaneous molecular neuronal activation and the strength of song learning during sleep. These results suggest that the caudal pallium contains the neural substrate for tutor song memory, which is activated during sleep when the young bird is in the process of learning its song. The findings provide insight into the formation of auditory memories that guide vocal production learning, a process fundamental for human speech acquisition.
Keywords: memory formation, sleep, speech, learning, sensorimotor, birdsong
1. Introduction
Songbirds learn their songs from an adult conspecific ‘tutor’ through a process that has parallels with human speech acquisition (Doupe & Kuhl 1999; Bolhuis & Wynne 2009). In both cases, there is a sensitive period for auditory learning, and vocal learning proceeds through a transitional sensorimotor phase that is called ‘babbling’ in human infants and ‘subsong’ in songbirds. In songbirds, during auditory learning an internal representation of the tutor song is formed that has been called a ‘template’ (Konishi 1965). During the sensorimotor phase, the young bird starts to vocalize, and it is thought that its song output is matched with the template that was formed in the memorization phase. Eventually, the bird will sing a crystallized song that resembles the song of the tutor to a certain degree. Nottebohm (1981) made the distinction between auditory memory and what he called ‘the conversion of that memory into a motor program’. In the case of age-limited learners such as the zebra finch, crystallized song does not change substantially during adulthood.
A network of interconnected forebrain nuclei, known as the ‘song system’, is necessary for vocal learning and song production during the sensorimotor phase and in adulthood (Nottebohm et al. 1976; Bottjer et al. 1984; Scharff & Nottebohm 1991). In Nottebohm's (1981) terms, the song system is involved in learning of the motor program. In contrast, brain regions outside the song system, in the nidopallium and mesopallium, are involved in secondary auditory processing (Mello et al. 1992; Mello & Clayton 1994) and they have been suggested to contain the neural substrate for tutor song memory acquired in the memorization phase (Bolhuis et al. 2000, 2001; Terpstra et al. 2004; Phan et al. 2006; London & Clayton 2008). In adult zebra finch males, molecular neuronal activation in the caudomedial nidopallium (NCM) in response to tutor song is related to the strength of song learning (Bolhuis et al. 2000, 2001; Terpstra et al. 2004; Phan et al. 2006). In addition, lesions to the NCM of adult male zebra finches impaired recognition of the tutor song (Gobes & Bolhuis 2007). The males' own song production was not affected by the lesions, suggesting a dissociation between regions in the brain that contain a neural representation of the bird's own song (BOS), or ‘motor program’, and are involved in song production, and those involved in song perception and memory, which contain a neural representation of the tutor's song, or ‘template’ (Gobes & Bolhuis 2007). Taken together, these findings in adults are consistent with the suggestion that the NCM contains the neural substrate for tutor song memory (Bolhuis & Gahr 2006).
If this hypothesis is correct, neurons in the NCM are expected to be activated in juvenile males that are learning the song of their tutor. To test this hypothesis, we investigated molecular neuronal activation, measured as the expression of the immediate early gene (IEG) ZENK (an acronym of Zif-268, Erg-1, NKFI-A and Krox-24), of juveniles in response to tutor song, novel song or silence. Although IEG expression is not a necessary concomitant of neuronal firing (Mello & Jarvis 2008), it has proved to be a useful indicator of neuronal activation when different treatment groups are compared, especially in the context of perception and learning. We measured molecular neuronal activation in the caudal pallium, specifically the NCM and caudomedial mesopallium (CMM). We also measured molecular neuronal activation in two nuclei of the posterior pathway of the song system: HVC (abbreviation used as a proper name) and the robust nucleus of the arcopallium (RA). Finally, we measured molecular neuronal activation when the juveniles were asleep, as it has been suggested that sleep plays a crucial role in avian learning and memory (Gobes & Bolhuis 2008; Jackson et al. 2008; Shank & Margoliash 2009).
2. Material and methods
(a). Subjects
Fifty-seven male zebra finches were obtained from the central animal facility (GDL) of Utrecht University. Birds were maintained on a 15∶9 light∶dark cycle, lights on at 06.00 h. All birds were kept in breeding cages with their parents and siblings until 47 days post-hatching (dph). At 47 dph, all sons from a clutch were taken to a different room and kept in communal cages (60 × 40 × 40 cm). Mean age at the day of the experiment was 56 days (range 54–59 dph; s.d. 1.8). In zebra finches, the memorization phase occurs between approximately 25 and 65 days after hatching, during which 10 days of exposure to a song tutor has been shown to be sufficient to acquire an adult song (Eales 1985, 1989; Roper & Zann 2006). In this species, the sensorimotor learning phase is thought to start around 30 days after hatching, and the song crystallizes when the bird is about 90 days old (Immelmann 1969; Johnson et al. 2002). Thus, all experimental birds were in the middle of the process of learning the song of the biological father up to the time of removal from their parents. Experimental procedures were in accordance with European law and approved by the Animal Experiments Committee of Utrecht University (DEC 06/296).
