Abstract
Spatio-temporal patterns of snowmelt and flowering times affect fruiting success in Erythronium grandiflorum Pursh (Liliaceae) in subalpine western Colorado, USA. From 1990 to 1995, I measured the consistency across years of snowmelt patterns and flowering times along a permanent transect. In most years since 1993, I have monitored fruit set in temporal cohorts (early- to late-flowering groups of plants) at one site. To assess ‘pollination limitation’, I have also conducted supplemental hand-pollination experiments at various times through the blooming season. The onset of blooming is determined by snowmelt, with the earliest years starting a month before the latest years owing to variation in winter snowpack accumulation. Fruit set is diminished or prevented entirely by killing frosts in some years, most frequently but not exclusively for the earlier cohorts. When frosts do not limit fruit set, pollination limitation is frequent, especially in the earlier cohorts. Pollination limitation is strongest for middle cohorts: it tends to be negated by frost in early cohorts and ameliorated by continuing emergence of bumble-bee queens in later cohorts. This lily appears to be poorly synchronized with its pollinators. Across the years of the study, pollination limitation appears to be increasing, perhaps because the synchronization is getting worse.
Keywords: Bombus, climate change, Erythronium, frost, phenological synchrony, pollinator decline
1. Snow, phenology, pollination and fruiting success
Studies of flowering phenology often focus on variation across years, but the reproductive success of plants can vary within populations depending on when they bloom (e.g. Augspurger 1981; Schmitt 1983; Dieringer 1991; Ehrlen & Munzbergova 2009). This variation is particularly striking in alpine and subalpine habitats, where the weather is harsh, the growing season short and where heterogeneous melting of the winter snowpack determines the onset of early growth (Billings & Mooney 1968; Inouye & McGuire 1991). Patchiness in snowmelt produces a spatio-temporal mosaic in blooming phenology (Kudo 1993; Stanton et al. 1994; Kudo & Hirao 2006) such that different patches of spring flowers are exposed to different pollinator availabilities and different abiotic stresses, particularly late storms and frosts. This variation, therefore, may exert selection on plant characteristics (Widén 1991) and influence plant responses and adjustments to changing environments. For example, climate change has been suggested to increase plants' susceptibility to late frosts (Inouye 2000, 2008) and pollination deficits (Saavedra et al. 2003; deficits are postulated to arise from phenological mismatches between plants and pollinators; cf. Kudo et al. 2004; Memmott et al. 2007; Williams & Jackson 2007). These consequences, however, depend on whether the same phenological patterns are observed consistently across years (among other things; see Ollerton & Lack 1992). This makes multiyear studies essential, but few such studies exist. Here, I present a set of long-running observations and experiments focused intensively on the vagaries of fruit set and pollination limitation in a small subpopulation of an abundant subalpine lily. Although the original intention was to study within-year variation, the data suggest a noteworthy increase in pollination deficits from 1993 to 2009. Such deterioration of pollination service is relevant to concerns about declining pollinators (Ghazoul 2005; National Research Council 2006), and these results appear to be unique. A review by Knight et al. (2005) found only one study (Primack & Stacy 1998) that assessed pollination limitation in 10 or more years (T. Knight 2009, personal communication), and that study was designed to assess costs of reproduction in repeatedly stressed plants, not natural variation.
2. Material and Methods
(a). Study site
I conducted observations and experiments on and immediately adjacent to private property (Block 28, Lots 7–14) in the town site of Irwin, Colorado (elevation 3170 m), in a roughly triangular area (coordinates 38°52.566′ N 107°06.050′ W, 38°52.553′ N 107°05.988° W, 38°52.625′ N 107°05.997′ W) of about 3 ha. The site is 11.7 km southwest of the Rocky Mountain Biological Laboratory, a centre for much pollination research and reference weather data (www.rmbl.org). The area receives locally heavy snowfall; snow cover frequently persists into early June on open ground and into July in forested areas. Irwin received some intermittent cattle grazing in the late twentieth century, but there has been none since at least 1987. The study site is nearly level, with a slightly southern exposure. Because other parts of the surrounding habitat have steeper southern exposures, flowering is well advanced in those areas before it begins in the study site proper. My characterization of plants as ‘early’ or ‘late’ applies only within my study area; none of these plants are truly early within the larger meadow system.
The usual progression of spring at the site includes unpredictable wintry weather through May and into early June, with hard frosts and snowstorms interspersed with brief breaks of sun. This unsettled pattern then yields to ‘the June drought’, which usually brings several weeks of long sunny days before yielding in turn to a monsoonal pattern of afternoon thunderstorms in early July.
From field notes, I have extracted two phenological indicators relating to the earliness of different springs since 1990. The first is the day on which the last winter snow disappears from the property referenced above; the second is the date of winter ice breakup on Lake Irwin, approximately 100 m from the study area.
(b). Study plant
The glacier lily Erythronium grandiflorum Pursh (Liliaceae) is a long-lived, spring-ephemeral geophyte, abundant in the meadows at Irwin. Seedlings comprise a single, grass-like cotyledonary leaf in their first growing season; older plants produce broader bladed leaves. A non-flowering plant makes a single leaf and a flowering plant makes a pair. (Occasionally, a two-leafed plant does not produce a flowering scape, but this seems to represent a developmental aberration: remnants of the aborted scape persist between the leaf bases.) Shoot emergence coincides with the recession of snowpack, and flowers bloom within a few days. The blooming period is about four weeks, typically spanning the end of the bad ‘May’ weather and extending into the ‘June drought’.
The principal pollinators are bumble-bee queens of the early-emerging species Bombus bifarius and Bombus occidentalis (Hymenoptera: Apidae); the latter species is larger and, therefore, more likely to contact the stigma and deposit pollen (Thomson 1986). Queens seek nectar. Although they become dusted with pollen, they typically groom it off their bodies rather than packing it into corbicular pellets. Salix is the preferred pollen source, and is abundant at the site. I have observed active pollen collection from E. grandiflorum only once, in the unusually late spring of 1995. In open meadow habitats, flowering is finished before Bombus workers emerge. Workers may visit very late-flowering patches in the forests, but I have not observed this. Broad-tailed hummingbirds (Selasphorus platycercus) take nectar by visiting the pendent flowers from the side, and smaller solitary bees harvest pollen by alighting on the large anthers, but neither transfers much pollen to stigmas. By midsummer, leaves and the scapes of unfertilized flowers wither and the plant withdraws into a discrete underground corm. If flowers are pollinated, the scape elongates to ca 25 cm and bears capsules that dehisce terminally, spreading seeds by a salt-shaker mechanism in late July–August. Plants grown from seed on site take at least 6 years to reach flowering size. Mature plants make one to three flowers, rarely more, depending on corm size; successful fruit production diminishes corm size, so flower production is partially regulated by the cost of fruiting (J. Thomson 1990–2009, unpublished data, available at http://rmbl.info/jthomson). Unlike some congeners, E. grandiflorum does not form clonal patches through the lateral spread of rhizomes, but some vegetative reproduction occurs by the splitting of corms, especially larger ones. Seeds lack elaiosomes. Flowering plants tend to be more abundant in areas of shallow soil on rock outcrops, a pattern hypothetically driven by predation by pocket gophers (Thomomys talpoides; Thomson et al. 1996).
