Abstract
Timing is essential, but circadian clocks, which play a crucial role in timekeeping, are almost unaddressed in evolutionary ecology. A key property of circadian clocks is their free-running period length (τ), i.e. the time taken for a full cycle under constant conditions. Under laboratory conditions, concordance of τ with the ambient light–dark cycle confers major fitness benefits, but little is known about period length and its implications in natural populations. We therefore studied natural variation of circadian traits in a songbird, the great tit (Parus major), by recording locomotor activity of 98 hand-raised, wild-derived individuals. We found, unexpectedly, that the free-running period of this diurnal species was significantly shorter than 24 h in constant dim light. We furthermore demonstrate, to our knowledge for the first time in a wild vertebrate, ample genetic variation and high heritability (h2 = 0.86 ± 0.24), implying that period length is potentially malleable by micro-evolutionary change. The observed, short period length may be a consequence of sexual selection, as offspring from extra-pair matings had significantly shorter free-running periods than their half-siblings from within-pair matings. These findings position circadian clocks in the ‘real world’ and underscore the value of using chronobiological approaches in evolutionary ecology. Evolutionary ecologists study variation and its fitness consequences, but often have difficulties relating behavioural variation to physiological mechanisms. The findings presented here open the possibility that properties of internal, circadian clocks affect performance in traits that are relevant to fitness and sexual selection.
Keywords: clock, circadian, heritability, sexual selection, extra-pair, Parus major
1. Introduction
The importance of accurate timing for living organisms is widely acknowledged (e.g. Cuthill & Houston 1997; Purves et al. 2001), but biological clocks are poorly understood in terms of evolutionary ecology (Kyriacou et al. 2008). Daily timing processes in many organisms, from bacterial photosynthesis to wake-up time in humans, are based on underlying, endogenous rhythms (Dunlap et al. 2004; Foster & Kreitzman 2005; Koukkari & Sothern 2006). This becomes evident if all temporal information is removed and organisms are exposed to constant light and temperature. Under such conditions, clocks continue ticking but drift progressively from the solar day, assuming an internal rhythm with a period length that differs slightly from 24 h (hence, circadian, from circa = about, and dies = day). Circadian rhythms help organisms anticipate upcoming environmental conditions and are essential for the integration of internal fluctuations in physiology (Daan & Aschoff 1982; Decoursey 2004; Koukkari & Sothern 2006; Brown et al. 2008; Martino et al. 2008; Turek 2008). In the remarkably successful study of circadian rhythms, free-running period length τ has been a focal trait (Kenagy 1980; Koukkari & Sothern 2006; Brown et al. 2008; Takahashi et al. 2008; von Schantz 2008). Period length varies within groups of study organisms, but also between species (Aschoff 1979; Daan & Beersma 2002). For example, τ tends to be longer than 24 h in diurnal animals and shorter than 24 h in nocturnal animals at low light levels (Aschoff 1979).
(a). Circadian period length and daily timing
Under normal daylight conditions, circadian rhythms synchronize to the daily change in light conditions. However, individuals differ markedly in how their circadian rhythm aligns itself with the light–dark (LD) cycles (the so-called ‘phase of entrainment’). Individuals can hence be classified into ‘chronotypes’, depending on whether they are active relatively early or late in the day (Duffy et al. 2001; Koukkari & Sothern 2006; Allebrandt & Roenneberg 2008). In many species, these individual differences in chronotype are related to free-running period length τ (Duffy et al. 2001; Allebrandt & Roenneberg 2008; Brown et al. 2008; von Schantz 2008). Under LD cycles, individuals with shorter τ tend to be active earlier in the day and to display more or longer lasting activity than those with longer τ (Aschoff & Wever 1966; Fleury et al. 2000; Brown et al. 2008). In humans, individuals with shorter τ tend to define themselves as earlier chronotypes than those with longer τ (Duffy et al. 2001; Brown et al. 2008). Corresponding information from wild organisms under natural conditions exists for species or populations, but not for individuals (Fleury et al. 2000; Kronfeld-Schor & Dayan 2003; Kyriacou et al. 2008). Thus, variation in τ is expected to have consequences for timing in free-living animals, but robust information is sorely missing.
(b). Fitness consequences of τ
Fitness implications of τ under natural conditions are not well known. Slight deviations of τ from 24 h could be adaptive because they may affect phase of entrainment and promote adjustment to seasonally changing daylength (Daan & Aschoff 1982; Fleury et al. 2000; Daan & Beersma 2002; Johnson et al. 2003). By contrast, large deviations of τ from the 24 h day appear to be harmful (Daan & Beersma 2002). If τ is genetically altered, or if organisms are exposed to artificial LD cycles that differ notably from 24 h, fitness and health decrease (Ouyang et al. 1998; Koukkari & Sothern 2006; Emerson et al. 2008; Martino et al. 2008). We therefore expect that in natural populations, τ will have a fitness peak near environmental rhyhmicity of 24 h (Daan & Beersma 2002). Slight deviations from 24 h could be favoured by selection for particular clock properties, e.g. for advantages of early chronotype or of easy adjustment to changing conditions (Johnson et al. 2003). If differences in τ are associated with a steep fitness peak (e.g. under stabilizing selection), variation in τ could be low. Heritable variation could then be depleted and evolutionary change constrained (i.e. genostasis; Bradshaw 1991; Price & Boag 1993). These ideas can be evaluated by studying τ in natural populations. Information on distribution of τ may provide insight into adaptiveness, while information on genetic contributions to variation, and ideally on fitness implications of differences in τ, is crucial for understanding micro-evolutionary processes (Price & Boag 1993; Roff 1997; Daan & Beersma 2002).