(b). Experimental procedures
Before stimulus exposure, the birds had been in a sound-proof chamber for two nights and one full day. After the second night, subjects were exposed to one of the three treatments: a recording of the song of the father (‘tutor song’, TUT), a recording of the song of a novel zebra finch male (‘novel song’, NOV) or silence (SIL; see electronic supplementary material for details on stimulus construction). For the main experimental group (group A; n = 19 birds: SIL = 6, TUT = 6, NOV = 7), on the day of the experiment, the lights were automatically turned on at 06.00 h and off at 08.00 h. Stimulus presentation started at 11.00 h and lasted approximately 1 h. The birds were sacrificed at 12.30 h, 30 min after the end of the last stimulus presentation. Between 08.00 and 12.30 h, the birds were kept in darkness to stop them from vocalizing and so to prevent their own vocalizations evoking molecular neuronal activation. Data from three animals in group A could not be collected because of technical failure (see electronic supplementary material). A second group of birds (group B; n = 20: SIL = 7, TUT = 7, NOV = 6) was treated identically to the main experimental group except that the lights remained switched on between 06.00 h and 12.30 h. These birds were thus exposed to the same auditory stimuli as the main experimental group, but they also continued to vocalize when the lights remained on after 08.00 h. This group made it possible to investigate effects that were attributable to the birds' own vocalizations. Birds in group B that did not vocalize themselves (n = 5) were added to group A for analysis. A third group of birds (group C; n = 18: SIL = 6, TUT = 6, NOV = 6) was treated identically to the birds in the second group (i.e. exposed to auditory stimuli at 11.00 h with lights on) with the exception that they were not sacrificed 30 min after the end of the last stimulus presentation. This third group of birds was kept with the lights on until 21.00 h, after which the lights were turned off and the birds were allowed to sleep for 3 h. These birds were sacrificed at 00.00 h (midnight), 12 h after the end of the last stimulus presentation. Owing to technical failure with sectioning, data for the NCM of one animal in group C could not be collected.
The stimulus songs were broadcast through a speaker (Vifa MG10SD, Rødovre, Denmark) with a flat frequency–response curve between 0.1 and 15 kHz. A Hypex PC amplifier (Hypex Electronics BV, Groningen, The Netherlands) and Windows Media Player controlled the sound pressure level at 65 dB mean SPL at 30 cm from the speaker. Video and sound recordings were made throughout the experiment (see the electronic supplementary material for details on monitoring behaviour) to ensure that birds were awake during stimulus presentation and sleeping at night-time, and to monitor singing behaviour during the day. During stimulus exposure, all birds were awake.
(c). Immunocytochemistry
At 12.30 h (30 min after the end of exposure to the stimulus set) or 00.00 h (12 h after the end of exposure to the stimulus set), the experimental subjects were anaesthetized with 0.06 ml Natriumpentobarbital (intramuscular; Nembutal, Ceva Sante Animale, Libourne, France) and subsequently perfused with phosphate-buffered saline (PBS), followed by fixation with 4 per cent paraformaldehyde in PBS (see the electronic supplementary material for details on tissue collection). Immunocytochemistry for Zenk was done in three runs, including parasaggital sections of the left hemisphere of all animals in one experiment (main experiment, lights-on experiment, night experiment). The protocol used for immunocytochemistry is described in a previous report (Gobes et al. 2009).
(d). Image analysis
Quantification of Zenk-immunopositive cells was performed for NCM and CMM on 150 × 200 µm images of three sections at the medial position (between 260 and 440 µm from the midline; see the electronic supplementary material for representative photomicrographs) and three sections at the lateral position (between 740 and 920 µm from the midline) at regular intervals (every second section for medial and every fourth section for lateral). For the NCM, a counting frame was placed at the extreme caudal pole of the nidopallium (Terpstra et al. 2004). For the CMM, the frame was placed adjacent to the ventricle and the lamina mesopallialis. Distance from the midline was assessed by calculating the number of serial sections and this location was verified using the atlas of Vates et al. (1996), an unpublished atlas of the zebra finch brain by A. M. den Boer-Visser (which was also used in previous studies; Terpstra et al. 2004, 2006) and a stereotaxic atlas that is available online (Nixdorf-Bergweiler & Bischof 2007). For three sections containing the HVC, as well as for the RA, the photomicrographs were taken in the centre of the nucleus. Digital photographs were taken using a Leica DFC 4206 camera and the Leica Application Suite program on an Axioskop (Zeiss, Germany) with 20× objective. Image analysis was carried out with a PC-based system equipped with the KS400 v. 3.0 software (Carl Zeiss Vision, Oberkochen, Germany). A program was developed in KS400 to quantify immunoreactive cells semi-automatically, which is described in detail in a previous report (Gobes et al. 2009). Counts of three sections per region per animal were averaged for further statistical analysis. Image analysis was performed ‘blind’ as to the experimental history of the subject.
(e). Behavioural analyses
Sound recordings were analysed to quantify the amount of singing for each bird, while the fidelity of tutor song imitation was determined using Sound Analysis Pro (Tchernichovski & Mitra 2004; see the electronic supplementary material for additional information on sound analysis and similarity measurements). For the birds that were sacrificed at midnight, we analysed video data to quantify the time a bird had been in deep sleep and the time a bird was awake/in REM-sleep during the 3 h prior to sacrifice (see the electronic supplementary material for additional details of the behavioural measures of sleep).
(f). Statistical analysis
All data were first normalized to the mean levels of Zenk expression in the appropriate silent control group because the sampled regions had different basal expression levels and we were interested in Zenk induction (‘fold-change’) resulting from the exposure. We conducted repeated-measures analysis of variance (ANOVA) to examine the effects of playback stimulus on the Zenk response in the different brain regions. When appropriate, brain regions were analysed separately using one-way ANOVAs with Bonferroni-corrected post hoc tests. Data were analysed using SPSS 15.0.0.