(c). Patterns of snowmelt and flowering: permanent transect
I established a permanently marked 2 × 200 m belt transect that ran from the open meadow, past several trees and into a matrix of forest and forest gaps. I placed the transect subjectively to include very early-flowering areas and very late-flowering areas. The transect was divided into 40 quadrats of 2 × 5 m each, with the edges marked by a nylon cord. The positions of trees or clumps of trees (all Picea engelmannii or Abies lasiocarpa) near the transect were also noted. In 1990–1995, I counted open flowers in each quadrat at 2 day intervals. To determine whether flowering patterns along the transect were consistent across years, within each year's dataset, I ranked the 40 quadrats according to their median date of flower production, and then correlated the ranks across years.
In 1992–1995, I also made sketch maps (by eye) of the course of snowmelt along the transect by recording the edges of bare ground. It was evident from comparing these sketch maps visually that snowmelt patterns were highly consistent from year to year, even though this sample included an early year (1992, first open ground 28 May) and a very late one (1995, first open ground 4 July). To quantify this consistency more objectively, I began with the sketch map for 1994, which was an intermediate year (first open ground 4 June). There were nine snow-mapping dates in 1994. On the map, I arbitrarily chose one spatial point for which the first open ground appeared on each of the nine census dates. I then transferred these nine locations to the maps for the other years and determined the dates on which snow disappeared at those locations in the other years. If sections along the transect melt in the same relative sequence in different years, despite large differences in absolute dates of melting, the dates of the first open ground at the nine reference locations will be correlated across years.
On 22 June of the extremely heavy snow year 1995, I also measured snow depth directly at 2 m intervals along the midline of the transect. At that date, the first open ground in the transect had just appeared and was limited to one 4 m patch.
As part of the transect studies, I also counted fruits of E. grandiflorum in the quadrats along the transect. Those data, not presented here, suggested interesting temporal patterns in fruiting success, possibly attributable to lack of early pollination. However, flowering time was strongly confounded with spatial position in those data sets. Because the spatial patterns of snowmelt and flowering times along the transect were virtually identical across years (see §3), I discontinued monitoring the transect after 1995 and concentrated instead on more spatially distributed observations of fruiting success. As explained below, the protocols for these experiments evolved slightly over the years.
(d). Observational study of fruit set patterns: phenological cohorts
Beginning in 1993, I marked successive flowering cohorts of approximately 100 single-flowering plants each at intervals through the flowering period. In the first year, I used 5 day intervals; in later years, I increased the sampling frequency to 4 day intervals, and later to 3 or 4 day intervals, depending on weather. A 3 day interval approximates the length of anthesis of individual flowers in the dry weather that is characteristic for June at this site. In cooler or rainy weather, flowers last longer, so a 4 day interval is more appropriate for separating non-overlapping cohorts. Because flowering lasts approximately a month, the total number of cohorts in a year typically ranges from eight to 10, depending on whether that year's blooming period was extended or compressed. Plants were marked with surveyor's pin flags during flowering, and capsules were collected when fully developed but not yet dehisced. I selected plants haphazardly, subject to the constraints that (i) they were fully open, with all six anthers dehisced and (ii) they were neither especially small nor large. (In this population, the smallest flowers frequently have poorly developed ovaries, are unlikely to set fruit even if pollinated and seem to be acting effectively as males.) I have repeated this procedure in most years since 1993. Plants for the earliest cohort are always in the same locations, on two south-facing slopes, and the latest cohort is always in another particular site that accumulates drifting snow and is partly shaded by conifers. The middle cohorts are less tightly associated with particular, extreme microsites. Therefore, they are larger in extent and more variable in location from year to year. I have not mapped the positions of the cohorts; nevertheless, there has been considerable consistency across years in the spatial locations of the cohorts.
For the first years of the study, I marked cohorts using red pin flags. Flags for different cohorts were distinguishable by spots of different colours of paint applied inconspicuously to the steel shafts. In 2003, complaints about the gaudiness of the red flags impelled me to switch to brown, still with colour-marked shafts. I do not think that pollinators reacted to the colour spots, which were ca 30 cm above the associated flowers, but I randomized the order of shaft colours across years as a precaution against confounding. Sample sizes approximate 100 plants per cohort, but vary somewhat. I used bundles that nominally contained 100 flags but often included a few extras; also, I did not always find and recover all of the flags set out each spring.
(e). Experimental study of fruit set patterns: supplemental pollinations
In these experiments, I selected adjacent pairs of single-flowered plants, using the same criteria as in the cohort study but also matching the members of the pair for stature. These plants were marked with green flags, alternating between two shaft colours. Plants with one colour of flag were supplementally pollinated by hand, while those with the other colour were left as unmanipulated controls. I prepared pollen mixtures by harvesting anthers from at least 12 different donor plants into a polystyrene vial. Erythronium anthers are ca 15 mm long and dehisce when mature by everting along a longitudinal suture (‘unzipping’) from the distal to the proximal end. I collected half-dehisced anthers as a way of standardizing pollen freshness (Thomson et al. 1994). I let dehiscence go to completion in the vial, and mixed the pollens by shaking the vial. As the mixture became depleted during the pollination process, I occasionally added fresh anthers. Until 2001, I applied pollen with applicators made of nylon fishing line; after that, I switched to MFH10 microbrush applicators (Microbrush International). I applied pollen over all three stigma lobes until a dense coating was visible by eye. Both tools applied equivalent coatings, but the microbrush was faster to load with pollen. The sample size for the supplementation experiment was about 150 single-flowered plants for each of the treatments, supplementation and control. Although plants were selected as pairs, I did not analyse the experiment as a paired design. It would have been logistically burdensome to keep paired fruits associated through harvest, and many pairs would be broken up by frost or herbivory.
In 1993–1995, I did a single supplementation experiment at approximately the middle of the flowering period. In 1997, I added a second (smaller, N = 50) experiment late in flowering. In all later years, I expanded to three experiments corresponding to the early, middle and late portions of flowering. The earliest and latest cohort dates always fell outside the period during which supplementations were done. The supplements can be viewed as representing the first third, middle third and last third of the flowering period.
For both the cohort and supplementation studies, I missed some years, as shown in subsequent tables.
(f). Harvesting and scoring fruit and seed set
I harvested capsules when they were dry and straw-coloured but not yet dehisced. In all years, I classified scapes into three categories: fruiting, failed or grazed. Failed scapes were thread-like, unelongated and retained remnants of the flowers. Failure was usually caused by lack of pollination or by frost; the causes were not distinguishable by the condition of the scape. The condition of grazed scapes depended on the stage at which herbivores attacked. Those that were attacked while flowering were thread-like and unelongated but retained no trace of flower tissue, having instead a cleanly snipped apex. This damage was typically caused by flower-eating chipmunks, which tended to attack only the earliest blooming cohorts. Later, grazing of developing fruits by deer left stiff, straight scapes with roughly torn ends. Neither form of grazing took more than a few per cent of flowers, and numerous harvests lost no flowers to grazers. Very rarely, developing fruits were attacked internally by caterpillars that destroyed some or all of the ovules. These were counted as successful fruits in tabulations for fruit set, but were eliminated from tabulations of seed set (below).
In some years, I also scored seed set by dissecting fruits that had been preserved in 70 per cent ethanol. At the stage at which I harvested, Erythronium ovules appear as small and white, medium and shrivelled brown or large, turgid and green; these correspond to unfertilized, fertilized but aborted and successfully matured seeds, respectively (Rigney et al. 1993). I calculated the fractional seed set as the number of successful seeds divided by the total number of ovules.
