Abstract
Terrestrial plants are powerful climate sentinels because their annual cycles of growth, reproduction and senescence are finely tuned to the annual climate cycle having a period of one year. Consistency in the seasonal phasing of terrestrial plant activity provides a relatively low-noise background from which phenological shifts can be detected and attributed to climate change. Here, we ask whether phytoplankton biomass also fluctuates over a consistent annual cycle in lake, estuarine–coastal and ocean ecosystems and whether there is a characteristic phenology of phytoplankton as a consistent phase and amplitude of variability. We compiled 125 time series of phytoplankton biomass (chlorophyll a concentration) from temperate and subtropical zones and used wavelet analysis to extract their dominant periods of variability and the recurrence strength at those periods. Fewer than half (48%) of the series had a dominant 12-month period of variability, commonly expressed as the canonical spring-bloom pattern. About 20 per cent had a dominant six-month period of variability, commonly expressed as the spring and autumn or winter and summer blooms of temperate lakes and oceans. These annual patterns varied in recurrence strength across sites, and did not persist over the full series duration at some sites. About a third of the series had no component of variability at either the six- or 12-month period, reflecting a series of irregular pulses of biomass. These findings show that there is high variability of annual phytoplankton cycles across ecosystems, and that climate-driven annual cycles can be obscured by other drivers of population variability, including human disturbance, aperiodic weather events and strong trophic coupling between phytoplankton and their consumers. Regulation of phytoplankton biomass by multiple processes operating at multiple time scales adds complexity to the challenge of detecting climate-driven trends in aquatic ecosystems where the noise to signal ratio is high.
Keywords: phenology, primary producer, chlorophyll a, aquatic systems, periodicity, wavelet analysis
1. Introduction
The past decade has seen explosive growth in the science of phenology (i.e. timing of periodic life-cycle events), largely because terrestrial plants are sensitive indicators of climate variability that now ‘provide some of the most compelling evidence that species and ecosystems are being influenced by global environmental change’ (Cleland et al. 2007; see also contributions to this volume, e.g. Ibáñez et al. 2010; Richardson et al. 2010). Observations of life-stage transitions of individual species (Menzel & Fabian 1999; Penuelas & Filella 2001; Ibáñez et al. 2010) and satellite-based indices of vegetation greenness (Myneni et al. 1997; Cleland et al. 2007) on land show that spring onset has advanced, autumn senescence has delayed, the growing season has lengthened, and these changes are correlated with rising temperatures in boreal and temperate ecosystems. Terrestrial plants are powerful climate sentinels because their annual cycles of growth, reproduction and senescence are finely tuned to the annual climate cycle. Although the timing of these life-history transitions varies among species (Menzel & Fabian 1999; Penuelas & Filella 2001) and regions (White et al. 2009), biomass of vegetation on land follows a recurrent cycle of growth and senescence with a 12-month periodicity (Myneni et al. 1997; Richardson et al. 2010). At mid and high latitudes, canopy greenness is controlled by temperature and photoperiod (Jolly et al. 2005), so a temperature increase of about 0.8°C in Eurasia and North America has advanced spring green-up by 4–6 days and delayed onset of senescence by 8–11 days over the past two decades (Zhou et al. 2001). Our ability to detect large-scale phenological shifts at this resolution is based on key life-history attributes of temperate-boreal plants: seasonal transitions between growth and senescence that have a fixed, 12-month periodicity; and recurrent timing of those transitions strongly cued to seasonal climate. This inherent consistency in the annual cycle of land plants (Lieh 1974) provides a low-noise background from which we can extract real signals of phenological change measured at the resolution of days, and then attribute those shifts to changes in climate (Richardson et al. 2010).
Whereas land plants have life histories adapted to the 12-month climate cycle, phytoplankton biomass turns over on the order of 100 times each year as a result of fast growth and equally fast consumption by grazers (Calbet & Landry 2004; Behrenfeld et al. 2006). Based on differences in their characteristic time scales of biomass turnover, we might expect differences in the periodicity of terrestrial plants and phytoplankton. However, a plankton phenology is not well developed. Phytoplankton blooms (Smayda 1997) are identifiable signals of the annual growth activity in pelagic systems. A well-described pattern is the spring bloom, a response to seasonal increases in temperature and solar radiation (Cushing 1959; Sommer et al. 1986) and regarded as the canonical phytoplankton pattern. This peak typically persists for a few weeks to months as nutrient limitation, cell sinking and grazing cause bloom collapse. A secondary biomass peak stimulated by excess nutrients can develop in late summer or autumn (Sommer et al. 1986; Longhurst 1995). These recurring annual phytoplankton cycles can be sensitive to changes in the climate system (Edwards & Richardson 2004; Winder & Schindler 2004b; Thackeray et al. 2008), analogous to climate-driven phenological shifts on land. However, annual phytoplankton patterns differ across ecosystems (Pratt 1959; Scheffer 1991; McQuatters-Gollop et al. 2008), have large year-to-year variability (Cloern & Jassby 2008; Paerl & Huisman 2008; Garcia-Soto & Pingree 2009) and are not strongly expressed in all aquatic ecosystems (Smayda 1998; Cloern & Jassby 2010). Whereas high variability of phytoplankton seasonal patterns is documented, no systematic analysis of phytoplankton annual cycles has been conducted to identify their characteristic periods of biomass variability and the recurrence strength at those periods.
The longest observations of phytoplankton in lakes, near shore coastal and oceanic waters extend back a few decades, with the earliest records starting in the 1930s (Hays et al. 2005). These observations were made during a period of significant global warming (IPCC 2007) and now provide an opportunity to determine if shifts in annual growth cycles of plankton are sentinels of warming across freshwater and marine ecosystems (Edwards & Richardson 2004; Winder & Schindler 2004a). From these observational programmes we compiled time series of phytoplankton biomass measured as chlorophyll-a (Chl-a) concentration from 125 estuarine–coastal, lake and oceanic sites in the temperate and subtropical region. We used wavelet analysis to extract the periodic components of variability in each series. Our purpose is to examine multi-year records of phytoplankton biomass to determine (i) if pelagic plants have a repeating annual cycle, (ii) the characteristic periods and (iii) recurrence strength at those periods. Do these communities have a characteristic annual pattern with a periodicity consistent enough so that phase shifts can be attributed to secular trends of global temperature? Or, does phytoplankton biomass have its own distinct phenological responses to climate variability?