3. Results
(a). Song learning
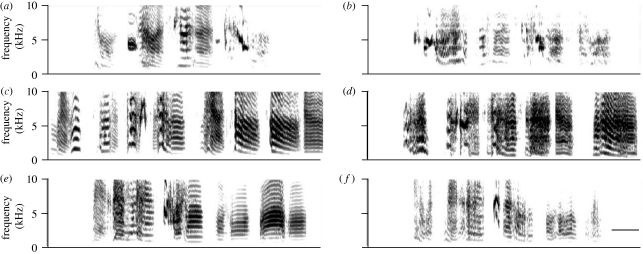
The juvenile males had already copied parts of the song of their father, but their songs were still different from those of adults. That is, the songs of the juveniles recorded in the morning prior to the stimulus exposure shared significantly more characteristics with the song of the father than with the songs of novel, unrelated males (similarity: 58.5 ± 4.1% (s.e.m.) with the tutor and 44.9 ± 2.8% with unrelated males; t11 = 2.5, p < 0.05), indicating that the juveniles had memorized (part of) the song from their father before they were separated (see figure 1 for representative examples from each group). There were no significant differences in similarity scores between the three groups (A, B, C: F2,45 = 0.68, p > 0.05) nor between birds exposed to different stimuli (TUT, NOV, SIL: F2,45 = 0.15, p > 0.05). In addition, 1 h exposure to the song stimuli did not significantly alter similarity scores (SIL: t9 = 0.1, p > 0.05; TUT: t10 = 1.2, p > 0.05; NOV: t8 = 1.4, p > 0.05), indicating that 1 h of exposure on the experimental day did not affect song learning. In comparison, the mean similarity of songs of adult zebra finch males (90 dph, n = 39) in our laboratory (separated from their father after the memorization phase at 67 dph) had a mean similarity score with the tutor song of 67.3 ± 3.1%. There was a significant difference between the similarity scores of the juveniles and adult males in our colony (n = 44 juveniles raised with their biological father, separated at 47 dph and similarity score determined between 54 and 59 dph [similarity: 55.5 ± 2.4%] and n = 39 adults; t81 = 3.02, p < 0.01). In addition, the juveniles in the main experiment had not copied the songs of their tutors with similar accuracy as the adult birds in our laboratory (n = 12 juveniles and n = 39 adults; t49 = 3.84, p < 0.0001). Our findings indicate that the juveniles in this study were still in the process of learning songs when compared with adults.
Figure 1.
Juveniles have learned song characteristics from their father. Spectrograms of song motifs of (a,c,e) some fathers (tutors) and (b,d,f) their respective sons. A representative example is shown of one bird in each group. Scale bar, 100 ms.
(b). A neural dissociation in song-induced molecular neuronal activation
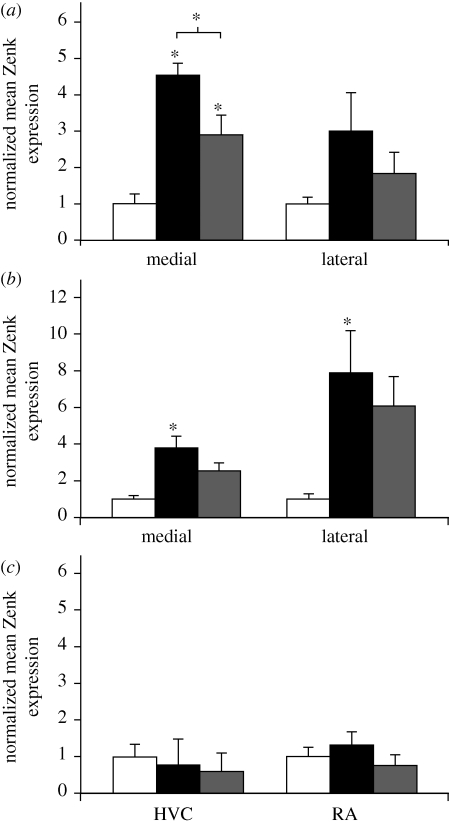
Visual inspection of the sections showed that there was song-induced expression of Zenk (the protein product of the IEG ZENK) in the two secondary auditory regions (NCM and CMM) but not in the two song system nuclei HVC and RA (robust nucleus of the arcopallium) of juveniles that did not vocalize themselves (figure 2; data of group A combined with five birds from group B that had not sung during and after stimulus exposure). This impression was confirmed by a repeated-measures ANOVA with two functional divisions of the sampled forebrain regions as a main factor: the secondary auditory regions (NCM and CMM) and the song system nuclei (HVC and RA). That is, there was a significant interaction between the factors Functional division and Stimulus (F2,18 = 7.64, p < 0.01, n = 21). Subsequent ANOVAs revealed a significant effect of Stimulus (F2,18 = 10.05, p < 0.01, n = 21) in the secondary auditory regions, but not in the song system (F2,18 = 0.46, p > 0.05, n = 21).
Figure 2.
Zenk expression in the NCM and CMM is induced by tutor song. Mean Zenk expression normalized to control levels (±s.e.m.) in (a) the NCM, (b) the CMM and (c) the song system for groups of juvenile male zebra finches exposed to tutor song (black bars, n = 7), novel song (grey bars, n = 8) or silence (white bars, n = 6). The number of Zenk-positive nuclei per square millimetre for each subject was divided by the mean number of Zenk-positive nuclei per square millimetre of the silent control group. Significant effects of exposure to auditory stimuli (compared with silence), as well as significant differences between song-exposed groups, are indicated with an asterisk.
(c). Localized learning-related molecular neuronal activation in the secondary auditory regions
On the basis of previous findings (Terpstra et al. 2004), we sampled Zenk expression in the secondary auditory regions NCM and CMM at two levels: medial and lateral. A repeated-measures ANOVA with the factors Region (CMM and NCM), Medial-lateral (as within-region levels) and Stimulus revealed significant main effects of Region (F1,18 = 4.84, p < 0.05, n = 21), medial-lateral (F1,18 = 4.93, p < 0.05, n = 21) and Stimulus (F2,18 = 10.05, p < 0.01, n = 21). There was a significant interaction between the factors Region and medial-lateral (F1,18 = 8.51, p < 0.01, n = 21). We continued with separate analyses of the different sub-regions.