(g). Dependence on pollinators
Although ‘pollinator limitation’ of fruit and seed set is widely assessed by conducting supplemental hand pollinations with outcross pollen (reviews by Burd 1994; Knight et al. 2005), the procedure does not truly mimic the improvement in pollination service that could be achieved by an increase in visitation: animal visitors will typically deposit a mixture of self- and outcross pollen that may be inferior to the pure outcross pollen usually used in supplementation experiments (Thomson 2001; Aizen & Harder 2007). To examine the response of E. grandiflorum fruit and seed set to direct manipulation of bumble-bee visitation, on 6 June 1991, I set up a small exclusion/enrichment experiment as follows. Through a dense stand of plants in bud, I laid out three contiguous, parallel belt transects of 0.6 × 3.7 m, and tagged all flower buds in each strip. Two of the transects were caged with side walls of 20 cm lumber and tops of fibreglass mosquito screen. One of them was kept closed to exclude bees; to the other, I added one queen bumble-bee (B. occidentalis, B. bifarius or Bombus flavifrons) per day until all buds had opened. The third strip was left uncaged as an open-pollinated control. All flowers had wilted by 20 June, when I removed the cages and let fruits develop without further intervention. When fruits were mature, I determined the fates of the flowers (aborted or successfully fruited) and counted the seeds produced by the successful fruits. This study plot was in a late-melting area, equivalent to the last or penultimate cohorts.
3. Results
(a). Snowmelt and flowering
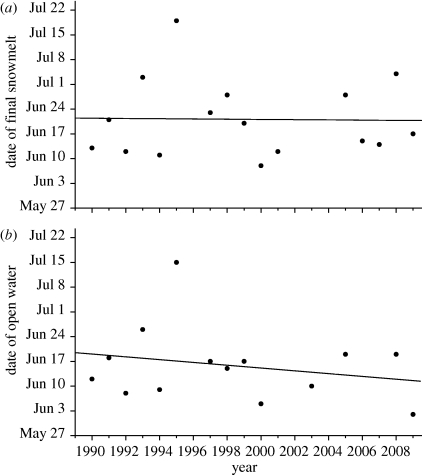
The study period included early and late years, with the greatest contrast between 2006 and the very snowy 1995, in which local winter residents estimated over 3 m of new snow during the month of May. Indeed, the last cohort of 2006 was marked on 21 June, whereas the first cohort of 1995 was marked on 28 June. The Erythronium bloom was offset by a full month in these 2 years, a noteworthy displacement in a habitat where the entire growing season is considered to last only about three months. Although local summer high temperatures increased over the study period, this warming did not translate to earlier springs at the Irwin site: neither local indicator showed a trend (figure 1; cf. Inouye et al. 2000.) In a longer time series of snowmelt data from the RMBL, Forrest et al. (2010) do show a significant trend towards earlier springs since 1973. That trend may be unapparent at Irwin because of the shorter time series; Irwin's snows are also heavier and may therefore show higher variance.
Figure 1.
Phenological indicators at the study site during the period studied. Data are missing for some years. (a) The date on which the last winter snowpack disappeared in the property that formed the core of the study area. The property is mostly an open meadow with scattered trees. Snow remained beyond this date in nearby forests. (b) The day on which ice broke up on Lake Irwin, adjacent to the study area. Lines are simple linear regressions; neither indicator shows a significant trend over the years of the study. The slight negative trend for ice breakup disappears if the abnormally snowy year of 1995 is ignored.
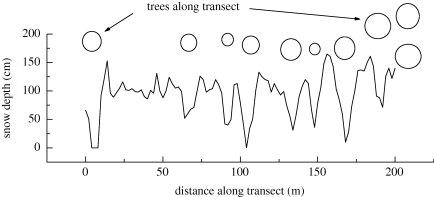
Despite large timing differences in the onset of snowmelt, the spatial patterns of snowmelt across the study area were strikingly consistent across years, as shown in table 1 for the 4 years for which I compiled maps of the snow's recession. Beyond those 4 years, qualitative observations confirmed this consistency. It was obvious that trees and clumps of trees strongly influenced the pattern (figure 2): bare ground appeared first through sublimation driven by long-wave radiation from these conifers (Marchand 1991), especially on the south–southeast sides. For example, the spot where snow last disappeared from the property was always at the same place each year from 1991 to 2009, within a metre. This high-drifting spot is just north of a 70 cm diameter spruce. Interestingly, the melt hole to the south of the same tree is consistently one of the earliest patches to melt. Although only 7 m apart, these two patches have no temporal overlap in flowering. In parts of the meadow farther away from trees, the depths to which snow drifts are important in determining the sequence of melting out, and these drifting patterns are also consistent from year to year.
Table 1.
Consistency of spatial pattern of snowmelt across 4 years ranging from early to late. (Nine points along the transect were chosen, each corresponding to open ground on each of the nine snow-mapping dates in the intermediate year 1994 (first open ground 4 June). The date of open ground at each of the nine points was determined for the maps from each of the other years, and the sets of open-ground dates correlated for all pairs of years. Values in the table are Pearson correlation coefficients. All correlations are highly significant, 7 d.f., p < 10−5.)
| 1992 | 1993 | 1994 | |
|---|---|---|---|
| 1993 | 0.977 | ||
| 1994 | 0.996 | 0.979 | |
| 1995 | 0.969 | 0.964 | 0.980 |
Figure 2.
Patterns of snow depth along the permanent transect for 1995. The circles (not to scale) diagrammatically suggest the approximate positions and relative sizes of large trees near the transect. The trees were all to the northwest of the transect, and strongly influenced snowmelt patterns.
Shoots of E. grandiflorum typically emerged through the last few centimetres of snow at the receding edges, and plants frequently opened their flowers within a metre of the snow's edge. This visually obvious tight linkage between snowmelt and lily bloom, coupled with the across-year consistency of snowmelt patterns (table 1), yields across-years consistency in the timing of flowering along the transect (table 2).
Table 2.
Consistency across 6 years of flowering in 40 2 × 5 m quadrats along the permanent transect. (Values in the table are Pearson correlation coefficients of the ranks of quadrats by their date of median bloom. All correlations are highly significant, 38 d.f., p < 10−12.)
| 1990 | 1991 | 1992 | 1993 | 1994 | |
|---|---|---|---|---|---|
| 1991 | 0.932 | ||||
| 1992 | 0.913 | 0.956 | |||
| 1993 | 0.880 | 0.910 | 0.900 | ||
| 1994 | 0.887 | 0.954 | 0.942 | 0.959 | |
| 1995 | 0.863 | 0.933 | 0.902 | 0.945 | 0.943 |
(b). Role of pollination in fruit and seed set
Flowers caged to exclude animals showed negligible capacity for autogamous fruit production (table 3), although E. grandiflorum is partially self-compatible (Rigney et al. 1993). The 1991 cage experiment did not include a hand-outcross treatment, which would have been necessary to assess rigorously whether visitation by bumble-bees could approach the success of pure outcross pollination. A rough comparison can be made, however, by comparing the fruit set and seed numbers from the 1991 bee-addition experiment to comparable late hand pollinations from other years of the supplementation experiments (table 4). The mean seed number (26.9) from the 1991 bee-addition experiment is comparable to the late supplementation experiments in 1997, 1999, 2003 and 2008 (13.6, 28.5, 23.7 and 20.5, respectively). The 1991 fruit set (0.52) is lower than that for the late supplementation experiments in 7 of 9 years, but is higher in 2003 and 2008. Although comparing different years is unwise because of different environmental conditions, we can tentatively conclude that the pollination efficacy of bumble-bees can approach that of pure outcross pollen, despite the fact that bees deliver substantial fractions of self-pollen (Thomson & Stratton 1985).