2. How can we extract periodic components in ecological time series?
(a). Spectral analysis
Spectral analysis is an effective tool to search for cyclical behaviour in time series of unknown periodicities (Chatfield 1989). It decomposes data measured over time as the sum of sine waves of different frequency. In the spectrum, each frequency explains a proportion of the temporal variance of the series. A series that oscillates at a fixed frequency will have a large proportion of its variance explained by this frequency compared with a series oscillating at multiple frequencies. Spectral analysis has been used for detecting frequency-driven periodic fluctuations in time-series data. For example, Beninca et al. (2008) and Vasseur & Fox (2007) used Fourier transforms to extract cyclical patterns in plankton populations.
These traditional spectral analyses are well suited for stationary time series in which the statistical properties do not vary with time, but are unable to characterize signals whose frequency content changes over time as it uses sinusoidal functions that repeat continuously. However, ecological data are typically noisy, irregular and non-stationary. Wavelet analysis has emerged as a tool for characterizing periodicities in non-stationary time series, as it unfolds a time series not only in frequency, but also in time (Percival & Walden 2000). This method gives us the possibility of investigating and quantifying the temporal evolution of time series with different rhythmic components. Rather than a sinusoid, the method is based on a wavelet function that narrows when high-frequency features are present and widens on low-frequency structures (Daubechies 1992; Torrence & Compo 1998). Wavelet analysis has been widely applied across disciplines since its introduction in the early 1980s (for a historical account see Percival & Walden 2000), particularly in atmospheric and oceanic sciences and more recently to ecological time series (Jenouvrier et al. 2005; Keitt & Fisher 2006; Wittemyer et al. 2008).
(b). Wavelet analysis to determine phenological attributes
Here we introduce the basic approach of using wavelet analysis to extract periodic components from phytoplankton time series (more details are provided in the electronic supplementary material). Wavelet analysis performs a time-scale decomposition of the signal by estimating its spectral characteristics as a function of time (Torrence & Compo 1998). This approach reveals how the different scales (periodic components) of the time series change over time as the wavelet function is stretched in time by varying its scale (Daubechies 1992). In the present analysis, we used the continuous Morlet wavelet transform as the wavelet base function since it provides a good balance between time and frequency localization, which is desirable for feature extraction purposes (Grinsted et al. 2004). The Morlet function is essentially a damped complex exponential, which can capture local (in time) cyclical fluctuations in the time series. The frequency or the time range over which it fluctuates is set by a scale parameter. In general, wavelet scale is related to the conventional Fourier period of oscillations. For analyses of Chl-a series, a start scale of two months (twice the sampling interval) was specified and the spacing between the discrete scales, δj, was chosen as 1/12 (12 suboctaves per octave), and the number of octaves was set to 3.65, resulting in 44 scales ranging from two to 25 months.
Analogous to the traditional smoothed periodogram, the wavelet power spectrum can be averaged over time (Torrence & Compo 1998). The time-averaged or global wavelet spectrum identifies the scales or periods that are the most important sources of variability of the complete series. It is estimated by averaging the local wavelet power spectrum over the series duration, and it gives the distribution of power (or, equivalently, variance) among different frequencies. The wavelet spectra are scale dependent, so wavelet analysis can produce distorted power spectra by underestimating short-period peaks (Torrence & Compo 1998; Liu et al. 2007). Normalizing the power spectra by the corresponding scale corrects this problem so that spectral peaks can be compared across scales (Liu et al. 2007). We used the time-averaged wavelet spectrum corrected by scale to extract the dominant period and the recurrence of cyclical fluctuations in each Chl-a series. In order to compare the variance across sites, each series was normalized to zero mean and unit variance. The Matlab functions by Torrence & Compo (http://atoc.Colorado.edu/research/wavelets/) and Grinsted et al. (2004) were used for the wavelet analysis.
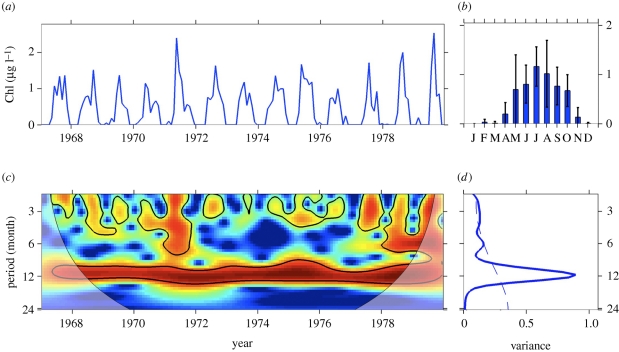
We illustrate application of wavelet analysis using a Chl-a time series from the North Atlantic (figure 1). The monthly series showed a recurrent cycle with an apparent 12-month period, and additional components of variability in some years. The mean annual cycle (monthly average Chl-a) at this site showed peak biomass in July. The wavelet power spectrum shows the decomposition of this series in time (along the x-axis) and period (along the y-axis) scale. It identified a strong annual cycle and confirmed a dominant 12-month periodicity (shown in red), and additional components of variability at shorter periods. The time-averaged spectrum showed that the 12-month periodicity was highly significant and explained the largest proportion of the variance of the time series.
Figure 1.
Wavelet analysis for the characterization of periodic frequencies of the North Atlantic Chl-a time series (57–62° N, 20–10° W) from 1967 to 1979. (a) Series of the Chl-a data, (b) monthly averages (± s.d.) over the sampling period, (c) continuous wavelet power spectrum showing the periodicity (shaded area indicates the region of time and frequency affected by the edges of the data and should not be considered; solid lines are significant (p < 0.05) coherent time–frequency regions) and (d) time-averaged wavelet spectrum of the series showing the dominance of the periods (dashed line shows the 95% significance level). Both the continuous and the time-averaged wavelet power spectra are shown in the base 2 logarithm. The continuous wavelet spectrum illustrates how the strength of the periodicities changed over time; colours indicate differing degrees of variance (dark red indicates high intensity; dark blue indicates low intensity). The time-averaged spectrum depicts the periods that explain a high proportion of the temporal variance of the series (y-axis) and the recurrence strength of the periods (x-axis). David Johns supplied data for the Chl-a time series as recorded by the Continuous Plankton Recorder green index, Sir Alister Hardy Foundation for Ocean Science, Plymouth (http://www.sahfos.org).