A one-way ANOVA for the medial NCM revealed a significant effect of Stimulus (F2,20 = 15.59, p < 0.001, n = 21). Post hoc tests showed that there was significantly greater Zenk expression in the NCM of juveniles that had been exposed to tutor song (p < 0.0001) and novel song (p = 0.019) when compared with the silent control group (figure 2a). The difference in Zenk expression in the medial NCM between juveniles that were exposed to tutor or novel song was also significant (p = 0.037) and apparent in the fold-change in Zenk expression, which was 57 per cent greater in the TUT group (fold-change of 4.53 compared with silence) than in the NOV group (fold-change of 2.89 compared with silence). There were no such effects in the lateral NCM (F2,20 = 1.79, p > 0.05, n = 21).
In the CMM, one-way ANOVAs revealed a significant effect of Stimulus (medial CMM: F2,20 = 7.63, p < 0.01, lateral CMM: F2,20 = 3.991, p < 0.05; n = 21). Post hoc tests revealed significant differences between the tutor group and the silence group (medial: p = 0.003; lateral: p = 0.04; see figure 2b). There were no significant differences between the tutor and the novel group (medial: p = 0.199; lateral: p = 0.260), nor the novel group and the silence group.
(d). Singing induced molecular neuronal activation in the song system in juveniles
As shown previously, the act of singing drives Zenk expression in HVC and RA of the developing songbird brain (Jin & Clayton 1997; Whitney et al. 2000) as it does in adults (Jarvis & Nottebohm 1997). The second group of juvenile males (group B; see §2) was exposed to tutor song, novel song or silence. Birds in the main experiment (group A) were kept in darkness to prevent them from singing. In contrast, birds in group B were kept in light. Out of 20 birds that were kept with the lights on, 15 sang during the last 2 h prior to sacrifice. The birds that had not sung themselves showed the same pattern of Zenk expression as the birds in the main experimental group (group A), which were kept in darkness. For this reason, IEG data from these five birds (two NOV, two TUT, one SIL) in group B were combined with the data from group A. None of these five birds showed up as an outlier when compared with the normalized data of group A. For the birds that did sing, the total amount of singing did not differ between stimulus groups (F2,12 = 0.25, p > 0.05, n = 15) and was thus independent of the stimulus to which they had been exposed.
There was no significant effect of Stimulus (tutor song, novel song or silence) on Zenk expression (F2,12 = 0.35, p > 0.05, n = 15) in the song system nuclei HVC and RA of birds that had been singing themselves. Zenk expression in RA, and not in HVC, was significantly greater in these subjects than in the birds that had not been singing (t34 = 3.16, p < 0.001, n = 21 for silent and n = 15 for singing), suggesting that the expression of Zenk in RA is induced by the act of singing, and not by hearing song. There was no significant correlation between the amount of singing in the 2 h prior to sacrifice and Zenk expression in RA and HVC, when looking across all animals or within stimulus groups (p > 0.05 for all groups and regions). Visual inspection of the expression of Zenk in the secondary auditory regions showed that there was high expression in all singing birds, independent of exposure to auditory stimuli. ANOVA confirmed that there were no effects of Stimulus in the secondary auditory regions (NCM and CMM) of birds that had been singing themselves (p > 0.05 in all regions). In addition, there was no significant correlation between the amount of singing and Zenk expression in the auditory regions in any of the groups (p > 0.05 for all groups and regions).
(e). Learning-related molecular neuronal activation during sleep
Because it has been suggested that sleep is important for learning (Dave & Margoliash 2000; Deregnaucourt et al. 2005; Stickgold & Walker 2005; Gobes & Bolhuis 2008; Jackson et al. 2008; Shank & Margoliash 2009), the third group of birds (group C) were exposed to their tutor song, novel song or no song during the day and sacrificed 12 h later, at midnight, after they had been allowed to sleep for 3 h. Behavioural methods were used to quantify sleep (see §2e). The birds had spent 152 min 3 s ± 3 min 41 s (s.e.m.) in sleep posture in the 3 h before they were sacrificed. In the third hour, 51 min 52 s ± 1 min 50 s were spent in sleep posture. There were no significant differences between the three groups in total time sleeping, time sleeping in any of the 3 h prior to sacrifice, nor the number of transitions between sleep and REM/awake (ANOVA, n = 18, p > 0.05 for all variables). As described in §3a, there were no differences between the similarity scores of birds exposed to TUT, NOV or SIL, nor did the similarity score change directly after 1 h of stimulus exposure, indicating that tutor song copying had taken place before the start of the experiment.
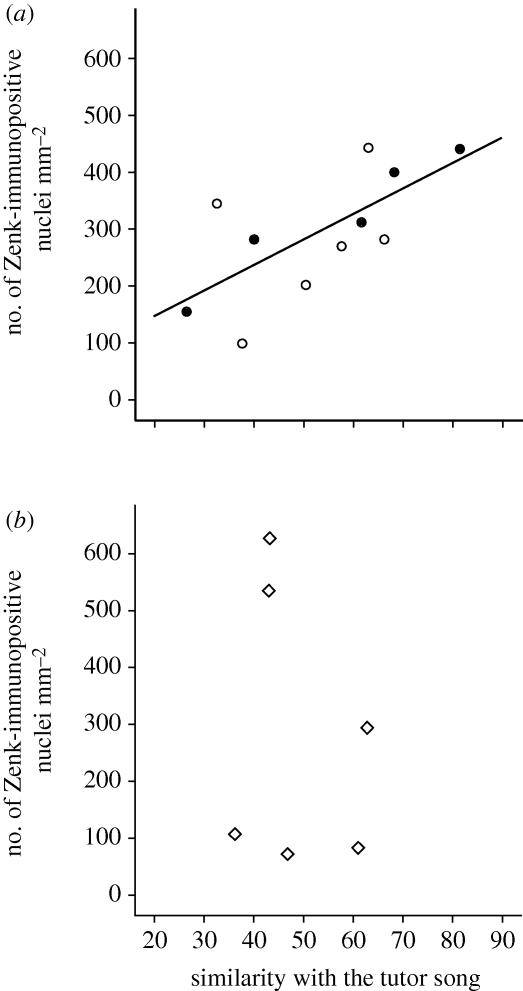
There were no significant effects of Stimulus in any of the auditory regions or song system nuclei (ANOVA, p > 0.05, n = 17 for all regions). We found a significant correlation between the similarity scores (with the tutor song) of the songs sung in the preceding afternoon and spontaneous Zenk expression in the lateral part of the NCM in birds that were not exposed to song in the stimulus session (r = 0.962, p = 0.009, n = 5). This correlation was also significant in all of the birds that had not been exposed to tutor song (i.e. birds exposed to novel song and birds kept in silence) during the previous day (r = 0.689, p = 0.019, n = 11; see figure 3a). The correlation was neither significant for birds that had been exposed to their tutor's song (r = −0.233, n.s., n = 6; figure 3b) nor was the correlation significant in either of the two groups exposed to song or silence and sacrificed during the day (p > 0.05 for all groups and regions); it should be added that in the group that was exposed to tutor song in darkness there were only four subjects with known similarity scores.