Table 3.
Response of fruit and seed set to experimental exclusion or enrichment of bumble-bee queens in caged groups of plants, late 1991. (Fruit set did not differ between the control and bees-added treatments (2 × 2 contingency χ2 = 0.36, p = 0.55), although both differed sharply from the bees-excluded treatment. Control and bees-added produced equivalent numbers of seeds per fruit (two-tailed t-test, p = 0.34).)
| control | bees added | bees excluded | |
|---|---|---|---|
| flowers that successfully fruited | 31 | 30 | 0 |
| flowers that aborted | 23 | 28 | 27 |
| seeds per successful fruit (mean, s.d.) | 23.7, 11.2 | 26.9, 13.8 | undefined |
Table 4.
Fruit set and seed set in phenological cohorts of supplementally pollinated flowers and matched open-pollinated controls. (In 1993–1995, a single experiment was done near the peak date of flowering. In 1995, a second, smaller experiment was added after the peak. In subsequent years, three experiments of ca 300 flowers were done, before, at and after the peak. ‘Failed fruits’ include those lost to frost and to abortion. Fruit set is calculated as the number of fruits divided by the total number of successful and failed fruits. The index of ‘fruit set limitation’ equals the fruit set for the supplemented flowers divided by the sum of the fruit sets for supplemented and control flowers; values of 0.5 indicate equal success of supplemented and control flowers, whereas higher values indicate greater success of supplemented flowers, i.e. pollination limitation, with significant values in boldface. The significance of pollination limitation (p-value (fruits)) reflects the χ2-squared statistic (not shown) for a 2 × 2 contingency table of (successful versus failed fruits) × (supplemented versus control). For years in which ovule fates were determined for successful fruits, ‘seed set’ is the number of matured seeds divided by the total number of ovules; the ‘seed set limitation’ index is calculated analogously to ‘fruit set limitation’, and the significance of seed set limitation is determined by a t-test (for unequal sample variances) of seed set, after arcsine-square-root transformation.)
| year | date | treatment | total sample | eaten | fruits | failed fruits | fruit set | fruit set limitation | p-value (fruits) | seed set | seed set limitation | p-value (seeds) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1993 | 28 Jun | supp. | 150 | 1 | 5 | 144 | 0.034 | 0.386 | 0.4018 | |||
| control | 150 | 0 | 8 | 142 | 0.053 | |||||||
| 1994 | 16 Jun | supp. | 150 | 12 | 121 | 17 | 0.877 | 0.498 | 0.8879 | |||
| control | 150 | 14 | 120 | 16 | 0.882 | |||||||
| 1995 | 11 Jul | supp. | 150 | 0 | 85 | 65 | 0.567 | 0.521 | 0.4172 | |||
| control | 150 | 0 | 78 | 72 | 0.520 | |||||||
| 1997 | 20 Jun | supp. | 175 | 5 | 105 | 65 | 0.618 | 0.582 | 0.0012 | 0.435 | 0.520 | 0.0031 |
| control | 180 | 2 | 79 | 99 | 0.444 | 0.402 | ||||||
| 30 Jun | supp. | 50 | 1 | 30 | 19 | 0.612 | 0.586 | 0.0819 | 0.356 | 0.480 | 0.517 | |
| control | 50 | 6 | 19 | 25 | 0.432 | 0.385 | ||||||
| 1998 | 12 Jun | supp. | 150 | 2 | 0 | 148 | 0 | undefined | n.s. | |||
| control | 150 | 7 | 0 | 143 | 0 | |||||||
| 20 Jun | supp. | 149 | 0 | 0 | 149 | 0 | undefined | n.s. | ||||
| control | 151 | 0 | 0 | 151 | 0 | |||||||
| 28 Jun | supp. | 149 | 0 | 121 | 28 | 0.812 | 0.503 | 0.8135 | ||||
| control | 151 | 0 | 121 | 30 | 0.801 | |||||||
| 1999 | 12 Jun | supp. | 150 | 0 | 39 | 111 | 0.260 | 0.886 | <0.0001 | 0.411 | 0.753 | <0.0001 |
| control | 150 | 0 | 5 | 145 | 0.033 | 0.135 | ||||||
| 20 Jun | supp. | 150 | 0 | 115 | 35 | 0.767 | 0.714 | <0.0001 | 0.489 | 0.645 | <0.0001 | |
| control | 150 | 0 | 46 | 104 | 0.307 | 0.269 | ||||||
| 28 Jun | supp. | 150 | 0 | 82 | 68 | 0.547 | 0.617 | 0.0003 | 0.568 | 0.559 | <0.0001 | |
| control | 150 | 0 | 51 | 99 | 0.340 | 0.449 | ||||||
| 2003 | 7 Jun | supp. | 147 | 3 | 114 | 30 | 0.792 | 0.736 | <0.0001 | 0.582 | 0.650 | <0.0001 |
| control | 149 | 1 | 42 | 106 | 0.284 | 0.313 | ||||||
| 15 Jun | supp. | 118 | 1 | 64 | 53 | 0.547 | 0.660 | <0.0001 | 0.540 | 0.675 | <0.0001 | |
| control | 124 | 0 | 35 | 89 | 0.282 | 0.260 | ||||||
| 23 Jun | supp. | 150 | 0 | 69 | 81 | 0.460 | 0.620 | 0.0014 | 0.434 | 0.529 | 0.26 | |
| control | 149 | 0 | 42 | 107 | 0.282 | 0.386 | ||||||
| 2005 | 18 Jun | supp. | 144 | 10 | 84 | 50 | 0.627 | 0.558 | 0.0294 | |||
| control | 149 | 8 | 70 | 71 | 0.496 | |||||||
| 22 Jun | supp. | 106 | 4 | 85 | 17 | 0.833 | 0.611 | <0.0001 | ||||
| control | 103 | 3 | 53 | 47 | 0.530 | |||||||
| 27 Jun | supp. | 159 | 12 | 128 | 19 | 0.871 | 0.566 | <0.0001 | ||||
| control | 150 | 8 | 95 | 47 | 0.669 | |||||||
| 2006 | 1 Jun | supp. | 148 | 7 | 81 | 60 | 0.574 | 0.673 | <0.0001 | |||
| control | 149 | 9 | 39 | 101 | 0.279 | |||||||
| 9 Jun | supp. | 145 | 4 | 46 | 95 | 0.326 | 0.563 | 0.1776 | ||||
| control | 145 | 3 | 36 | 106 | 0.254 | |||||||
| 15 Jun | supp. | 137 | 2 | 92 | 43 | 0.681 | 0.535 | 0.1311 | ||||
| control | 134 | 4 | 77 | 53 | 0.592 | |||||||
| 2008 | 18 Jun | supp. | 144 | 11 | 89 | 44 | 0.669 | 0.643 | <0.0001 | 0.475 | 0.572 | 0.0011 |
| control | 142 | 10 | 49 | 83 | 0.371 | 0.356 | ||||||
| 22 Jun | supp. | 148 | 9 | 100 | 39 | 0.719 | 0.676 | <0.0001 | 0.351 | 0.589 | 0.0004 | |
| control | 149 | 10 | 48 | 91 | 0.345 | 0.245 | ||||||