This example illustrates the power of wavelet analysis for visualizing the periodic components of variability in a time series, the strength of those periodic components, and their consistency over time. Patterns visualized with the wavelet power spectrum are integrated over time in the time-averaged spectrum, which extracts the dominant period(s) of variability in the full record and the explained variance of each period. We analysed synthetic time series (see the electronic supplementary material) to demonstrate that the explained variance of the time-averaged spectrum is a useful index to extract from time series two characteristic attributes of phenology: the important period(s) of variability (shown on the y-axis of the time-averaged wavelet spectrum in figure 1), and the recurrence strength of the dominant period(s) from year to year (shown on the x-axis of the time-averaged wavelet spectrum in figure 1). We applied this technique to Chl-a time series described below, and then compared results across sites.
3. Sources and limitations of chl-a time series data
(a). Data sources and screening
Chl-a concentration is a measure of phytoplankton biomass and proxy for primary production (Field et al. 1998). We compiled Chl-a measurements from monitoring and research programmes in lakes, estuarine–coastal waters, and oceanic sites. Satellite imagery has been used over the past two decades to routinely measure Chl-a variability in the ocean and on land (Garcia-Soto & Pingree 2009; Kahru et al. 2009). However, there are no operational programmes of remote sensing for measuring Chl-a in small lakes or estuaries and bays because of interference of the chlorophyll reflectance signal from dissolved coloured substances, suspended sediments and bottom reflectance (Lunetta et al. 2009). In addition, remote sensing is not feasible in ecosystems having dimensions of the same scale as pixel size of satellite imagery. Therefore, studies of phytoplankton biomass variability in lake and shallow coastal ecosystems are based largely on direct measurements of Chl-a in discrete water samples.
Time series of Chl-a were selected for analysis based on length and completeness of the data record. Each selected series had at least 8 years of data with at least 10 months of data for each year, and each month was sampled for at least 6 years. For lake sites where Chl-a measurements were consistently missing during one or two months of the year (usually the months of ice-on and ice-off) we relaxed the latter criterion. Chl-a series meeting these criteria were compiled across a wide geographical, trophic and morphological spectrum that included 70 estuarine–coastal, 50 lakes and five ocean time series (yielding 2,236 site-years) from sites between 22° and 60° latitude and from sea level to 1994 m elevation. The length of the Chl-a series ranged from 8 to 50 years and had a median of 16 years duration. Site location, description, and sources of Chl-a time series are provided in the electronic supplementary material, table S1 and figure S3.
The depth and frequency of Chl-a sampling varied across sites. We used surface measurements from estuarine–coastal ecosystems, oceanic sites and shallow lakes, but mean values over the euphotic zone for deep lakes (except Lake Constance), and the Hawaii and Bermuda Atlantic series. Most sites were sampled at monthly frequency; series collected at higher frequency were aggregated on a monthly basis using the mean. Missing monthly values were interpolated by the long-term mean of that month adjusted for that year's mean Chl-a using the decomposition method described by Cloern & Jassby (2010) (see the electronic supplementary material for more details). We used R v. 2.10.0 (R Development Core Team 2009) and its contributed packages for data preparation for wavelet analysis.
(b). Monthly sampling frequency as a limitation to assess annual cycles
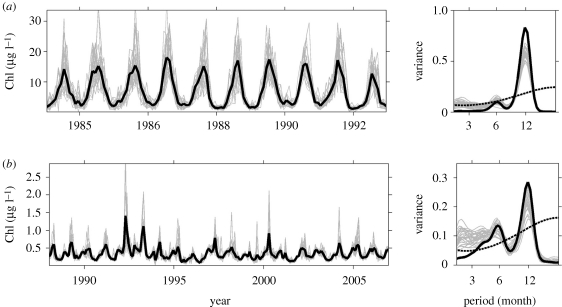
Phytoplankton biomass varies across the full spectrum of time scales at which we can make measurements (Cloern 1996), but most research and monitoring programmes sustained longer than a decade have measured phytoplankton biomass at monthly frequency. This limitation of the available data is a (potentially large) source of error in analyses to extract periodicity of within-year Chl-a variability. We used a bootstrap approach to estimate this error, using Chl-a series measured (near-) daily in North Inlet Estuary and Gulf of Aqaba. We resampled each of these series at a monthly sampling interval that varied randomly for 7 days to construct simulated series of monthly sampling. We then extracted the dominant period(s) of variability in each simulated monthly series using wavelet analysis (as described above). These simulated time series were compared with the actual monthly Chl-a series generated from the daily data (figure 2). Per cent deviations of simulated averages (xsim) from actual Chl-a averages (xobs) were calculated as 100 |xsim—xobs| / xobs.
Figure 2.
Comparison of monthly time series and periodicity of Chl-a based on daily data (thick black line) and simulated monthly sampling (thin grey lines). For each series (a,b), the left panel shows the Chl-a series and the right panel the time-averaged wavelet spectrum of the series showing the dominance of the periods (dashed line shows the 95% significance level of the actual data based on daily sampling). The Chl-a series are from (a) North Inlet Estuary (site OL), 1983–1992; (b) Gulf of Aqaba (Eilat), 1988–2006. Data are provided by (a) http://links.baruch.sc.edu/Data/CoastalData.html and (b) Amatzia Genin (Steinitz Marine Biology Laboratory, The Hebrew University).
Simulated monthly averages of Chl-a differed from actual monthly averages by 34 ± 4 per cent for the North Inlet and 19 ± 3 per cent for the Gulf of Aqaba Chl-a series. Although monthly frequency sampling over- or underestimated bloom peaks (figure 2, left panels), it provided a basis for identifying the dominant period(s) of variability at sites where annual cycles were present (figure 2, right panels). The actual dominant periodicity was always captured with discrete monthly sampling, but the explained variance of the spectra was often lower compared with sampling at daily frequency. This implies that discrete monthly sampling may underestimate the recurrence strength of the actual annual cycle. Jassby et al. (2004) used a similar bootstrap approach and concluded that much of the basic structure of phytoplankton variability is captured with sampling at monthly frequency. Further, sampling over many years increases the robustness of time-averaged spectra as the annual pattern is resampled many times. However, a definitive measure of the magnitude of the sampling error will not be feasible until decadal records of high-frequency Chl-a variability become available across a range of ecosystem types.