Figure 3.
Zenk expression during sleep is related to the strength of song learning. Scatter plots of the number of Zenk-immunoreactive nuclei per square millimetre in the lateral NCM in relation to the similarity with the tutor song (‘song similarity score’ measured with Sound Analysis Pro) for groups of birds exposed to (a) novel song (open circles) or no song (filled circles), or to (b) tutor song. The trend line in (a) is for the correlation of the whole sample.
To test whether the significant correlations between similarity score and Zenk expression (figure 3a,b) were because of differences in singing behaviour during the previous day between the group exposed to tutor song and the novel and silence groups, we investigated whether singing behaviour affected Zenk expression the following night. We measured the total amount of singing during the previous day, the number of songs sung during the hour in which the birds were exposed to the auditory stimuli or silence, the number of songs sung during the last hour in which the bird had sung (i.e. the number of songs sung in the hour preceding the bird's last song of the day independent of when the lights were turned off) and the total time (number of hours) in which the bird had been quiet before the lights went off. None of these variables differed significantly between the experimental groups (ANOVA, n = 18, p > 0.05 for all variables); nor did any of these variables correlate with the expression of Zenk in the auditory regions for any of the groups (p > 0.05 for all groups and regions). In the group of birds that had been exposed to tutor song during the day, we found a significant correlation between Zenk expression in the nuclei of the posterior pathway of the song system during sleep and the number of songs in the last hour that the bird sang (HVC: r = 0.937, p = 0.006, n = 6; RA: r = 0.822, p = 0.045, n = 6), which was independent of how long the birds had been quiet before the lights were turned off (p > 0.05 for both regions). There were no such correlations in the other two experimental groups.
4. Discussion
These results show that in juvenile zebra finches that were in the process of learning their songs, there was song-induced molecular neuronal activation in the auditory forebrain (cf. Jin & Clayton 1997; Stripling et al. 2001; Bailey & Wade 2005) that, in the medial NCM, was greatest in response to tutor song. During sleep, spontaneous molecular neuronal activation in the NCM was positively correlated with the strength of song learning.
Jin & Clayton (1997) reported that basal expression of ZENK in the NCM of male zebra finches early in the memorization phase (20–30 dph) was greater than in adults (Jin & Clayton 1997; Stripling et al. 2001). At 30 and 45 dph, which is when the memorization phase and sensorimotor learning phase in the zebra finch overlap, there was significantly greater Zenk expression after exposure to conspecific song than in silence (Jin & Clayton 1997; Stripling et al. 2001; Bailey & Wade 2005). Here, we show that later in the sensorimotor learning phase, this response is greatest for the memorized tutor song. The tutor song elicited a greater mean neuronal response than an unfamiliar song in birds that were in the sensorimotor learning phase, which is indicative of recognition of the memorized tutor song. Such a mean increase was not found in a previous study of adult birds (Terpstra et al. 2004). In adults, there was a correlation between the strength of song learning and molecular neuronal activation after exposure to the tutor's song, while there were no differences in the levels of molecular neuronal activation between groups exposed to tutor and novel song. This difference between adults and juveniles might be the result of ongoing memorization of tutor song in juveniles or higher salience for tutor song in the sensorimotor learning period.
London & Clayton (2008) have shown that song learning was impaired when an inhibitor of activation of the extracellular signal-regulated kinase (ERK)—an enzyme that regulates the transcription of ZENK (Velho et al. 2005), as well as other genes—was infused into the NCM of juveniles in the sensorimotor learning period (between 40 and 50 dph) when they were first exposed to tutor song (Velho et al. 2005; London & Clayton 2008). This work, taken together with the results of the present study, shows that in juvenile zebra finches, neurons in the NCM are responsive to song, and that this molecular neuronal activation is important for song learning. In addition, the present study shows that in males that were not exposed to tutor song, during subsequent sleep at night there was spontaneous molecular neuronal activation in the NCM, proportional to the strength of song learning. These findings suggest that in juvenile songbirds that are in the process of learning to sing a song, the NCM is (part of) the neural substrate for the representation of tutor song, which is activated when the bird is exposed to that song. In addition, the results suggest that sleep may play a role in song learning in juveniles.
In the CMM, there was also an increase in mean levels of molecular neuronal activation in juveniles that were exposed to tutor song, when compared with silence, while novel song elicited an intermediate response. In adult male zebra finches, there was no such difference (Terpstra et al. 2004), while in female zebra finches, which do not sing themselves, mean levels of molecular neuronal activation were greater for the father's song than for a novel song (Terpstra et al. 2006). The present results indicate that the CMM may also be important while juveniles are learning their song. The CMM may be the brain region from which auditory information related to song learning is relayed to the nuclei of the song system (Bauer et al. 2008).