| 26 Jun | supp. | 145 | 6 | 93 | 46 | 0.669 | 0.649 | <0.0001 | 0.370 | 0.535 | 0.0859 | |
| control | 148 | 10 | 50 | 88 | 0.362 | 0.322 | ||||||
| 2009 | 4 Jun | supp. | 145 | 0 | 10 | 135 | 0.069 | 0.909 | 0.0059 | |||
| control | 146 | 2 | 1 | 143 | 0.007 | |||||||
| 12 Jun | supp. | 132 | 1 | 27 | 104 | 0.206 | 0.904 | <0.0001 | ||||
| control | 139 | 2 | 3 | 134 | 0.022 | |||||||
| 17 Jun | supp. | 145 | 0 | 75 | 70 | 0.517 | 0.640 | 0.0001 | ||||
| control | 148 | 0 | 43 | 105 | 0.291 |
(c). Fruit set within and across year: cohorts and supplementations
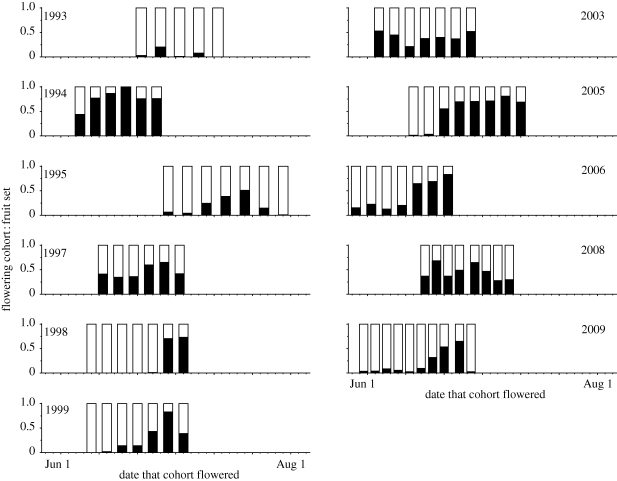
The ‘cohort’ data in figure 3 reveal sharp differences among years in the temporal pattern of fruit set. Herbivory was usually low and restricted to chipmunks eating early flowers, although deer grazing became more important in 2005–2008, perhaps owing to fewer domestic dogs near the study site. Fruiting failures reflect both frost damage and pollination deficits, so it is best to consider the among-year variation in conjunction with the supplementation experiments (table 4 and figure 4), and with observations on frost effects. Erythronium grandiflorum flowers can withstand mild frosts, but harder freezes kill flowers. Although frozen flowers appear superficially normal the day after a killing frost, the style and ovary lose turgor and become wrinkled. Such flowers never recover to set fruits. Buds and developing fruits are more resistant to frost than flowers in anthesis. Therefore, frosts of intermediate severity can kill later-opening flowers while sparing earlier ones that have started maturing fruits. Extremely hard frosts kill a wider range of developmental stages. For example, a hard frost and snowstorm on 5 June 2007 killed virtually all flowers in the study area, leading me to cancel the regular experiments in that year. The destruction in 1993 was almost as complete, although some flowers survived in two cohorts, probably by being somewhat sheltered from longwave radiative cooling to cold night skies (Leuning & Cremer 1988; Inouye 2000). In contrast, plants in 1994, 2003 and 2008 escaped strong frosts and showed roughly similar fruit set in all cohorts; in 1998, 1999 and 2005, earlier cohorts failed completely but later ones succeeded. In 1995, 1999 and 2009, fruit set increased more gradually over time. Pollinator availability probably interacted with frost damage in these years. The year 1995 was quite anomalous because of the greatly delayed bloom; 2009 was anomalous because the June drought pattern never materialized: after warm weather in May; June 2009 was uncharacteristically cold and stormy. Therefore, flowering started early, but the date of snow disappearance was pushed back (figure 1b).
Figure 3.
Patterns of fruit set over 11 years of observation of numerous temporal cohorts per year of unmanipulated, open-pollinated, single-flowered plants of E. grandiflorum. (Sample sizes approximate 100 plants for each cohort. The black portions of bars indicate the fraction of flowers that successfully set fruits, and the white portions indicate flowers that failed for any cause other than grazing. A few flowers were typically lost to herbivory (see table 4 for the magnitude of herbivory in different years); these were removed from this tabulation.)
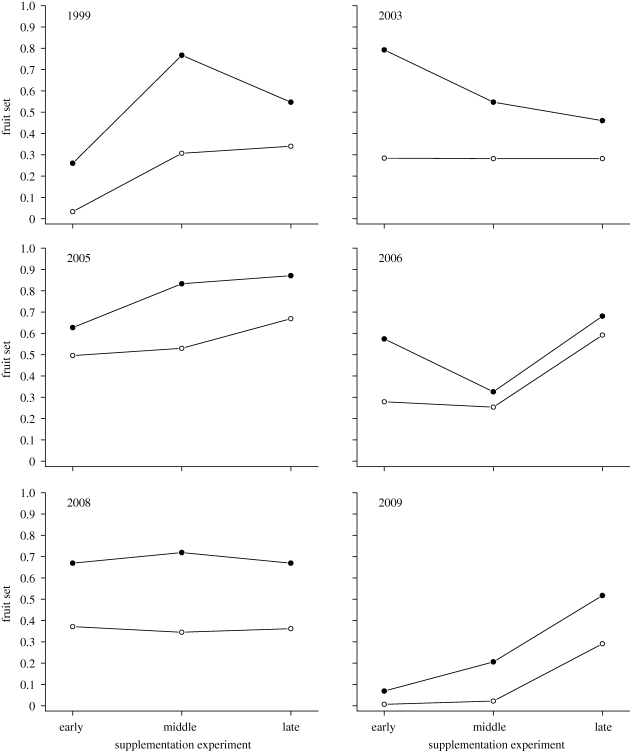
Figure 4.
Temporal patterns of response to supplemental hand-pollination experiments for the 6 years in which three experiments were done in the early, middle and late portions of the flowering period (table 4). (Filled circles represent fractional fruit set of flowers receiving supplemental outcross pollen; open circles represent open-pollinated controls.)
Considered only by themselves, patterns of early failure and later success are consistent with either frost damage or insufficient pollination. Unfortunately, I have no direct measures of frost damage, so I cannot formally deconfound these two sources of failure. Their effects can be partially disentangled, however, by considering the temporal patterns of the pollen supplementation experiments, at least in the later years in which I conducted early, middle and late supplementations (figure 4). If supplemented and control flowers both fail heavily, as in the early experiments from 1999 and 2009, pollination deficits can be ruled out. On the other hand, substantial differences between control and supplemented treatments suggest pollination deficits. Significant limitation of fruit set by pollination is evident in most but not all years and cohorts. In particular, cases in which pollination limitation—the gap between control and supplemented flowers—decreases from the middle to the late experiment suggest that pollinators were emerging too late to service the earlier flowers fully (figure 4, 1999, 2003, 2006).
In aggregate, the data suggest a hierarchy of effects. First, frost can reduce or prevent fruit set in any cohort, but is more likely to affect earlier cohorts. By killing flowers regardless of their pollination status, severe frosts render pollination limitation moot. In years when frost spares earlier cohorts, those flowers may suffer pollination deficits. Like frost, pollination deficits are more probable early in bloom. Therefore, both abiotic and biotic factors conspire to produce the pattern of greater fruiting success in later cohorts, which is evident in figure 3.