(c). Approach
We examined circadian traits in a songbird species, the great tit (Parus major), which is widely studied by evolutionary ecologists (Nussey et al. 2005; Charmantier et al. 2008; Van Oers et al. 2008; Visser 2008). We recorded free-running period length τ of birds that hatched in the wild from free-living parents and were raised in captivity to minimize environmental effects. Birds were studied under constant dim light (LL, ca 0.5 lux) to assess circadian period length in the absence of environmental rhythmicity (Dunlap et al. 2004; Foster & Kreitzman 2005; Koukkari & Sothern 2006). For exploratory analyses of possible implications of τ, we collected additional data on three aspects of the birds' biology that are associated with variation in clocks.
We addressed the relationship between τ and chronotype by brief sampling of activity patterns under LD cycles. Great tits and birds of several other species are thought to be under pressure during the breeding season to commence daily activities early in the morning when territorial and reproductive behaviours peak (Daan & Aschoff 1982; Dolan et al. 2007; Poesel et al. 2007; Murphy et al. 2008). Consequently, a short τ, which is associated with early chronotype, could be advantageous.
We also assessed paternity because extra-pair (EP) young are common in our study population (Van Oers et al. 2008). Information on paternity is important for calculation of heritability, and EP paternity itself may be related to timing. In several avian species, including blue tits (Cyanistes caeruleus), EP success was highest for males that initiated morning song the earliest (Daan & Aschoff 1982; Dolan et al. 2007; Poesel et al. 2007; Murphy et al. 2008).
Last, we related τ to reproductive timing by recording lay date in captivity. Circadian clocks contribute to seasonal timing by providing reference time for photoperiodic time measurement and possibly also by links to circannual rhythms (i.e. rhythms that underlie annual processes; Gwinner 1986; Sharp 2005; Bradshaw & Holzapfel 2007; Foster & Kreitzman 2009; Liedvogel et al. 2009). Our study population has been extensively monitored for seasonal timing under climate change. Despite high selection pressure for early breeding, the birds' reproductive schedules do not keep pace with an advancing food peak (Visser et al. 1998; Visser 2008). Limited flexibility of breeding schedules could arise from mechanistic constraints, including circadian contributions, which we hereby examine (Silverin et al. 1993; Majoy & Heideman 2000; Bradshaw & Holzapfel 2007; Liedvogel et al. 2009; Visser et al. in press).
(d). Objectives
Our primary goal was investigation of individual variation in period length. Based on data of 98 great tits from 20 broods, we first derived information on the distribution of τ within the population. We then used these phenotypic data and information on relatedness to address genotypic contributions to variation in τ. This is based on the rationale that resemblance between family members (e.g. parents and offspring, siblings) allows an estimate of the relative importance of genetic variation. Using data from genetically confirmed full siblings, we calculated broad-sense heritability (h2) to estimate the extent to which phenotypic variation is determined by genotypic variation (Conner & Hartl 2004). However, broad-sense h2 can be inflated by environmental variation (Roff 1997). We therefore used the differences in paternity to explore common-environment effects on τ. EP young share maternal origin and a common nesting environment until hand-raising with their within-pair (WP) half-siblings. If differences in τ between broods were largely owing to a common environment, there should be little difference between EP and WP young. Conversely, strong effects of paternity on τ would support high additive genetic variation and emphasize genotypic contributions of WP and EP fathers. A secondary goal of our study was an exploratory analysis of implications of period length. We therefore related, on an individual basis, findings on τ to those on chronotype and lay date in captivity.
2. Material and methods
(a). Birds and housing conditions prior to circadian experiments
Great tits were collected in spring of 2005 and 2006 in the Hoge Veluwe study population (52°05′ N, 05°50′ E) of the Netherlands Institute of Ecology (NIOO-KNAW) as entire broods at day 10 after hatching. Parents were caught prior to removing the brood and blood-sampled to obtain a DNA sample. The nestlings were transported to the NIOO-KNAW and hand-raised and housed as described elsewhere (Drent et al. 2003). Young birds were paired in December and were allowed to breed and moult in 2 × 2 × 2 m aviaries (see Visser et al. 2009 for a description). All seasonal activities were closely monitored, and for the 86 birds that initiated broods, exact lay dates are known. Temperatures were controlled and recorded every 10 min. Aviaries were illuminated by artificial light. From December until the beginning of circadian experiments, photoperiod was increased twice a week following the natural, local increase in day length (i.e. exposing birds to daily LD cycles from a minimum of 7.45 L : 16.15 D to a maximum of 16.30 L : 7.30 D at the winter and summer solstice, respectively). The main sources of light were two ‘True Light’ high-frequency fluorescent tubes, and additional natural light was provided using a tubular daylighting device (SolaTube) that was opened and closed when the True Light tubes were turned on and off, respectively. In combination, this provided about 700 lux at perch level. For half an hour before the True Light tubes were switched on, and half an hour after they were turned off, an 8 W light bulb mimicked dusk and dawn.