4. Characteristics and periodicities of phytoplankton time series
(a). Phytoplankton biomass variability within and between sites
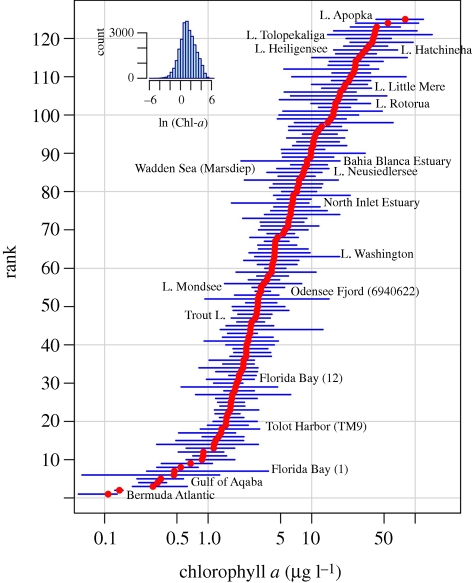
The Chl-a series analysed here were collected across a broad range of trophic states. Median annual Chl-a concentration ranged from 0.11 µg l−1 in oligotrophic waters such as Bermuda Atlantic (BATS) to 80 µg l−1 in ultraproductive systems such as Lake Apopka (figure 3). Annual mean Chl-a concentration at individual sites was highly variable from year to year, ranging, for example, between 2.8 and 18.6 µg l−1 in Lake Washington and between 6.2 and 53 µg l−1 in lake Little Mere. Ten-fold variability of mean annual biomass and, therefore, amplitude of the annual cycle, appears to be a common feature of phytoplankton dynamics (Cloern & Jassby 2008).
Figure 3.
Median (filled dots) and range (bars) of the annual mean phytoplankton biomass (Chl-a) at 70 estuarine–coastal sites, 50 lakes and five ocean sites. Sites are ordered by their medians and described in the electronic supplementary material, table S1. Although the length of the records varied (from 8 to 50 years) there was no relationship between the length of the dataset and the coefficient of variation of annual mean Chl-a (R2 = 0.01, p = 0.19). The insert shows the frequency distribution of log-transformed monthly Chl-a values across all sites. L. = Lake.
(b). Annual patterns revealed by wavelet analysis
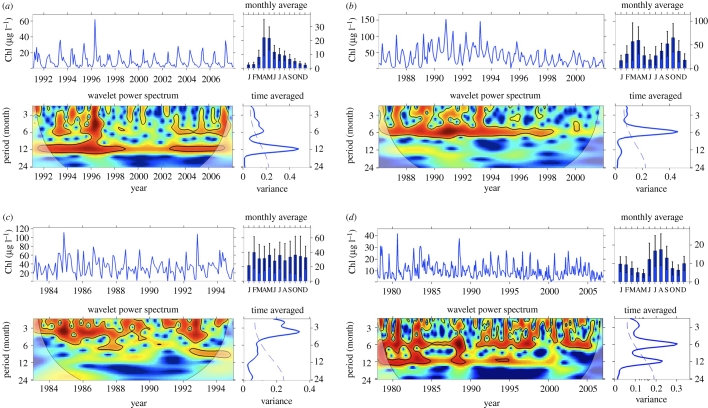
Analyses of 125 Chl-a time series revealed four annual patterns in the wavelet power spectra as peaks at periods of either 12, 6 or 2–4 months, or mixed patterns, such as 12- and six-month periodicity. We selected examples to illustrate these four patterns. The Wadden Sea (site Marsdiep noord) time series (figure 4a) exemplified an annual cycle with one dominant bloom, in this case with peak biomass in April and May. The continuous wavelet power spectrum revealed a persistent 12-month periodicity, which explained the largest amount of variability over the sampling period. It also revealed periodic patterns at six-month scale, but these explained a small amount of the variance. In contrast, the Chl-a series from Lake Heiligensee (figure 4b) exemplified a six-month periodicity of a spring and autumn bloom pattern of temperate lakes. The continuous wavelet spectrum showed that the six-month pattern was persistent in Heiligensee until the late 1990s and then weakened. The continuous spectrum also identified variability at periods less than six months, but these disappeared in the mid 1990s. The time-averaged spectrum showed that the six-month period explained most of the overall variability in this lake (figure 4b).
Figure 4.
Four examples of Chl-a series that illustrate common annual patterns of variability. For each series (a–d), the top left panel shows the Chl-a series, the top right monthly averages (± standard variation), bottom left the continuous wavelet power spectrum, and bottom right panel the time-averaged wavelet spectrum. The Chl-a series are from (a) Wadden Sea (Marsdiep nord), 1991–2007; (b) Lake Heiligensee, 1986–2001; (c) Lake Hatchineha, 1983–1994 and (d) Bahia Blanca Estuary (site Cuatreros), 1978–2006 (the years 1985 and 1986 were interpolated to obtain a continuous time series). See figure 1 for further information on wavelet spectra. The series were normalized so the explained variances of the time-averaged spectra can be compared across sites. Data are provided by (a) Jacco Kromkamp (http://www.waterbase.nl), (b) Rita Adrian (Leibniz-Institute of Freshwater and Inland Fisheries), (c) Karl Havens (UF/IFAS Fisheries and Aquatic Sciences) and (d) Hugo Freije (Universidad Nacional del Sur).
Phytoplankton biomass variability in Lake Hatchineha did not follow either of the canonical patterns of six- or 12-month cycles. Instead, Chl-a fluctuated here at periods from two to four months (figure 4c), indicative of a noisy time series with high frequency variability. The series of monthly average Chl-a also showed that phytoplankton biomass did not fluctuate over a regular annual cycle in this lake. The explained variance of this Chl-a series was small; thus the less than four-month pattern was not strongly expressed. Weakness of the less than four-month pattern was also reflected in the continuous wavelet power spectrum showing that it disappeared in 1990. The 12- and six-month periods (figure 4a,b) are well-established features of phytoplankton variability, but variability in the 2–4 month band is not. We interpret this short-period pattern as either: (i) variability dominated by irregular, short-term bloom events, (ii) variability at periods shorter than six months and attributable to serial correlation of the series, or (iii) sampling error, especially at sites where only one or a few measurements were taken per month and the short-period components of variability cannot be resolved.
The fourth pattern revealed by wavelet analysis was mixed, with variability detected at both six- and 12-month periods, exemplified in the time-averaged spectrum of the Chl-a series from Bahia Blanca Estuary (figure 4d). The six-month period explained the highest absolute amount of variance over the sampling period, but the 12-month period was also significant. The continuous wavelet transform identified a shift that occurred around 1990, from a dominant 12-month period (and its possible subharmonics at six and three months) to a dominant six-month period. This example illustrates the non-stationary character of phytoplankton time series, and the utility of the wavelet power spectrum for visualizing changes in periodic behaviour over time. In this case, the presence of two dominant periods shown in the time-averaged spectrum is explained in the continuous spectrum as a shift from a 12- to a six-month cycle. A few sites (e.g. Florida Bay (site 1), Lake Apopka) had no detectable periodic components, indicative of irregular biomass variability.