There was no increased molecular neuronal activation in the song system nuclei HVC and RA when the young males were exposed to song, including tutor song, but there was increased molecular neuronal activation in RA when the birds were singing (cf. Jin & Clayton 1997; Whitney et al. 2000). In contrast to previous reports (Jin & Clayton 1997; Whitney et al. 2000), we did not find a significant correlation between the amount of singing in the 2 h prior to sacrifice and Zenk expression in RA and HVC. The lack of a correlation might be the result of the relatively long time period during which birds were allowed to sing, leading to a saturation of the Zenk response (Jarvis & Nottebohm 1997). The lack of molecular neuronal activation in response to the tutor song does not exclude the possibility that the nuclei in the posterior pathway of the song system are (part of) the neural substrate for tutor song memory (Bolhuis & Gahr 2006). However, the present results, combined with a substantial body of previous work (for reviews see Bolhuis & Gahr 2006; Hahnloser & Kotowicz in press), are consistent with the suggestion that there is a neural dissociation between the cognitive systems of vocal production and auditory memory in both juveniles and adults.
Bolhuis & Gahr (2006) suggested that during song learning there is continual interaction between regions in the auditory forebrain and the song system. Electrophysiological analyses have shown that during the first half of the sensorimotor phase (35–69 dph), neurons in the HVC of male zebra finches respond preferentially to the tutor song, whereas during the second half of the sensorimotor phase, and in adult zebra finches and white-crowned sparrows, there is preferential responding to the BOS (Volman 1993; Nick & Konishi 2005). Recently, it was found that lesions to the HVC in zebra finch males in the early sensorimotor phase (33–44 dph) did not impair the production of subsong (Aronov et al. 2008). Lesions in HVC late in the sensorimotor learning phase (45–73 dph) did affect the production of so-called ‘plastic song’ (Aronov et al. 2008). These findings suggest that the HVC is important for song production in the plastic song phase and that during this phase neurons in HVC acquire their preferential responsiveness to the BOS.
At this stage, we cannot rule out the possibility that the NCM of juvenile males contains the neural substrate for the representation of BOS, rather than, or in addition to, the memory of the tutor song. Previous studies have shown that in adult zebra finch males, it is unlikely that the NCM contains a neural representation of BOS. Exposure of adult males to BOS does not lead to greater molecular neuronal activation than when they are exposed to tutor song or to novel song, and molecular neuronal activation (in the awake state) correlated with song similarity only when the birds were exposed to tutor song, not when they were exposed to BOS (Terpstra et al. 2004). In addition, lesions to the NCM of adult zebra finch males impaired recognition of the tutor song, but did not affect production of the BOS (Gobes & Bolhuis 2007), rendering it unlikely that the NCM contains the neural substrate for BOS, at least in adults (Gobes & Bolhuis 2007).
The present findings suggest an effect of sleep on molecular neuronal activation related to song learning. The correlation between molecular neuronal activation and the strength of song learning was found in the same brain region (lateral NCM) as in adult zebra finch males (Bolhuis et al. 2000, 2001; Terpstra et al. 2004). However, in adult males, learning-related molecular neuronal activation in awake animals was found in response to exposure to the tutor song. In contrast, in the juveniles in the present study, molecular neuronal activation during sleep was spontaneous and related to how much was learned from the song tutor before the experiment. There was no learning-related molecular neuronal activation during sleep after the juveniles had been exposed to tutor song the previous day. Such exposure may have caused neuronal habituation to the tutor song, similar to habituation found in the NCM of adult zebra finches (Mello et al. 1995; Phan et al. 2006). In the NCM of adult zebra finch males, there was a rapid decline in IEG responsiveness with repeated exposure to a song, while there was dishabituation with exposure to a novel song (Mello et al. 1995). The decline in responsiveness persisted for a day (Mello et al. 1995). There may have been similar habituation to the familiar tutor song in the juveniles in the present study, causing the absence of spontaneous molecular neuronal activation in the birds that had been exposed to tutor song. In addition, molecular neuronal activation during sleep in the song system nuclei correlated significantly with the number of song bouts that were produced in the last hour in which the birds had sung only in the group that had been exposed to tutor song during the day. These results are reminiscent of the spontaneous ‘replay’ (patterns of spiking) of neuronal activity in the song system nucleus RA during sleep (Dave & Margoliash 2000). In adult zebra finches, replay in RA resembles pre-motor activity in RA during daytime singing, and is driven by neuronal bursting activity in HVC and the nuclei that project to HVC (Dave & Margoliash 2000; Hahnloser et al. 2006; Hahnloser & Fee 2007). A detailed behavioural analysis of the development of song in juvenile zebra finch males revealed that sensorimotor learning benefits from post-sleep variability in song output (Deregnaucourt et al. 2005). That is, there was a positive correlation between the magnitude of post-sleep deterioration of song (measured as the difference between acoustically stable evening songs and variable morning songs) and the strength of subsequent song learning (Deregnaucourt et al. 2005). The present findings give further support to the suggestion that information processing related to song learning may occur during sleep. Similarly, it was found that tutor-song-specific neuronal bursting activity in RA of sleeping juvenile zebra finches preceded the changes in singing observed the next day and was dependent on normal sensorimotor feedback (Shank & Margoliash 2009). Alternatively, the correlation between Zenk expression and strength of song learning in the NCM might be indicative of changes in general patterns of nocturnal brain activity related to the developmental process, such as that shown in the song system (Crandall et al. 2007; Shank & Margoliash 2009). Taken together, these different findings suggest that sleep is important for sensorimotor learning of the BOS and auditory memory of the tutor song. As such, they reinforce the hypothesis that sleep is crucial for learning and memory in birds (Gobes & Bolhuis 2008; Jackson et al. 2008; Shank & Margoliash 2009), as well as mammals (Stickgold & Walker 2005), including human infants (Gomez et al. 2006; Hupbach et al. 2009).