(b). Circadian experiments
Birds were examined for temporal behaviour in the breeding aviaries in their second autumn at the end of moult. In order to collect data on individual circadian clocks, birds had to be tested in isolation from all external time cues including those from conspecifics (Dunlap et al. 2004; Foster & Kreitzman 2005; Koukkari & Sothern 2006). Hence, birds were tested in batches during times when aviary space was available. In 2006, female activity was examined from 15 September, and males were subsequently examined from 13 October. In 2007, females were monitored from 28 August (see below for details). All birds were first briefly recorded under LD cycles. Light conditions were kept constant based on the amount of natural daylight on the day the experiment began. Accordingly, for the groups tested in 2006, day length was fixed at 12.7 h and for the group tested in 2007, at 14.4 h.
Subsequently, LD cycles were replaced by constant conditions to measure circadian rhythms. Lights were dimmed because constant bright light disrupts clocks (Dunlap et al. 2004; Foster & Kreitzman 2005; Koukkari & Sothern 2006). During exposure to constant LL, light was provided using a green light night lamp of 0.5 lux at perch level. Light intensity was measured at perch level using a lux light meter (Nieaf Instruments NI-L204) at least once per experiment. Food was constantly available and replenished at random times between 8.00 and 15.00 to avoid entrainment to the feeding regime. To avoid possible communication between family members, birds were placed randomly into neighbouring aviaries. Furthermore, testing by spatial autocorrelation methods indicated that social effects between neighbouring birds were absent. Movements were measured using radar-based detectors (Conrad Electronics) connected to a computer. If the bird moved within a 2 s interval, a movement was detected, and every 2 min the total number of 2 s intervals in which a movement was detected was stored (i.e. a number between 0 and 60). Activity counts in 2 min bins were smoothed by a 5 bin running average. Overall, data were available for 98 individuals tested in three sets. In 2006, female activity was recorded for 4 days under LD cycles and subsequently for 20.5 days under LL. Males were recorded for 3 days under LD cycles but owing to technical problems, for only 12.3 days under LL. To attain a longer series of measurements, we recorded females in 2007 for 32.4 days under LL following 4 days under LD cycles. In some cases, equipment failure created missing data. Estimates of τ were unaffected by missing values, but for phase estimates, short gaps (up to 8 bins) were filled by means of preceding and subsequent values. For a single male, phase could not be estimated owing to a larger gap in the data.
(c). Derivation of timing traits and statistical analyses
We derived τ by the Lomb–Scargle periodogram analysis (Ruf 1999), implemented in the software program Chronoshop (courtesy of Kamiel Spoelstra). Several individuals showed some degree of internal desynchronization under LL, but in all cases, a dominant τ exceeded the significance threshold (p = 0.05). We used the long recording of females in 2007 to examine whether the differences in series length affected estimates of τ. We investigated at which time after onset of LL period length stabilized, and whether the estimated τ depended on length of recording (courtesy of Julia Diegmann). Even when estimated from the first 3 days after exposure to LL, period length was significantly correlated with estimates that were based on longer time series. From day 6, estimates of τ were stable and accurate. Thus, τ could be estimated with confidence from short series. Estimates based on 7 versus 27 days, respectively, differed maximally by ±0.17 min. Therefore, we removed the first 5 days after exposure to LL and calculated τ over 15.0 days in females and over 7.3 days in males.
We also estimated chronotype under LD cycles as phase of entrainment, given by centre of gravity (COG; Kenagy 1980), which is a particularly robust measure. However, data on chronotype are preliminary because the interval from which we estimated chronotype was short, and because sensors also recorded minor movements. We used an edge detector (Helm & Gwinner 2005) to determine, individually for each bird, the time of greatest increase in activity in the morning (within 2 h before and after lights-on) and of greatest decrease in activity in the evening (within 4 h because of greater variation). This method identifies the time of core activity, yielding a relatively late calculated onset and early calculated termination of activity. Duration of activity was the interval between onset and termination. Phase was calculated as a bird's activity timing relative to the time of lights-on, mid-light time and lights-off, respectively. Thus, a positive estimate of phase under LD cycles indicates early (i.e. phase-advanced) chronotypes, and a negative estimate indicates late (i.e. phase-delayed) chronotypes. The overall level of activity of a bird was the mean number of hops per 2 min bin. Activity levels were calculated separately under LL and LD cycles, but the two measures were closely correlated (r = 0.64; p < 0.001; n = 97).
Timing traits were analysed by linear mixed models implementing restricted maximum-likelihood estimation methods. These have high flexibility and are suitable for unbalanced designs, as was the case for unequal clutch sizes in our study (Genstat 6.0. VSN International, Genstat 1993). We modelled effects of sex, brood, EP paternity, year of study and lay date as fixed factors and covariates to test for statistical significance. Significance was derived from Wald statistics which asymptotically follow a χ2-distribution. Since significance levels can depend on the order of entry of factors, we tested each factor by adding as well as removing it from the model, but found no discrepancies. Experiments in 2006 and 2007 differed in initial LD conditions, but because we found no effects of study year on τ we pooled the data. In broods with both EP and WP young, we tested for effects of EP paternity, brood and their interaction. Variance components and estimates of heritability (h2) were derived from a random model. We used data from all WP young to estimate broad-sense h2, reducing the model to the single remaining significant factor, family. We derived h2 from the variance component associated with family divided by the total phenotypic variation (Falconer & Mackay 1996) as twice the intra-class correlation coefficient, treating the 20 families as class (Sokal & Rohlf 1995), and computed standard errors by an approximate formula for unequal family sizes (Roff 1997). We then used data from broods with both WP and EP young to attain relative proportions of variance associated with a common environment (shared nest and same mother), and with paternal genotype. In addition, we conducted similar, exploratory analyses of chronotype. Data were characterized by descriptive methods (mean ± s.d.) and correlational techniques.