(c). Identification of the dominant period
The Bahia Blanca Estuary Chl-a series demonstrated that annual cycles of phytoplankton variability can change over time. This implies that series length can be critical if we select the dominant periods as those with highest explained absolute variance, particularly since series lengths ranged from 8 to 50 years. To account for different sampling duration, we standardized the length of the time series by applying a sliding window bootstrap approach of 10 years (or the entire series for sites with 8 and 9 years of observation, respectively). This approach yielded 1146 10-year records of monthly Chl-a from which we extracted the dominant periods of variability and the explained variance of each period. We then identified the dominant period of variability in each 10-year window and counted proportional occurrences of each pattern (no, 2–4, 6- and 12-month period) over the entire sampling period for each site. The counts in each pattern type were summed across sites and divided by the total number of sites to calculate overall proportions of each period. The wavelet variance of each site for each period was averaged to calculate the explained variance of a site.
(d). Phytoplankton periodicity in lake, estuarine–coastal and ocean ecosystems
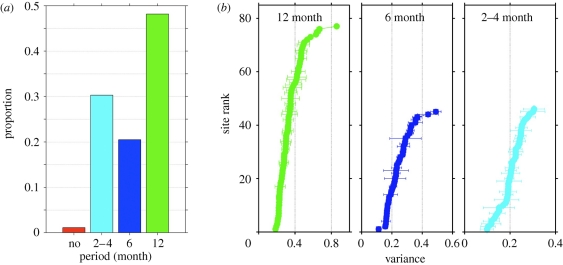
Nearly half (48%) of the Chl-a series examined here had a dominant 12-month cycle (figure 5a) corresponding to one peak per year. The phasing of these annual cycles varied, however, across ecosystems, which can have peak biomass any month of the year (Cloern & Jassby 2008). We showed, for example, summer Chl-a maxima in the North Atlantic (figure 1) but winter–spring peaks in the Bahia Blanca Estuary (figure 4d). The six-month period, diagnostic of two peaks per year (such as spring–autumn or summer–winter cycles), was dominant in about 20 per cent of the series and more common in lake (25%) and oceanic (50%) sites than in estuarine–coastal (16%) ecosystems (data not shown). A third (31%) of the series showed a pattern best attributed to the 2–4 month band periodicity, and for 1 per cent no cyclic patterns were detected (see the electronic supplementary material, table S1 for the dominant period of each site).
Figure 5.
Dominant periodicities and recurrence strength of phytoplankton biomass (Chl-a) patterns. (a) Proportion of dominant periodicities over all estuarine–coastal, lake and oceanic sites. (b) Strength of the recurrence pattern of the 12-, 6-, and 2–4-month periodicities. Horizontal bars show the mean (± s.d.) of the 10-year sliding bootstrap window.
The 12-month period had the strongest recurrence, with an average explained variance of 0.35 ± 0.12 (figure 5b), suggesting that one bloom per year is the most predictable signal. However, explained variance of the 12-month cycle varied across sites, ranging from 0.2 to 0.9 and was highest at North Inlet Estuary and Dorset Ontario Lakes, and lowest at sites such as Lake Rotorua. Therefore, even where 12-month cycles were present their phase and amplitude were not necessarily recurrent from year to year. This finding is consistent with observations that the seasonal timing of biomass peaks is variable within some estuarine–coastal ecosystems (Cloern & Jassby 2008). Analysis of the longer Chl-a series also showed that recurrence strength of the 12-month cycle varied among their 10-year segments, illustrated as error bars around the mean recurrence strength at each site (figure 5b).
The 6- and 2–4-month periods explained smaller proportions of Chl-a variance (0.25 ± 0.08 and 0.21 ± 0.05, respectively) compared with the 12-month cycle (figure 5b). Trout Lake, Lake Heiligensee and Odense Fjord (site 6940622) had strongly recurring six-month cycles, whereas Lake Mondsee had weakly recurring six-month cycles. The 2–4 month scale was most recurrent in the series from Lake Hatchineha, Lake Tolopekaliga and coastal site Tolo Harbor (site TM9) and least recurrent at Lake Neusiedlersee and Florida Bay (site 12).
5. Annual cycles of phytoplankton biomass
Our analysis and comparison of many Chl-a series revealed the following annual patterns of phytoplankton variability:
— 12-month periodicity. The most commonly observed pattern was one phytoplankton peak per year, the canonical annual cycle of temperate lakes and oceans (Cushing 1959; Sommer et al. 1986). Spring blooms are tuned to the seasonal increase in solar radiation and thermal stratification after winter mixing redistributes nutrients to surface waters. In large estuaries, such as Chesapeake Bay, the spring bloom is a response to high river flow that delivers nutrients and freshwater to establish salinity stratification (Harding & Perry 1997). The phasing, duration and intensity of annual blooms can vary from year to year within single ecosystems; for example, the annual phytoplankton maximum in Narragansett Bay occurred between winter–spring and mid-August and its magnitude ranged more than 10-fold over the last decades (Smayda 1998). High interannual variability of the 12-month cycle is reflected in the low observed wavelet variance detected in many Chl-a series (figure 5). This variability arises from many interactive processes of bloom dynamics, including external forcing (meteorological conditions, resource availability) and internal multispecies interactions (Dakos et al. 2009; Platt et al. 2010).
— Six-month periodicity. This bimodal pattern is characteristic of two peaks per year, such as spring and autumn or summer and winter blooms. It is also associated with seasonal changes in mixing intensity, nutrient availability, grazing and shifts in phytoplankton community structure. Nutrient depletion and increasing zooplankton grazing typically cause breakdown of spring blooms and maintain low phytoplankton biomass during summer; and in nutrient-rich systems grazing-resistant algal species can give rise to a second bloom later in the year (Sommer et al. 1986). In temperate lakes (Reynolds 2006), oceans (Longhurst 1995) and coastal basins (Longhurst 1995; Li et al. 2010), a secondary bloom in autumn is often fuelled by transport of nutrient-rich deep waters to the surface as stratification is eroded by surface cooling and convective mixing. Further, in turbid estuaries where phytoplankton is controlled by light availability, winter blooms can be triggered by increasing solar penetration caused by a reduction of suspended sediments owing to low river inflow or reduced wind stress (Guinder et al. 2009). Similar to the unimodal patterns, these bimodal patterns are highly variable and secondary blooms may not appear regularly.