(d). Ovule fates and limitation of seed set
Erythronium grandiflorum flowers typically mature only about half of their ovules, even when supplementally pollinated (table 4). In all 4 years for which ovule fates were scored, however, supplemented flowers in earlier experiments set significantly more seeds than open-pollinated controls, i.e. there was significant pollination limitation of seed set (always in addition to limitation of fruit set). In all years, moreover, seed set limitation declined in the late experiments; in 3 of the 4 years, it declined to statistical insignificance. This pattern is consistent with the late emergence of bumble-bee pollinators.
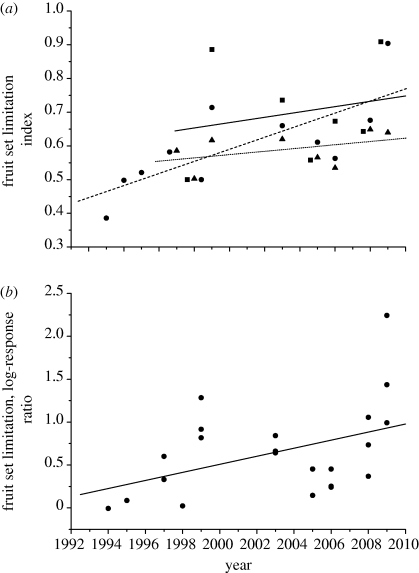
(e). Trends across years in pollination limitation
Given that pollination limitation is an important factor for E. grandiflorum at this study site, one can ask whether it is changing in importance with time. For each set of experiments, the pollination limitation index tends to increase over the period of the study (figure 5), suggesting a deterioration of pollination service. Following a suggestion from an anonymous referee, I have examined the significance of these trends by calculating the log-response ratio measure of effect size (Knight et al. 2005), which has better statistical properties than the bounded proportional index I use in table 4 and the figures. I assessed trends by calculating Pearson correlations between log-response ratios (ln (supplemented proportional fruit set/control proportional fruit set)) and the year of sampling. Data from early and middle experiments in 1998 could not be included because frost damage eliminates all fruit set in both treatments, rendering the log–response ratio undefined. The single 1993 datum is also problematic, being based on very few surviving fruits in either treatment. It is true to say that there was no pollination limitation in those years, but those data contribute little information about pollination service.
Figure 5.
Temporal trends in the degree of pollination limitation over the course of the study. (a) Indices of pollen limitation for supplementation experiments done in the early, middle or late portions of the flowering period are indicated by squares, circles and triangles, respectively. Symbols that overlapped have been offset horizontally. Simple linear regression lines are presented to indicate trends within each dataset (early, solid; middle, long dash; late, short dash). These graphs include points from 1993 and 1998 experiments with such heavy frost damage that few or no fruits were produced in either treatment. These points are excluded from the analyses of significance (see text and table 4). (b) As above, but the data have been transformed to log-response ratios, the problematic 1993 and 1998 data removed and the early and late datasets rescaled by factors representing the average differences among the three temporal supplementation experiments within years. This allows an overall test of the hypothesis that pollination service has deteriorated over the study period (r = 0.434, n = 23, p = 0.039).
The trends for early and late supplementation experiments do not approach significance. The trend for mid-bloom supplementations is significant if the 1993 datum is included (r = 0.714, n = 10, p = 0.020), but not if it is discarded (r = 0.633, n = 9, p = 0.067). Doing a single test that combines early, middle and late datasets requires correcting for the tendency of pollination limitation to decline within a season. Averaged across all years for which all three measures are available, the log-response ratios for early and late experiments differ from that of the middle experiments by factors of 1.50 and 0.58, respectively. Therefore, I rescaled all early and late data by these factors to examine an overall temporal trend. For the rescaled data including 1993, r = 0.526, n = 24, p = 0.008; eliminating the dubious datum for 1993, r = 0.434, n = 23, p = 0.039. Therefore, the data strongly suggest a deterioration of pollination service, although the next several years' data will probably determine how robust it is. Why is the trend more significant for middle cohorts than for early or late cohorts? In part, there are more data points for the middle cohorts, but the differences may well reflect ecology, too: in early-flowering cohorts, pollination limitation will more frequently be negated by frost damage; in late cohorts, it may be ameliorated by the continuing emergence of more pollinators. The index of pollination limitation was uncorrelated with the date of last snowmelt (r = −0.30, n = 10, p = 0.4).
4. Discussion
(a). Timing of snowmelt and flowering
The E. grandiflorum plants in the study area do not comprise an idealized panmictic population but rather a snowpack-driven mosaic of different patches that bloom at different times and face different stresses (Yamagishi et al. 2005). Given the potential variability in such factors as the timing and character of snow storms, and the wind-driven drifting of snow, it seems somewhat surprising that the spatial pattern of spring snowmelt should be as repeatable as it is. However, similar repeatability has been reported from alpine regions (e.g. Kudo 1993, p. 1304; Stanton et al. 1994, p. 364, and references therein). It is also commonplace that the timing of flowering is correlated with the depth of snowpack (Billings & Mooney 1968; Inouye & McGuire 1991). What distinguishes the subalpine study population of E. grandiflorum from the tundra habitats studied by Kudo and Stanton et al. is the important role of large trees that are scattered through the Irwin meadows. By influencing drift patterns and creating early melt holes in spring, these trees contribute much to the spatio-temporal mosaic of lily bloom, with effects on both population-genetic structure and the exposure of different patches to different conditions of weather and pollinator availability. Early flowering plants growing in tree melt holes may be able to mate with each other, as early bees fly from one hole to another, but unable to exchange gametes with much closer neighbours. In treeless tundra, by contrast, the major structuring template would be the microtopography of the terrain itself. Of course, tree-based phenological structure will shift when the trees die, but the large trees at Irwin are old (e.g. one human-felled Engelmann spruce stump of 85 cm diameter had 349 annual rings), and they frequently grow with apparently self-perpetuating clusters of daughter stems around their original trunks. Recruitment of new trees is rare, and seedlings grow slowly (e.g. one spruce at the centre of the study area is at least 20 years old but is only 75 cm tall). Therefore, trees are a rather stable structuring element in these subalpine meadows.
Under present conditions, early flowering cohorts are less likely to set fruit in most years, which suggests a non-equilibrial situation that might induce selection against early flowering time if unopposed. To explain a similar situation in Rhododendron aureum, Kudo (1993) proposed that the pollination advantage of late flowering was countered by the disadvantage of maturing fruits in the face of autumn frosts and snow. This seems inapplicable to E. grandiflorum, which fruits in midsummer, with no obvious penalty for flowering later. However, response to selection on flowering time would be attenuated because any genetic basis for flowering time would tend to be swamped by non-genetic variation arising from snowpack effects. Where a seed lands is likely to affect the resulting plant's flowering time more than its genetic heritage.
(b). Pollination, frost and fruit failure
Reviews suggest that alpine tundra plants frequently suffer from pollination limitation (García-Camacho & Totland 2009) and that their reproductive success tends to increase as the season progresses (Molau 1993). The subalpine E. grandiflorum exemplifies both tendencies. Pollinating bees are critical to seed reproduction in E. grandiflorum. The 1991 cage experiment showed that autogamous fruit set is minimal, and that bumble-bee queens by themselves can produce fruit and seed set equivalent to open pollination late in flowering (when pollination is characteristically more sufficient). Pollination limitation of both fruit and seed set prevailed in most but not all years of the study, highlighting again the importance of replicating limitation studies, first stressed by Campbell (1987). Pollination limitation is clearly a characteristic of an ecological situation rather than a constitutional attribute of a plant species (Wilson et al. 1994). More precisely, E. grandiflorum tends to receive poor pollination service early in its blooming period, but pollination typically improves through the blooming period. That improvement correlates with increasing numbers of bumble-bees seen while conducting experiments, and is probably monotonic (although the latest lily flowers did poorly in the unusual years of 1999 and 2009, possibly because bees switched to other forage). Because early flowers are more frequently killed by frost, however, pollination limitation (as defined by the difference between control and supplemented flowers) is not monotonic, tending instead to be strongest in mid-bloom.