(d). Paternity analyses
Genomic DNA from 5 µl blood from both parents and the chicks was isolated using the PureGene DNA Isolation Kit (Gentra Systems, USA). Polymerase chain reactions (PCR) were carried out in a 10 µl volume using the Multiplex PCR kit (Qiagen, The Netherlands). We used a micro-satellite protocol with an annealing temperature at 60°C. Micro-satellite markers (for the 2006 birds: Pma CAn1, Pma GAn27, Pma GAn42, Pma GAn30 and Pma D22, and for the 2007 birds: Pma CAn1, Pma TAGAn86, Pma GAn27, Pma C25 and Pma D22) were selected as described by Saladin et al. (2003). Fluorescent PCR fragments were visualized by capillary electrophoresis on an ABI3130 Genetic Analyzer (Applied Biosystems) and the sizes of the PCR products were determined using GeneMapper 4.0 software (Applied Biosystems). Individuals were categorized as WP young if all loci or all but one locus matched those of the social father (none of the young had a single mismatch).
3. Results
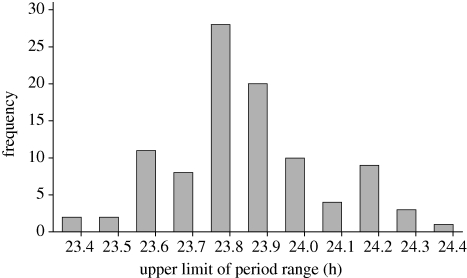
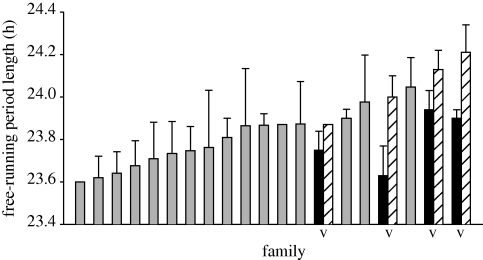
Circadian period length τ differed considerably between the 98 recorded great tits from 20 broods (figures 1 and 2). Overall, τ was significantly shorter than 24 h and its distribution was slightly left-skewed (figure 2; mean ± s.d.: 23.83 ± 0.21; range: 23.4–24.4 h; t97 = −8.33; p < 0.001; skewness: 0.42 ± 0.25; kurtosis: 0.07 ± 0.49). Birds with shorter τ had higher activity levels (under LL: r = −0.23; p = 0.025; under LD cycles: r = −0.17; p = 0.090). As detailed in table 1, τ did not differ between the sexes and was unrelated to the lay date of a bird, regardless of whether females only or all birds were analysed. The only factors that had significant effects on τ were brood of origin and paternity. Paternity analyses revealed that four of the 20 broods contained both EP young (EP; n = 11) and WP young (WP; n = 10). EP paternity occurred preferably in broods with slow clocks, as evident from longer τ of WP young with EP siblings compared with those without EP siblings (figure 3; EP broods: 24.12 ± 0.16; WP broods: 23.79 ± 0.19; t85 = 5.21; p < 0.001). EP young differed from their half-siblings by considerably shorter τ (figure 3; EP: 23.79 ± 0.14; WP: 24.12 ± 0.16; t21 = −5.21; p < 0.001), but were similar to WP young on a population level (EP: 23.79 ± 0.14; WP: 23.83 ± 0.21; t96 = −0.65; p = 0.517). Based on data from full genetic siblings (i.e. WP young only), τ had a high h2 of 0.86 ± 0.24. In clutches with both EP and WP young, brood, which combines maternal contribution and common environment, was associated with 23 per cent of variation, while EP paternity accounted for 59 per cent of overall phenotypic variation, although standard errors for these estimates were high (table 2).
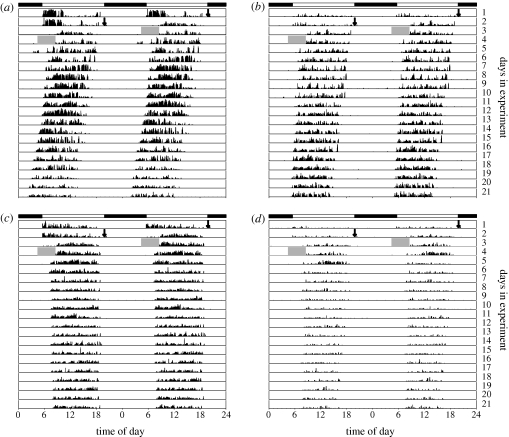
Figure 1.
Activity timing of great tits. Actograms illustrate the distribution of movements (as black marks) over time for four females (a–d) collected from a single brood in 2007. The graphs show the number of recorded movements during subsequent 2 min bins across the time of day (records of each day are duplicated for greater clarity). Each line represents a day in the experiment (column to the right: day number). Bar on top indicates initial LD cycles (white = light; black = darkness); black arrows indicate onset of LL; grey blocks show brief failure of sensor. (a,b) Extra-pair daughters; (c,d) within-pair daughters.