— Short-term fluctuation. About a third of the series we examined were dominated by short-periodic fluctuations. These were more common in coastal–estuarine sites (39%) compared with lakes (25%) and were not a dominant pattern in the ocean time series. Irregular blooms are often responses to short-term climatic events that change temperature and mixing dynamics. For example, storm series can cause a sequence of biomass oscillations by breaking down phytoplankton blooms that build during intervening calm periods of low turbulence (Garcia-Soto & Pingree 2009). Short-term fluctuations also result from individual species succession causing stochastic bloom occurrence: coccolithophores, dinoflagellates, cyanobacteria or diatoms can produce massive blooms under favourable growing conditions (Smayda 1998; Siegel et al. 2007; Paerl et al. 2010). Wind events that influence exchange of water masses with the ocean can also cause random, short-lived blooms in estuaries and coastal lagoons (Abreu et al. 2010).
— Shift in periodicity. Our analysis showed that approximately a third of the sites had different dominant periods of variability within a 10-year sampling window (data not shown), indicating that shifts in the annual phytoplankton cycle are common. The example of the Bahia Blanca Estuary (figure 4c) showed that annual cycles can change abruptly. In this estuary, the once dominant 12-month cycle (winter blooms) was replaced by a six-month periodicity. Shifts in annual cycles have been observed in other ecosystems, such as weakening of the autumn bloom in the Dutch Wadden Sea over past decades (Philippart et al. 2010), or a shift from winter biomass maxima to pronounced summer or autumn biomass buildup in Narragansett Bay (Karentz & Smayda 1998; Borkman & Smayda 2009).
— No periodic pattern. Some sites revealed episodic bloom events with no recurring pattern or suppressed cycles. For example, no dominant annual pattern was apparent in hypereutrophic Lake Apopka over the entire sampling period, and when periodic cycles (12-month or short-term fluctuations) were present they only persisted for a few years. The absence of a periodic pattern in this shallow nutrient-rich lake is probably associated with the year-round dominance of cyanobacteria and low zooplankton grazing due to high fish predation (Havens et al. 2009).
All these pattern types were found in both lakes and estuarine–coastal sites. In contrast, the open ocean series had 12- and six-month periodicities, although the Hawaii Ocean time series (HOT) contained episodic short-term variability (data not shown). In addition, the predominant annual bloom pattern was not related to Chl-a concentration (ANOVA; F1,120 = 1.36, p = 0.2), suggesting that trophic condition did not affect the annual phytoplankton cycle. These observed patterns of phytoplankton periodicity in the temperate and subtropical zone probably extend to the tropics. Melack (1979) found no uniform pattern in seasonal phytoplankton dynamics of tropical lakes. In these low-latitude sites with absence of distinct seasonal change in radiation and temperature, phytoplankton dynamics can vary from strong seasonal fluctuations to more damped fluctuations and sudden change in the seasonal dynamics. A latitudinal gradient in annual patterns is expected (e.g. Harris 1986), with a consistent unimodal pattern predominating at high latitudes and progressive weakening and irregularity of an annual cycle at lower latitudes. Our results were consistent with this expectation as the 12-month periodicity dominated in northern sites and the less than four-month periodicity was most common in lakes and estuarine–coastal sites below 30° latitude. However, almost all of the subtropical sites were located in Florida (see the electronic supplementary material, figure S3). A definitive identification of latitudinal gradients in the phasing and amplitude of phytoplankton annual cycles will require time series across a more globally representative suite of low-latitude ecosystems.
Our findings show that phytoplankton biomass does not follow one annual cycle, that the phase and amplitude of annual cycles vary from year-to-year, and that phytoplankton time series are non-stationary. High annual variability appears to be a fundamental attribute of phytoplankton growth–senescence cycles. These fast-growing cells can respond rapidly to favourable environmental conditions by exponential population growth, and tight predator–prey coupling can cause equally rapid bloom collapse. In addition, phytoplankton boom–bust cycles are not always a response of the entire community but often a reflection of population succession of individual taxa. Species-specific variation in resource requirement and trophic interactions can generate complex dynamical behaviours and trigger episodic bloom events (Dakos et al. 2009). Consequently, fast biomass turnover of this physiologically and morphologically diverse group suggests that blooms can occur with varying intensity and at different times each year.
6. Phytoplankton phenology as an indicator of global change
Phytoplankton biomass fluctuates in synchrony with the annual climate cycle in many lakes, oceans and estuaries, analogous to terrestrial plants (Richardson et al. 2010). These periodic annual cycles are linked to annual fluctuations of mixing, temperature, light and precipitation (Smetacek 1985; Sommer et al. 1986; Cloern 1996). Changing climatic conditions can modify these environmental factors and alter phytoplankton annual cycles directly or indirectly by altering resource availability and trophic interactions. For example, vernal warming advanced the timing of stratification onset and the spring bloom in Lake Washington by more than 20 days over the past four decades (Winder & Schindler 2004b). Shifts in bloom timing were also observed in the Western Scheldt Estuary, where earlier onset of blooms paralleled increasing temperature over the past 30 years (Kromkamp & Van Engeland 2010). Similarly, a shift to the warm phase of the North Atlantic Oscillation caused advancement of stratification onset and the spring bloom in the Baltic Sea (Smayda et al. 2004; Alheit et al. 2005), and accelerated early summer algal suppression due to faster growth of herbivores in warmer water across central European lakes (Straile 2002). Similarly, new autumn phytoplankton blooms developed in San Francisco Bay through a trophic cascade induced by a shift of the east Pacific to its ‘cool’ phase in 1999 (Cloern et al. 2007). So, phytoplankton time series are beginning to reveal responses to trends or multidecadal oscillations of climate variability.
However, phytoplankton biomass also responds strongly to climatic variability at shorter time scales. Heatwaves, nutrient run-off from storms, upwelling events or drought-induced increases of water residence time can all trigger algal blooms. Massive blooms of cyanobacteria and dinoflagellates (red tides) are often responses to anomalous warm temperatures and stable stratification (Huisman et al. 2004; Hall et al. 2008; Jöhnk et al. 2008). Extreme nutrient run-off produced by tropical storms triggered dinoflagellate blooms in the Neuse River estuary (Hall et al. 2008) and increased Chl-a in Florida Bay (Briceño & Boyer 2010). In the Black Sea warm temperature and low wind stress can trigger unusually large blooms throughout the entire growing period (McQuatters-Gollop et al. 2008). Similarly, increased wind stress, water temperature and reduced water run-off affected the phytoplankton dynamics in Boreal lakes (Schindler et al. 1990).