Has recent climate change affected this system? Specifically, do we see earlier spring melts, more killing frosts (as proposed by Inouye 2000) or more pollination deficits (as proposed by Price & Waser 1998; Dunne et al. 2003; Saavedra et al. 2003; Memmott et al. 2007)? No, no and yes. Considering only data from the Irwin study site, neither abiotic (figure 1) nor biotic (figure 2) events have advanced. It may be that the effects of warming have been counteracted by concomitant increases in snowpack depth, as suggested by Inouye et al. (2000). On the other hand, Miller-Rushing & Inouye (2009) have documented a longer term trend towards earlier snowmelt in the broader area around the Irwin study area since 1973. This trend has not registered at Irwin over the course of my study, however. Nor is it apparent that killing frosts have increased, at least insofar as these have affected the reproductive success of E. grandiflorum. Admittedly, the recent years of 2007 and 2009 delivered killing frosts, but 2003, 2006 and 2008 were relatively benign.
On the other hand, pollination service has apparently deteriorated, especially for plants that flower during the middle of the bloom (figure 5). Because such deterioration has been anticipated on the grounds of pollinator declines (National Research Council 2006) or climate-driven phenological shifts (Memmott et al. 2007), this first documentation of a progressive decline may warrant further discussion despite its borderline significance. The most likely possibilities are that (i) pollinator populations have declined, (ii) lily flowering and pollinator emergence have become less synchronous, or (iii) pollinators have shifted their activity away from the lilies, presumably by visiting other plant species. Rigorously evaluating these possibilities would require direct estimates of pollinator abundance, which are utterly lacking. Nevertheless, I speculate that the third explanation is unimportant. The only other significant native floral resources for bumble-bees during the lily peak are Mertensia fusiformis Greene and Salix spp., and neither of these appear to have changed in density or timing. Turning to pollinator declines, the best pollinator of E. grandiflorum, B. occidentalis, has declined over much of its range (Williams & Osborne 2009), and some RMBL researchers believe that it may have become rarer in the study area. No estimates of absolute abundance are available. However, replicated quantitative surveys near the RMBL show no decline in the relative abundance of B. occidentalis as a fraction of all bumble-bees: Pyke (1982) found 3.8 per cent (502/13 136) in 1974; J. Thomson & E. Long (unpublished data) found 6.9 per cent (42/611) in 1998; and J. Thomson & B. Thomson (unpublished data) found 5.2 per cent (30/579) in a late-season sample from 2007. Because this species has not declined relative to other Bombus spp., the increasing pollination deficits are more probably attributable to a phenological mismatch or to a general decline in bumble-bee species. Casual observations are inconsistent with an overall decline in bumble-bees. Essentially, all flowers of Corydalis caseana Gray in a large stand near the Irwin study area show holes from nectar robbing, almost certainly done by B. occidentalis workers, with no decline in attack rates evident from 2007 to 2009 (J. Thomson, unpublished data). It appears that healthy populations of effective pollinators remain in the area.
Therefore, weak inference suggests a growing phenological mismatch between the blooming of E. grandiflorum and the emergence of its best pollinators. Hegland et al. (2009, p. 184) argue that such mismatches ought to be rare because the ‘onset of flowering in plants and first appearance dates of pollinators in several cases appear to advance linearly in response to recent temperature increases’, but there could certainly be exceptions. Kudo et al. (2004) reported that one particularly early spring in Hokkaido depressed seed set in two bee-pollinated spring ephemerals but not in two fly-pollinated ones. Even if emergence times remain in step, however, both the activity levels of queen bumble-bees and the longevity of flowers may be very sensitive to air temperatures, insolation, precipitation and wind. If these factors are changing, subtle dislocations of bees and flowers seem plausible. Further research is needed to see whether the trend continues, and what might be driving it.
Acknowledgements
This work was supported by grants to J.D.T. from the US National Science Foundation and the Canadian Natural Sciences and Engineering Research Council. I thank B. Thomson for help from fieldwork to analysis, G. Ernst and K. and R. Robbins for access to property, T. Anderson, R. Huralt, S. Keller, A. Lowrance, E. Long, M. Malzone, M. Somers, A Leslie and L. Arcila-Hernandez for assistance and J. Forrest, T. Knight and anonymous reviewers for comments.
Footnotes
One contribution of 11 to a Theme Issue ‘The role of phenology in ecology and evolution’.
References
- Aizen M. A., Harder L. D.2007Expanding the limits of the pollen-limitation concept: effects of pollen quantity and quality. Ecology 88, 271–281 (doi:10.1890/06-1017) [DOI] [PubMed] [Google Scholar]
- Augspurger C. K.1981Reproductive synchrony of a tropical shrub: experimental studies on effects of pollinators and seed predators on Hybanthus prunifolius (Violaceae). Ecology 62, 775–788 (doi:10.2307/1937745) [Google Scholar]
- Billings W. D., Mooney H. A.1968The ecology of arctic and alpine plants. Biol. Rev. 43, 481–529 (doi:10.1111/j.1469-185X.1968.tb00968.x) [Google Scholar]
- Burd M.1994Bateman's principle and plant reproduction: the role of pollen limitation in fruit and seed set. Bot. Rev. 60, 83–139 (doi:10.1007/BF02856594) [Google Scholar]
- Campbell D. R.1987Interpopulational variation in fruit production: the role of pollination-limitation in the Olympic mountains. Am. J. Bot. 74, 269–273 (doi:10.2307/2444029) [Google Scholar]
- Dieringer G.1991Variation in individual flowering time and reproductive success of Agalinis strictifolia (Scrophulariaceae). Am. J. Bot 78, 497–503 (doi:10.2307/2445259) [Google Scholar]
- Dunne J. A., Harte J., Taylor K.2003Subalpine meadow flowering phenology responses to climate change: integrating experimental and gradient methods. Ecol. Monogr. 73, 69–86 (doi:10.1890/0012-9615(2003)073[0069:SMFPRT]2.0.CO;2) [Google Scholar]
- Ehrlen J., Munzbergova Z.2009Timing of flowering: opposed selection on different fitness components and trait covariation. Am. Nat. 173, 819–830 (doi:10.1086/598492) [DOI] [PubMed] [Google Scholar]
- Forrest J., Inouye D. W., Thomson J. D.2010Flowering phenology in subalpine meadows: does climate variation influence community co-flowering patterns? Ecology 91, 431–440 (doi:10.1890/09-0099.1) [DOI] [PubMed] [Google Scholar]