Figure 2.
Distribution of free-running period τ in 98 great tits. Bars give frequency of τ for intervals of 0.1 h.
Table 1.
Factors influencing period length τ. (Results from linear mixed-model analysis of τ of locomotor activity in wild-derived great tits (n = 98), all factors and covariates modelled as fixed effects; results on lay date are based on the subset of 86 laying birds.)
| variable | Wald statistic | p-value |
|---|---|---|
| brood | Wald19= 77.1 | <0.001 |
| extra-pair paternity | Wald1 = 11.6 | <0.001 |
| chronotype (COG) | Wald1 = 2.0 | 0.159 |
| sex | Wald1 = 1.7 | 0.197 |
| lay date | Wald1 = 0.1 | 0.772 |
Figure 3.
Free-running period of great tit broods. Bars show family means with s.e.m. ordered by τ of within-pair (WP) young. Broods with exclusively WP young are shown by grey bars. Broods with extra-pair (EP) young have two bars joined by a V: black bars, EP young; hatched bars, WP young.
Table 2.
Estimated variance components (±s.e.m.) from linear mixed-model analysis of τ in the four broods of great tits (n = 21) that contained both extra-pair and within-pair young.
| random term | variance component | proportion of total variance (%) |
|---|---|---|
| brood | 0.01394 ± 0.0134 | 22.9 |
| extra-pair paternity | 0.03571 ± 0.0523 | 58.7 |
| residual (individual) | 0.01120 ± 0.0040 | 18.4 |
| total phenotypic variance | 0.06085 | 100 |
From the data collected during brief exposure to simulated LD cycles, we derived preliminary estimates of chronotype. Activity was timed relatively early (figure 1). The COG, used as a proxy for chronotype, preceded midday by 52.5 ± 22.3 min. The morning increase in activity occurred on average 16.7 ± 11.2 min after lights-on, and the evening decrease 51.1 ± 22.3 min before lights-off. Early chronotypes had higher activity levels (r = −0.27; p = 0.007) and were active longer than late chronotypes (r = −0.38; p < 0.001). However, we found no relationship between chronotype and τ (r = −0.04; p = 0.701; table 1). When we subjected chronotype to full analysis of factors related to timing, we found no effects of brood (Wald18 = 18.2, p = 0.44), EP paternity (Wald1 = 0.02, p = 0.88) and lay date (Wald1 = 3.1, p = 0.08). We detected effects of two factors that were associated with differences in LD conditions, study year (Wald1 = 9.6, p = 0.002; earlier timing in 2007 than in 2006) and sex (Wald1 = 11.6, p < 0.001; earlier timing of male than female activity).
4. Discussion
Our data demonstrate that τ, a fundamental property of the circadian system, is variable and highly heritable in a natural bird population. High h2 of period length suggests that fast micro-evolutionary adjustments are conceivable (Bradshaw 1991; Price & Boag 1993). To our knowledge, evidence for genetic variation in τ is a novel finding for wild-derived vertebrates. In invertebrates, heritability was calculated from full-sibling analyses of τ in one population of nemobiine crickets, and from selective breeding in a second. Estimated h2 differed between 0.17 and 0.78, respectively, and τ and phase under LD cycles were uncorrelated (Shimizu & Masaki 1997). The findings of selectable variation in a clock trait converge with circumstantial evidence for adaptive adjustment of circadian systems, inferred from latitudinal clines in clock properties and gene variants (Daan & Aschoff 1982; Pittendrigh & Takamura 1989; Price & Boag 1993; Decoursey 2004; Johnsen et al. 2007; Allebrandt & Roenneberg 2008; Kyriacou et al. 2008). Such micro-evolutionary adjustments could counteract possible constraints on flexibility that are thought to be imposed by circadian clocks (Kronfeld-Schor & Dayan 2003).
In our study, the factors that mediate selection on τ are still unclear. With respect to seasonal timing, we found no relationship between τ and lay date in captivity, which in our great tit population is a good proxy for laying date of these females in the wild (Visser et al. 2009). Similarly, in studies of selection lines of rodents (Majoy & Heideman 2000), circadian differences were unrelated to individual differences in reproductive response to photoperiod. It has been argued that evolutionary adjustments of seasonal timing may occur downstream from the clock because circadian modifications are expected to have pleiotropic effects (Majoy & Heideman 2000; Bradshaw & Holzapfel 2007). By contrast, recent findings from a closely related songbird, the blue tit, do point to a possible link between clock gene variants and seasonal timing (Liedvogel et al. 2009).
With respect to daily behaviour, our study confirmed that birds with shorter τ had higher activity levels, but we did not detect the links between τ and chronotype shown in some other studies (Aschoff & Wever 1966; Fleury et al. 2000; Johnson et al. 2003; Brown et al. 2008). Lack of an association is probably a consequence of short sampling under LD cycles (figure 1), but could also be related to complex entrainment of multiple oscillators (Daan & Beersma 2002; Decoursey 2004; Koukkari & Sothern 2006; Beersma et al. 2008; Brown et al. 2008) and to the particular light simulation (Fleissner & Fleissner 2002). Our preliminary study of chronotype indicated differences between study years and sexes. Both factors were confounded with expected effects of the different initial LD cycles (Daan & Aschoff 1975). An alternative interpretation, possible sex differences in phase control, would merit closer investigation.