Acknowledgements
We thank Marita Dijkshoorn, Mirjam van Loon and Hanneke Poot for assistance with data collection, and Sidarta Ribeiro for stimulating discussions. This research was partly supported by a Rubicon fellowship from the Netherlands Organisation for Scientific Research (NWO) to S.M.H.G.
References
- Aronov D., Andalman A. S., Fee M. S.2008A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science 320, 630–634 (doi:10.1126/science.1155140) [DOI] [PubMed] [Google Scholar]
- Bailey D. J., Wade J.2005FOS and ZENK responses in 45-day-old zebra finches vary with auditory stimulus and brain region, but not sex. Behav. Brain Res. 162, 108–115 (doi:10.1016/j.bbr.2005.03.016) [DOI] [PubMed] [Google Scholar]
- Bauer E. E., Coleman M. J., Roberts T. F., Roy A., Prather J. F., Mooney R.2008A synaptic basis for auditory-vocal integration in the songbird. J. Neurosci. 28, 1509–1522 (doi:10.1523/JNEUROSCI.3838-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis J. J., Gahr M.2006Neural mechanisms of birdsong memory. Nat. Rev. Neurosci. 7, 347–357 (doi:10.1038/nrn1904) [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Wynne C. D. L.2009Can evolution explain how minds work? Nature 458, 832–833 (doi:10.1038/458832a) [DOI] [PubMed] [Google Scholar]
- Bolhuis J. J., Zijlstra G. G. O., den Boer-Visser A. M., Van der Zee E. A.2000Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc. Natl Acad. Sci. USA 97, 2282–2285 (doi:10.1073/pnas.030539097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis J. J., Hetebrij E., Den Boer-Visser A. M., De Groot J. H., Zijlstra G. G. O.2001Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches. Eur. J. Neurosci. 13, 2165–2170 (doi:10.1046/j.0953-816x.2001.01588.x) [DOI] [PubMed] [Google Scholar]
- Bottjer S., Miesner E., Arnold A.1984Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science 224, 901–903 (doi:10.1126/science.6719123) [DOI] [PubMed] [Google Scholar]
- Crandall S. R., Adam M., Kinnischtzke A. K., Nick T. A.2007HVC neural sleep activity increases with development and parallels nightly changes in song behavior. J. Neurophysiol. 98, 232–240 (doi:10.1152/jn.00128.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave A. S., Margoliash D.2000Song replay during sleep and computational rules for sensorimotor vocal learning. Science 290, 812–816 (doi:10.1126/science.290.5492.812) [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S., Mitra P. P., Feher O., Pytte C., Tchernichovski O.2005How sleep affects the developmental learning of bird song. Nature 433, 710–716 (doi:10.1038/nature03275) [DOI] [PubMed] [Google Scholar]
- Doupe A. J., Kuhl P. K.1999Birdsong and human speech: common themes and mechanisms. Ann. Rev. Neurosci. 22, 567–631 (doi:10.1146/annurev.neuro.22.1.567) [DOI] [PubMed] [Google Scholar]
- Eales L. A.1985Song learning in zebra finches: some effects of song model availability on what is learnt and when. Anim. Behav. 33, 1293–1300 (doi:10.1016/S0003-3472(85)80189-5) [Google Scholar]
- Eales L. A.1989The influences of visual and vocal interaction on song learning in zebra finches. Anim. Behav. 37, 507–508 (doi:10.1016/0003-3472(89)90097-3) [Google Scholar]
- Gobes S. M. H., Bolhuis J. J.2007Birdsong memory: a neural dissociation between song recognition and production. Curr. Biol. 17, 789–793 (doi:10.1016/j.cub.2007.03.059) [DOI] [PubMed] [Google Scholar]
- Gobes S. M. H., Bolhuis J. J.2008Bird brains key to the functions of sleep. Science 322, 1789–1789 [DOI] [PubMed] [Google Scholar]
- Gobes S. M. H., Ter Haar S. M., Vignal C., Vergne A. L., Mathevon N., Bolhuis J. J.2009Differential responsiveness in brain and behavior to sexually dimorphic long calls in male and female zebra finches. J. Comp. Neurol. 516, 312–320 (doi:10.1002/cne.22113) [DOI] [PubMed] [Google Scholar]
- Gomez R. L., Bootzin R. R., Nadel L.2006Naps promote abstraction in language-learning infants. Psychol. Sci. 17, 670–674 (doi:10.1111/j.1467-9280.2006.01764.x) [DOI] [PubMed] [Google Scholar]
- Hahnloser R. H. R., Fee M. S.2007Sleep-related spike bursts in HVC are driven by the nucleus interface of the nidopallium. J. Neurophysiol. 97, 423–435 (doi:10.1152/jn.00547.2006) [DOI] [PubMed] [Google Scholar]
- Hahnloser R. H. R., Kotowicz A.In press Auditory representations and memory in birdsong learning. Curr. Opin. Neurobiol. (doi:10.1016/j.conb.2010.02.011) [DOI] [PubMed] [Google Scholar]
- Hahnloser R. H. R., Kozhevnikov A. A., Fee M. S.2006Sleep-related neural activity in a premotor and a basal-ganglia pathway of the songbird. J. Neurophysiol. 96, 794–812 (doi:10.1152/jn.01064.2005) [DOI] [PubMed] [Google Scholar]
- Hupbach A., Gomez R. L., Bootzin R. R., Nadel L.2009Nap-dependent learning in infants. Dev. Sci. 12, 1007–1012 (doi:10.1111/j.1467-7687.2009.00837.x) [DOI] [PubMed] [Google Scholar]
- Immelmann K.1969Song development in the zebra finch and other estrildid finches. In Bird vocalizations (ed. Hinde R. A.), pp. 61–77 London, UK and New York, NY: Cambridge University Press [Google Scholar]
- Jackson C., McCabe B. J., Nicol A. U., Grout A. S., Brown M. W., Horn G.2008Dynamics of a memory trace: effects of sleep on consolidation. Curr. Biol. 18, 393–400 (doi:10.1016/j.cub.2008.01.062) [DOI] [PubMed] [Google Scholar]