These examples illustrate that the climate system induces changes in phytoplankton fluctuation through many processes operating at many time scales in addition to the annual cycle, affecting both the timing and intensity of blooms. However, phytoplankton variability is not tuned to the annual climate cycle at all sites, and large changes in phytoplankton patterns have been caused by processes disconnected from climate variability, most notably by human disturbances (Schindler 2001). Confounding factors and interacting effects of climate and local-scale variability can disrupt or mask phytoplankton responses caused by climate and meteorological forcing. Prominent local drivers of annual phytoplankton variability include alterations of nutrient loading and trophic interactions. Cultural eutrophication or reduction of nutrient loading can cause massive change in phytoplankton dynamics (Anneville et al. 2005; Jeppesen et al. 2005) and modify the temporal succession and amplitude of phytoplankton fluctuations (Sommer et al. 1993; Verity & Borkman 2010). Similarly, disruption of food-webs by introduced species can profoundly alter phytoplankton biomass (Alpine & Cloern 1992; Fahnenstiel et al. 1995; Makarewicz et al. 1999). Removal of top predators by fishing or introduction of non-indigenous species can also affect phytoplankton dynamics through trophic cascades (Frank et al. 2005; Casini et al. 2009). Rates and the relative importance of these processes vary across sites, leading to diverse patterns of biomass fluctuation. The exposure of phytoplankton to a range of global-change factors and local drivers explains the heterogeneity of annual fluctuations observed in our analysis, indicating that climate is one of several factors that shape pelagic seasonality. The processes driving variation in bloom formation are sometimes not fully understood as the interplay between physical, chemical and food-web processes can lead to complex annual patterns. Finally, chlorophyll measurements combine the response of many species, whereas single species can show opposing patterns, which can obscure phenological responses of phytoplankton to changing climate (Edwards & Richardson 2004).
The underlying basis for phenology as a sensitive biological indicator of climate change is that seasonal activity is finely tuned to the annual climate cycle. The emergence of multidecadal datasets is now beginning to reveal that phytoplankton communities undergo distinct seasonal and interannual changes in response to climate change. However, applications of phenology to detect gradual shifts in the annual growth–senescence cycle will be difficult at those sites where phytoplankton biomass does not closely track the annual climate cycle. The potential for detecting ecological responses to climate change therefore varies by site, depending on the relative importance of the annual climate cycle versus other drivers of phytoplankton variability. Phytoplankton variability is a key driver of biogeochemical variability (Cloern 1996; Behrenfeld et al. 2006) and fluctuations in annual fish recruitment (Platt et al. 2003). An improved understanding of the inherent natural variability of phytoplankton is therefore important for forecasting the extent of global change impact on aquatic ecosystem functioning.
Acknowledgements
We sincerely appreciate the generosity of the researchers and programme managers for sharing datasets for this comparative analysis. The institutions and site investigators are listed in the electronic supplementary material. We thank Alan D. Jassby for helpful advice and recommendations, Yonggang Liu for help with wavelet analysis and Daniel E. Schindler and three anonymous reviewers for valuable comments on the manuscript. This research was supported by the CALFED Science Program under Grant no. R/SF-36 (CalFed U-04-SC-005) and the US Geological Survey Toxic Substances Hydrology Program and the National Research Program for Hydrologic Research.
Footnotes
One contribution of 11 to a Theme Issue ‘The role of phenology in ecology and evolution’.
References
- Abreu P. C., Bergesch M., Proença L. A., Garcia C. A. E., Odebrecht C.2010Short- and long-term chlorophyll a variability in the shallow microtidal Patos Lagoon Estuary, Southern Brazil. Estuaries and Coasts 33, 554–569 (doi:10.1007/s12237-009-9181-9) [Google Scholar]
- Alheit J., Mollmann C., Dutz J., Kornilovs G., Loewe P., Mohrholz V., Wasmund N.2005Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. ICES J. Mar. Sci. 62, 1205–1215 (doi:10.1016/j.icesjms.2005.04.024) [Google Scholar]
- Alpine A. E., Cloern J. E.1992Trophic interactions and direct physical effects control phytoplankton biomass and production in an estuary. Limnol. Oceanogr. 37, 946–955 (doi:10.4319/lo.1992.37.5.0946) [Google Scholar]
- Anneville O., Gammeter S., Straile D.2005Phosphorus decrease and climate variability: mediators of synchrony in phytoplankton changes among European peri-alpine lakes. Freshwater Biol. 50, 1731–1746 (doi:10.1111/j.1365-2427.2005.01429.x) [Google Scholar]
- Behrenfeld M. J., et al. 2006Climate-driven trends in contemporary ocean productivity. Nature 444, 752–755 (doi:10.1038/nature05317) [DOI] [PubMed] [Google Scholar]
- Beninca E., Huisman J., Heerkloss R., Jöhnk K. D., Branco P., Van Nes E. H., Scheffer M., Ellner S. P.2008Chaos in a long-term experiment with a plankton community. Nature 451, 822–827 (doi:10.1038/nature06512) [DOI] [PubMed] [Google Scholar]
- Borkman D. G., Smayda T.2009Multidecadal (1959–1997) changes in Skeletonema abundance and seasonal bloom patterns in Narragansett Bay, Rhode Island, USA. J. Sea Res. 61, 84–94 (doi:10.1016/j.seares.2008.10.004) [Google Scholar]
- Briceño H. O., Boyer J. N.2010Climatic controls on phytoplankton biomass in a sub-tropical estuary, Florida Bay, USA. Estuaries and Coasts 33, 541–553 (doi:10.1007/s12237-009-9189-1) [Google Scholar]
- Calbet A., Landry M.2004Phytoplankton growth, microzooplankton grazing, and carbon cycling in marine systems. Limnol. Oceanogr. 49, 51–57 [Google Scholar]
- Casini M., Hjelm J., Molinero J. C., Lovgren J., Cardinale M., Bartolino V., Belgrano A., Kornilovs G.2009Trophic cascades promote threshold-like shifts in pelagic marine ecosystems. Proc. Natl Acad. Sci. USA 106, 197–202 (doi:10.1073/pnas.0806649105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatfield J. R.1989The analysis of time series: an introduction. London, UK: Chapman & Hall [Google Scholar]