Our findings offer a surprising clue to possible selection on τ. Data from EP young suggest that differences in τ may be associated with traits that in free-living great tits are implicated in sexual selection. EP young were found in broods with slow clocks and differed from their WP half-siblings by a significantly shorter τ (figures 1 and 3). Assuming that period lengths of offspring partly reflect those of their fathers, the data suggest that females choose males with fast clocks for EP matings, in particular if their social mate has a slow clock. Sexual selection for heritable, fast clocks could thereby contribute to adaptive adjustments of circadian rhythms. Studies of several avian species have demonstrated territorial and reproductive benefits of early activity, including high EP success (Daan & Aschoff 1982; Dolan et al. 2007; Murphy et al. 2008). EP matings are thought to be constrained by spatial limitations, but temporal niches, like early-morning hours, may provide opportunities to increase reproductive success for socially monogamous birds such as great tits (Dolan et al. 2007; Murphy et al. 2008). Our data further suggest that EP success could be related to more or longer lasting activity, rather than only to early rising because fast clocks were associated with high activity. EP paternity and sexual selection could potentially explain the finding that the τ of our wild bird population is significantly shorter than 24 h (Dolan et al. 2007). Directional selection for short τ is also suggested by the near-significant left-skew in its distribution (Price & Boag 1993).
We conclude that circadian systems in the wild may be highly variable and malleable in response to both natural and sexual selection. In great tits, slight deviations of τ from 24 h could be rewarded by reproductive benefits, but benefits of clocks with longer period lengths are still unclear. Reproductive benefits of particular chronotypes could be widespread. Many organisms show pronounced circadian rhythms in sexual traits, for example, flowering in plants and sperm production and sex steroid levels in mammals, including humans (Smale et al. 2005; Koukkari & Sothern 2006). Timing of these traits relative to mating opportunities and environmental conditions could confer major fitness benefits to individuals with particular circadian traits. Our findings are an important step forward in relating circadian biology to evolutionary ecology, and thereby contribute to the timely quest for the role of clocks in the real world (Menaker 2006).
Acknowledgements
All experiments were conducted under licence of the Animal Experimental Committee of the KNAW (DEC protocol no CTE 05-01).
We thank Kamiel Spoelstra for providing software and support in the analysis of circadian rhythms, and Julia Diegmann for taking a close, expert look at entrainment. Kees Van Oers, Daniel Nussey, Domien Beersma, Michaela Hau, Paul Heideman, Charlotte Förster-Helfrich, Mike Menaker, Sonja Schaper and Karla Allebrandt all helped by discussion of ideas and commented on an earlier version of the manuscript. The manuscript also profitted from advice by referees and editors, and from careful language editing by Tim Greives. Leonard Holleman assisted with the experiments, Christa Mateman carried out the paternity analysis, Marylou Aaldering, Floor Petit and Janneke Venhorst took good care of the birds and Ab and Gilles Wijlhuizen provided technical support with the aviaries. M.E.V. is supported by a NWO-VICI grant.
References
- Allebrandt K. V., Roenneberg T.2008The search for circadian clock components in humans: new perspectives for association studies. Braz. J. Med. Biol. Res. 41, 716–721 [DOI] [PubMed] [Google Scholar]
- Aschoff J.1979Circadian rhythms: influences of internal and external factors on the period measured in constant conditions. Z. Tierpsychol. 49, 225–249 [DOI] [PubMed] [Google Scholar]
- Aschoff J., Wever R.1966Circadian period and phase-angle difference in chaffinches (Fringilla coelebs L.). Comp. Biochem. Physiol. 18, 397–404 [DOI] [PubMed] [Google Scholar]
- Beersma D. G. M., Van Bunnik B. A. D., Hut R. A., Daan S.2008Emergence of circadian and photoperiodic system level properties from interactions among pacemaker cells. J. Biol. Rhyth. 23, 362–373 (doi:10.1177/0748730408317992) [DOI] [PubMed] [Google Scholar]
- Bradshaw A. D.1991The Croonian Lecture, 1991: genostasis and the limits to evolution. Phil. Trans. R. Soc. Lond. B 333, 289–305 (doi:10.1098/rstb.1991.0079) [DOI] [PubMed] [Google Scholar]
- Bradshaw W. E., Holzapfel C. M.2007Evolution of animal photoperiodism. Annu. Rev. Ecol. Evol. Syst. 38, 1–25 (doi:10.1146/annurev.ecolsys.37.091305.110115) [Google Scholar]