- Jarvis E. D., Nottebohm F.1997Motor-driven gene expression. Proc. Natl Acad. Sci. USA 94, 4097–4102 (doi:10.1073/pnas.94.8.4097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Clayton D. F.1997Localized changes in immediate-early gene regulation during sensory and motor learning in zebra finches. Neuron 19, 1049–1059 (doi:10.1016/S0896-6273(00)80396-7) [DOI] [PubMed] [Google Scholar]
- Johnson F., Soderstrom K., Whitney O.2002Quantifying song bout production during zebra finch sensory-motor learning suggests a sensitive period for vocal practice. Behav. Brain Res. 131, 57–65 (doi:10.1016/S0166-4328(01)00374-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M.1965The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z. Tierpsychol. 22, 770–783 [PubMed] [Google Scholar]
- London S. E., Clayton D. F.2008Functional identification of sensory mechanisms required for developmental song learning. Nat. Neurosci. 11, 579–586 (doi:10.1038/nn.2103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Clayton D. F.1994Song-induced Zenk gene-expression in auditory pathways of songbird brain and its relation to the song control-system. J. Neurosci. 14, 6652–6666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Jarvis E. D.2008Behavior-dependent expression of inducible genes in vocal learning birds. In Neuroscience of birdsong (eds Zeigler H. P., Marler P.), pp. 381–397 Cambridge, UK: Cambridge University Press [Google Scholar]
- Mello C. V., Vicario D. S., Clayton D. F.1992Song presentation induces gene-expression in the songbird forebrain. Proc. Natl Acad. Sci. USA 89, 6818–6822 (doi:10.1073/pnas.89.15.6818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. V., Nottebohm F., Clayton D. F.1995Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J. Neurosci. 15, 6919–6925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick T. A., Konishi M.2005Neural song preference during vocal learning in the zebra finch depends on age and state. J. Neurobiol. 62, 231–242 (doi:10.1002/neu.20087) [DOI] [PubMed] [Google Scholar]
- Nixdorf-Bergweiler B. E., Bischof J.2007A stereotaxic atlas of the brain of the zebra finch, Taeniopygia guttata–with special emphasis on telencephalic visual and song system nuclei in transverse and sagittal sections. Bethesda, MD: National Library of Medicine (US), NCBI [Google Scholar]
- Nottebohm F.1981A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science 214, 1368–1370 (doi:10.1126/science.7313697) [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M.1976Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 165, 457–486 (doi:10.1002/cne.901650405) [DOI] [PubMed] [Google Scholar]
- Phan M. L., Pytte C. L., Vicario D. S.2006Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc. Natl Acad. Sci. USA 103, 1088–1093 (doi:10.1073/pnas.0510136103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper A., Zann R.2006The onset of song learning and song tutor selection in fledgling zebra finches. Ethology 112, 458–470 (doi:10.1111/j.1439-0310.2005.01169.x) [Google Scholar]
- Scharff C., Nottebohm F.1991A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 11, 2896–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank S. S., Margoliash D.2009Sleep and sensorimotor integration during early vocal learning in a songbird. Nature 458, 73–74 (doi:10.1038/nature07615) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stickgold R., Walker M. P.2005Memory consolidation and reconsolidation: what is the role of sleep? Trends Neurosci. 28, 408–415 (doi:10.1016/j.tins.2005.06.004) [DOI] [PubMed] [Google Scholar]
- Stripling R., Kruse A. A., Clayton D. F.2001Development of song responses in the zebra finch caudomedial neostriatum: role of genomic and electrophysiological activities. J. Neurobiol. 48, 163–180 (doi:10.1002/neu.1049) [DOI] [PubMed] [Google Scholar]
- Tchernichovski O., Mitra P. P.2004Sound Analysis Pro (version 1.056). See http://ofer.sci.ccny.cuny.edu/sound_analysis_pro [Google Scholar]
- Terpstra N. J., Bolhuis J. J., den Boer-Visser A. M.2004An analysis of the neural representation of birdsong memory. J. Neurosci. 24, 4971–4977 (doi:10.1523/JNEUROSCI.0570-04.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra N. J., Bolhuis J. J., Riebel K., van der Burg J. M. M., den Boer-Visser A. M.2006Localized brain activation specific to auditory memory in a female songbird. J. Comp. Neurol. 494, 784–791 (doi:10.1002/cne.20831) [DOI] [PubMed] [Google Scholar]
- Vates G. E., Broome B. M., Mello C. V., Nottebohm F.1996Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata). J. Comp. Neurol. 366, 613–642 (doi:10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7) [DOI] [PubMed] [Google Scholar]
- Velho T. A. F., Pinaud R., Rodrigues P. V., Mello C. V.2005Co-induction of activity-dependent genes in songbirds. Eur. J. Neurosci. 22, 1667–1678 (doi:10.1111/j.1460-9568.2005.04369.x) [DOI] [PubMed] [Google Scholar]
- Volman S. F.1993Development of neural selectivity for birdsong during vocal learning. J. Neurosci. 13, 4737–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O., Soderstrom K., Johnson F.2000Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Mol. Brain Res. 80, 279–290 (doi:10.1016/S0169-328X(00)00178-9) [DOI] [PMC free article] [PubMed] [Google Scholar]