- García-Camacho R., Totland Ø.2009Pollen limitation in the alpine: a meta-analysis. Arct. Antarct. Alp. Res. 41, 103–111 (doi:10.1657/1523-0430-41.1.103) [Google Scholar]
- Ghazoul J.2005Buzziness as usual? Questioning the global pollination crisis. Trends Ecol. Evol. 20, 367–373 [DOI] [PubMed] [Google Scholar]
- Hegland S. J., Nielsen A., Lazaro A., Bjerknes A.-L., Totland Ø.2009How does climate warming affect plant–pollinator interactions? Ecol. Lett. 12, 184–195 (doi:10.1111/j.1461-0248.2008.01269.x) [DOI] [PubMed] [Google Scholar]
- Inouye D. W.2000The ecological and evolutionary significance of frost in the context of climate change. Ecol. Lett. 3, 457–463 (doi:10.1046/j.1461-0248.2000.00165.x) [Google Scholar]
- Inouye D. W.2008Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362 (doi:10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- Inouye D. W., McGuire A. D.1991Effects of snowpack on timing and abundance of flowering in Delphinium nelsonii (Ranunculaceae): implications for climate change. Am. J. Bot. 78, 97–1001 [Google Scholar]
- Inouye D. W., Barr B., Armitage K. B., Inouye B. D.2000Climate change is affecting altitudinal migrants and hibernating species. Proc. Natl Acad. USA 97, 1630–1633 (doi:10.1073/pnas.97.4.1630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight T. M., et al. 2005Pollen limitation of plant reproduction: pattern and process. Annu. Rev. Ecol. Evol. Syst. 36, 467–497 (doi:10.1146/annurev.ecolsys.36.102403.115320) [Google Scholar]
- Kudo G.1993Relationship between flowering time and fruit set of the entomophilous alpine shrub, Rhododendron aureum (Ericaceae), inhabiting snow patches. Am. J. Bot. 80, 1300–1304 (doi:10.2307/2445714) [Google Scholar]
- Kudo G., Hirao A. S.2006Habitat-specific responses in the flowering phenology and seed set of alpine plants to climate variation: implications for global-change impacts. Popul. Ecol. 48, 49–58 (doi:10.1007/s10144-005-0242-z) [Google Scholar]
- Kudo G., Nishikawa Y., Kasagi I., Kosuge S.2004Does seed production of spring ephemerals decrease when spring comes early? Ecol. Res. 19, 255–259 (doi:10.1111/j.1440-1703.2003.00630.x) [Google Scholar]
- Leuning R., Cremer K.1988Leaf temperatures during radiation frost. Part I. Observations. Agric. For. Meteorol. 42, 121–133 (doi:10.1016/0168-1923(88)90072-X) [Google Scholar]
- Marchand P.1991Life in the cold: an introduction to winter ecology. Hanover, NH: University Press of New England [Google Scholar]
- Memmott J., Craze P. G., Waser N. M., Price M. V.2007Global warming and the disruption of plant–pollinator interactions. Ecol. Lett. 10, 710–717 (doi:10.1111/j.1461-0248.2007.01061.x) [DOI] [PubMed] [Google Scholar]
- Miller-Rushing A., Inouye D. W.2009Variation in the impact of climate change on flowering phenology and abundance: an examination of two pairs of closely related wildflower species. Am. J. Bot. 96, 1821–1829 (doi:10.3732/ajb.0800411) [DOI] [PubMed] [Google Scholar]
- Molau U.1993Relationships between flowering phenology and life history strategies in tundra plants. Arctic Alpine Res. 25, 391–402 (doi:10.2307/1551922) [Google Scholar]
- National Research Council of the National Academies 2006Status of pollinators in North America. Washington, DC: National Academy Press [Google Scholar]
- Ollerton J., Lack A. J.1992Flowering phenology: an example of relaxation of natural selection? Trends Ecol. Evol. 7, 274–276 [DOI] [PubMed] [Google Scholar]
- Price M. V., Waser N. M.1998Effects of experimental warming on plant reproductive phenology in a subalpine meadow. Ecology 79, 1261–1271 (doi:10.1890/0012-9658(1998)079[1261:EOEWOP]2.0.CO;2) [Google Scholar]
- Primack R. B., Stacy E.1998Cost of reproduction in the pink lady's slipper orchid (Cypripedium acaule, Orchidaceae): an eleven-year study of three populations. Am. J. Bot. 85, 1672–1679 (doi:10.2307/2446500) [PubMed] [Google Scholar]
- Pyke G. H.1982Local geographic distributions of bumblebees near Crested Butte, Colorado: competition and community structure. Ecology 63, 555–573 (doi:10.2307/1938970) [DOI] [PubMed] [Google Scholar]
- Rigney L. P., Thomson J. D., Cruzan M. B., Brunet J.1993Differential success of pollen donors in a self-compatible lily. Evolution 47, 915–924 (doi:10.2307/2410194) [DOI] [PubMed] [Google Scholar]
- Saavedra F., Inouye D. W., Price M. V., Harte J.2003Changes in flowering and abundance of Delphinium nuttallianum (Ranunculaceae) in response to a subalpine climate warming experiment. Global Change Biol. 9, 885–894 (doi:10.1046/j.1365-2486.2003.00635.x) [Google Scholar]
- Schmitt J.1983Individual flowering phenology, plant size, and reproductive success in Linanthus androsaceus, a California annual. Oecologia 59, 135–140 (doi:10.1007/BF00388084) [DOI] [PubMed] [Google Scholar]
- Stanton M. L., Rejmánek M., Galen C.1994Changes in vegetation and soil fertility along a predictable snowmelt gradient in the Mosquito Range, Colorado, U.S.A. Arctic Alpine Res. 26, 364–374 (doi:10.2307/1551798) [Google Scholar]
- Thomson J. D.1986Pollen transport and deposition by bumble bees in Erythronium: influences of floral nectar and bee grooming. J. Ecol. 74, 329–341 [Google Scholar]
- Thomson J. D.2001Connecting pollination deficits and pollinator declines: can theory guide us? Cons. Ecol. 5, 6 See http://www.consecol.org/vol5/iss1/art6 [Google Scholar]
- Thomson J. D., Stratton D. A.1985Floral morphology and outcrossing in Erythronium grandiflorum. (Liliaceae). Am. J. Bot. 72, 433–437 (doi:10.2307/2443535) [Google Scholar]
- Thomson J. D., Rigney L. P., Karoly K., Thomson B. A.1994Pollen viability, vigor, and competitive ability in Erythronium grandiflorum (Liliaceae). Am. J. Bot. 81, 1257–1266 (doi:10.2307/2445401) [Google Scholar]
- Thomson J. D., Weiblen G., Thomson B. A., Alfaro S., Legendre P.1996Untangling multiple factors in spatial distributions: lilies, gophers, and rocks. Ecology 77, 1698–1715 (doi:10.2307/2265776) [Google Scholar]
- Widén B.1991Phenotypic selection on flowering phenology in Senecio integrifolius, a perennial herb. Oikos 61, 205–210 (doi:10.2307/3545338) [Google Scholar]
- Williams J. W., Jackson S. T.2007Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (doi:10.1890/070037) [Google Scholar]
- Williams P. H., Osborne J. L.2009Bumblebee vulnerability and conservation worldwide. Apidologie 40, 367–387 (doi:10.1051/apido/2009025) [Google Scholar]
- Wilson P., Thomson J. D., Stanton M. L., Rigney L. P.1994Beyond floral Batemania: sexual selection on pollination success. Am. Nat. 143, 283–296 (doi:10.1086/285604) [Google Scholar]
- Yamagishi H., Allison T. D., Ohara M.2005Effect of snowmelt timing on the genetic structure of an Erythronium grandiflorum population in an alpine environment. Ecol. Res. 20, 199–204 (doi:10.1007/s11284-004-0032-7) [Google Scholar]