- Brown S. A., Kunz D., Dumas A., Westermark P. O., Vanselow K., Tilmann-Wahnschaffe A., Herzel H., Kramer A.2008Molecular insights into human daily behavior. Proc. Natl Acad. Sci. USA 105, 1602–1607 (doi:10.1073/pnas.0707772105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantier A., McCleery R. H., Cole L. R., Perrins C., Kruuk L. E. B., Sheldon B. C.2008Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803 (doi:10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- Conner J. K., Hartl D. L.2004A primer of ecological genetics. Sunderland, MA: Sinauer [Google Scholar]
- Cuthill I. C., Houston A. I.1997Managing time and energy. In Behavioral ecology. An evolutionary approach (eds Krebs J. R., Davies N. B.), pp. 97–121 Oxford, UK: Blackwell Science [Google Scholar]
- Daan S., Aschoff J.1975Circadian rhythms of locomotor activity in captive birds and mammals: their variations with season and latitude. Oecologia 18, 269–316 (doi:10.1007/BF00345851) [DOI] [PubMed] [Google Scholar]
- Daan S., Aschoff J.1982Circadian contributions to survival. In Vertebrate circadian systems: structure and physiology (eds Aschoff J., Daan S., Groos G. A.), pp. 305–321 Berlin, Germany: Springer-Verlag [Google Scholar]
- Daan S., Beersma D. G. M.2002Circadian frequency and its variability. In Biological rhythms (ed. Kumar V.), pp. 24–37 New Delhi, India: Narosa [Google Scholar]
- Decoursey P.2004The behavioral ecology and evolution of biological timing systems. In Chronobiology: biological timekeeping (eds Dunlap J. C., Loros J. J., Decoursey P.), pp. 27–65 Sunderland, MA: Sinauer [Google Scholar]
- Dolan A. C., Murphy M. T., Redmond L. J., Sexton K., Duffield D.2007Extrapair paternity and the opportunity for sexual selection in a socially monogamous passerine. Behav. Ecol. 18, 985–993 (doi:10.1093/beheco/arm068) [Google Scholar]
- Drent P. J., Van Oers K., Van Noordwijk A. J.2003Realized heritability of personalities in the great tit (Parus major). Proc. R. Soc. Lond. B 270, 45–51 (doi:10.1098/rspb.2002.2168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy J. F., Rimmer D. W., Czeisler C. A.2001Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav. Neurosci. 115, 895–899 (doi:10.1037/0735-7044.115.4.895) [DOI] [PubMed] [Google Scholar]
- Dunlap J. C., Loros J. J., DeCoursey P. (eds) 2004Chronobiology: biological timekeeping. Sunderland, MA: Sinauer [Google Scholar]
- Emerson K. J., Bradshaw W. E., Holzapfel C. M.2008Concordance of the circadian clock with the environment is necessary to maximize fitness in natural populations. Evolution 62, 979–983 (doi:10.1111/j.1558-5646.2008.00324.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D. S., Mackay T. F. C.1996Introduction to quantitative genetics. London, UK: Longman [Google Scholar]
- Fleissner G., Fleissner G.2002Perception of natural zeitgeber signals. In Biological rhythms (ed. Kumar V.), pp. 83–93 New Delhi, India: Narosa [Google Scholar]
- Fleury F., Allemand R., Vavre F., Fouillet P., Bouletreau M.2000Adaptive significance of a circadian clock: temporal segregation of activities reduces intrinsic competitive inferiority in Drosophila parasitoids. Proc. R. Soc. Lond. B 267, 1005–1010 (doi:10.1098/rspb.2000.1103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster R. G., Kreitzman L.2005Rhythms of life: the biological clocks that control the daily lives of every living thing. New Haven, CT: Yale University Press [Google Scholar]
- Foster R. G., Kreitzman L.2009Seasons of life. London, UK: Profile Books [Google Scholar]
- Genstat 1993Genstat 5 release 3 reference manual. Oxford, UK: Clarendon Press [Google Scholar]
- Gwinner E.1986Circannual rhythms. Heidelberg, Germany: Springer [Google Scholar]
- Helm B., Gwinner E.2005Carry-over effects of day length during spring migration. J. Ornithol. 146, 348–354 (doi:10.1007/s10336-005-0009-5) [Google Scholar]
- Johnsen A., et al. 2007Avian clock gene polymorphism: evidence for a latitudinal cline in allele frequencies. Mol. Ecol. 16, 4867–4880 (doi:10.1111/j.1365-294X.2007.03552.x) [DOI] [PubMed] [Google Scholar]
- Johnson C. H., Elliott J. A., Foster R.2003Entrainment of circadian programs. Chronobiol. Int. 20, 741–774 [DOI] [PubMed] [Google Scholar]
- Kenagy G. J.1980Center-of-gravity of circadian activity and its relation to free-running period in two rodent species. J. Interdisciplinary Cycle Res. 11, 1–8 [Google Scholar]
- Koukkari W. L., Sothern B.2006Introducing biological rhythms. New York, NY: Springer [Google Scholar]
- Kronfeld-Schor N., Dayan T.2003Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 34, 151–181 [Google Scholar]
- Kyriacou C. P., Peixoto A. A., Sandrelli F., Costa R., Tauber E.2008Clines in clock genes: fine-tuning circadian rhythms to the environment. Trends Genet. 24, 124–132 (doi:10.1016/j.tig.2007.12.003) [DOI] [PubMed] [Google Scholar]
- Liedvogel M., Szulkin M., Knowles S., Wood M. J., Sheldon B. C.2009Phenotypic correlates of clock gene variation in a wild blue tit population: evidence for a role in seasonal timing of reproduction. Mol. Ecol. 18, 2444–2456 (doi:10.1111/j.1365-294X.2009.04204.x) [DOI] [PubMed] [Google Scholar]
- Majoy S. B., Heideman P. D.2000Tau differences between short-day responsive and short-day nonresponsive white-footed mice (Peromyscus leucopus) do not affect reproductive photoresponsiveness. J. Biol. Rhyth. 15, 501–513 (doi:10.1177/074873000129001611) [DOI] [PubMed] [Google Scholar]
- Martino T., et al. 2008Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, 1675–1683 [DOI] [PubMed] [Google Scholar]
- Menaker M.2006Circadian organization in the real world. Proc. Natl Acad. Sci. USA 103, 3015–3016 (doi:10.1073/pnas.0600360103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. T., Sexton K., Dolan A. C., Redmond L. J.2008Dawn song of the eastern kingbird: an honest signal of male quality? Anim. Behav. 75, 1075–1084 (doi:10.1016/j.anbehav.2007.08.020) [Google Scholar]
- Nussey D. H., Postma E., Gienapp P., Visser M. E.2005Selection on heritable phenotypic plasticity in a wild bird population. Science 310, 304–306 (doi:10.1126/science.1117004) [DOI] [PubMed] [Google Scholar]
- Ouyang Y., Andersson C. R., Kondo T., Golden S. S., Johnson C. H.1998Resonating circadian clocks enhance fitness in cyanobacteria. Proc. Natl Acad. Sci. USA 95, 8660–8664 (doi:10.1073/pnas.95.15.8660) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C., Takamura T.1989Latitudinal clines in the properties of a circadian pacemaker. In Biological clocks and environmental time (eds Daan S., Gwinner E.), pp. 105–123 New York, NY: Guilford Press; [PubMed] [Google Scholar]
- Poesel A., Kunc H., Foerster K., Johnsen A., Kempenaers B.2007Early birds are sexy: male age, dawn song and extra-pair paternity in blue tits, Cyanistes (formerly Parus) caeruleus. Animal Behaviour 72, 531–538 (doi:10.1016/j.anbehav.2005.10.022) [Google Scholar]
- Price T., Boag P. T.1993Selection in natural populations of birds. In Avian genetics. A population and ecological approach (eds Cooke F., Buckley P. A.), pp. 257–287 London, UK: Academic Press [Google Scholar]
- Purves W. K., Sadava D., Orians G. H., Heller H. C.2001Life: the science of biology. Sunderland, MA: Sinauer [Google Scholar]
- Roff D. A.1997Evolutionary quantitative genetics. New York, NY: Chapman & Hall [Google Scholar]
- Ruf T.1999The Lomb–Scargle periodogram in biological rhythm research: analysis of incomplete and unequally spaced time-series. Biol. Rhyth. Res. 30, 178–201 (doi:10.1076/brhm.30.2.178.1422) [DOI] [PubMed] [Google Scholar]
- Saladin V., Bonfils D., Binz T., Richner H.2003Isolation and characterization of 16 microsatellite loci in the great tit Parus major. Mol. Ecol. Notes 3, 520–522 (doi:10.1046/j.1471-8286.2003.00498.x) [Google Scholar]
- Sharp P. J.2005Photoperiodic regulation of seasonal breeding in birds. Ann. NY Acad. Sci. 1040, 189–199 (doi:10.1196/annals.1327.024) [DOI] [PubMed] [Google Scholar]
- Shimizu T., Masaki S.1997Geographical and species variation in circadian rhythm parameters in nemobiine crickets. Physiol. Entomol. 22, 83–93 (doi:10.1111/j.1365-3032.1997.tb01144.x) [Google Scholar]
- Silverin B., Massa R., Stokkan K. A.1993Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 90, 14–22 (doi:10.1006/gcen.1993.1055) [DOI] [PubMed] [Google Scholar]
- Smale L., Heideman P. D., French J. A.2005Behavioral neuroendocrinology in nontraditional species of mammals: things the ‘knockout’ mouse CAN'T tell us. Horm. Behav. 48, 474–483 (doi:10.1016/j.yhbeh.2005.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R. R., Rohlf F. J.1995Biometry. New York, NY: W. H. Freeman & Co [Google Scholar]
- Takahashi J. S., Shimomura K., Kumar V.2008Searching for genes underlying behavior: lessons from circadian rhythms. Science 322, 909–912 (doi:10.1126/science.1158822) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F.2008Circadian clocks: tips from the tip of the iceberg. Nature 456, 881–883 (doi:10.1038/456881a) [DOI] [PubMed] [Google Scholar]
- Van Oers K., Drent P. J., Dingemanse N. J., Kempenaers B.2008Personality is associated with extrapair paternity in great tits, Parus major. Anim. Behav. 76, 555–563 [Google Scholar]
- Visser M. E.2008Keeping up with a warming world; assessing the rate of adaptation to climate change. Proc. R. Soc. B 275, 649–659 (doi:10.1098/rspb.2007.0997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Van Noordwijk A. J., Tinbergen J. M., Lessells C. M.1998Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc. R. Soc. Lond. B 265, 1867–1870 (doi:10.1098/rspb.1998.0514) [Google Scholar]
- Visser M. E., Holleman L. J. M., Caro S. P.2009Temperature has a causal effect on avian timing of reproduction. Proc. R. Soc. B 276, 2323–2331 (doi:10.1098/rspb.2009.0213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Schaper S., Van Oers K., Caro S., Helm B.In press Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Schantz M.2008Phenotypic effects of genetic variability in human clock genes on circadian and sleep parameters. J. Genet. 87, 513–519 [DOI] [PubMed] [Google Scholar]