- Cleland E. E., Chuine I., Menzel A., Mooney H. A., Schwartz M. D.2007Shifting plant phenology in response to climate change. Trends Ecol. Evol. 22, 357–365 (doi:10.1016/j.tree.2007.04.003) [DOI] [PubMed] [Google Scholar]
- Cloern J. E.1996Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Rev. Geophys. 34, 127–168 (doi:10.1029/96RG00986) [Google Scholar]
- Cloern J. E., Jassby A. D.2008Complex seasonal patterns of primary producers at the land–sea interface. Ecol. Lett. 11, 1294–1303 (doi:10.1111/j.1461-0248.2008.01244.x) [DOI] [PubMed] [Google Scholar]
- Cloern J. E., Jassby A.2010Patterns and scales of phytoplankton variability in estuarine–coastal ecosystems. Estuaries and Coasts 33, 230–241 (doi:10.1007/s12237-009-9195-3) [Google Scholar]
- Cloern J. E., Jassby A. D., Thompson J. K., Hieb K. A.2007A cold phase of the East Pacific triggers new phytoplankton blooms in San Francisco Bay. Proc. Natl Acad. Sci. USA 104, 18 561–18 565 (doi:10.1073pnas.0706151104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushing D. H.1959The seasonal variation in oceanic production as a problem in population dynamics. J. Cons. Cons. Perm. Int. Explor. Mer. 24, 455–464 [Google Scholar]
- Dakos V., Benincà E., van Nes E. H., Philippart C. J. M., Scheffer M., Huisman J.2009Interannual variability in species composition explained as seasonally entrained chaos. Proc. R. Soc. B 276, 2871–2880 (doi:10.1098/rspb.2009.0584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubechies I.1992Ten lectures on wavelets. SIAM monographs. Philadelphia, PA: SIAM [Google Scholar]
- Edwards M., Richardson A. J.2004Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430, 881–884 (doi:10.1038/nature02808) [DOI] [PubMed] [Google Scholar]
- Fahnenstiel G. L., Bridgeman T. B., Lang G. A., McCormick M. J., Nalepa T. F.1995Phytoplankton productivity in Saginaw Bay, Lake Huron: effects of zebra mussel (Dreissena polymorpha) colonization. J. Great Lakes Res. 21, 465–475 [Google Scholar]
- Field C. B., Behrenfeld M. J., Randerson J. T., Falkowski P.1998Primary production of the biosphere: integrating terrestrial and oceanic components. Science 281, 237–242 (doi:10.1126/science.281.5374.237) [DOI] [PubMed] [Google Scholar]
- Frank K. T., Petrie B., Choi J. S., Leggett W. C.2005Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623 (doi:10.1126/science.1113075) [DOI] [PubMed] [Google Scholar]
- Garcia-Soto C., Pingree R. D.2009Spring and summer blooms of phytoplankton (SeaWiFS/MODIS) along a ferry line in the Bay of Biscay and western English Channel. Continental Shelf Res. 29, 1111–1122 (doi:10.1016/j.csr.2008.12.012) [Google Scholar]
- Grinsted A., Moore J. C., Jevrejeva S.2004Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 11, 561–566 (doi:10.5194/npg-11-561-2004) [Google Scholar]
- Guinder V. A., Popovich C. A., Perillo G. M. E.2009Particulate suspended matter concentrations in the Bahia Blanca Estuary, Argentina: implication for the development of phytoplankton blooms. Estuarine Coastal Shelf Sci. 85, 157–165 (doi:10.1016/j.ecss.2009.05.022) [Google Scholar]
- Hall N. S., Litaker R. W., Fensin E., Adolf J. E., Bowers H. A., Place A. R., Pearl H. W.2008Environmental factors contributing to the development and demise of a toxic dinoflagellate (Karlodinium veneficum) bloom in a shallow, eutrophic, lagoonal estuary. Estuaries and Coasts 31, 1559–2723 (doi:10.1007/s12237-008-9035-x) [Google Scholar]
- Harding L. W., Perry E. S.1997Long-term increase of phytoplankton biomass in Chesapeake Bay, 1950–94. Mar. Ecol. Progr. Ser. 157, 39–52 (doi:10.3354/meps157039) [Google Scholar]
- Harris G. P.1986Phytoplankton ecology: structure, function, and fluctuation. London, UK: Chapman and Hall [Google Scholar]
- Havens K. E., Elia A. C., Taticchi M. I., Fulton R. S.2009Zooplankton-phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 628, 165–175 (doi:10.1007/s10750-009-9754-4) [Google Scholar]
- Hays G. C., Richardson A. J., Robinson C.2005Climate change and marine plankton. Trends Ecol. Evol. 20, 337–344 (doi:10.1016/j.tree.2005.03.004) [DOI] [PubMed] [Google Scholar]
- Huisman J., Sharples J., Stroom J. M., Visser P. M., Kardinaal W. E. A., Verspagen J. M. H., Sommeijer B.2004Changes in turbulent mixing shift competition for light between phytoplankton species. Ecology 85, 2960–2970 (doi:10.1890/03-0763) [Google Scholar]
- Ibáñez I., Primack R. B., Miller-Rushing A. J., Ellwood E., Higuchi H., Don Lee S., Kobori H., Silander J. A.2010Forecasting phenology under global warming. Phil. Trans. R. Soc. B 365, 3247–3260 (doi:10.1098/rstb.2010.0120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC 2007Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press [Google Scholar]
- Jassby A. D., Müller-Solger A. B., Vayssières M.2004Short-term variability of chlorophyll and implications for sampling frequency in the San Joaquin River. IEP Newsletter 18, 21–28 [Google Scholar]
- Jenouvrier S., Weimerskirch H., Barbraud C., Park Y. H., Cazelles B.2005Evidence of a shift in the cyclicity of Antarctic seabird dynamics linked to climate. Proc. R. Soc. B 272, 887–895 (doi:10.1098/rspb.2004.2978) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen E., Sondergaard M., Jensen A. P., Havens K., et al. 2005Lakes' response to reduced nutrient loading—an analysis of contemporary data from 35 European and North American long term studies. Freshwater Biol. 50, 1747–1771 (doi:10.1111/j.1365-2427.2005.01415.x) [Google Scholar]
- Jöhnk K. D., Huisman J., Sharples J., Sommeijer B., Visser P. M., Stroom J. M.2008Summer heatwaves promote blooms of harmful cyanobacteria. Global Change Biol. 14, 495–512 (doi:10.1111/j.1365-2486.2007.01510.x) [Google Scholar]
- Jolly W., Nemani R., Running S.2005A generalized, bioclimatic index to predict foliar phenology in response to climate. Global Change Biol. 11, 619–632 (doi:10.1111/j.1365-2486.2005.00930.x) [Google Scholar]
- Kahru M., Kudela R., Manzano-Sarabia M., Mitchell B. G.2009Trends in primary production in the California current detected with satellite data. J. Geophys. Res.—Oceans 114, C02004 (doi:10.1029/2008JC004979) [Google Scholar]
- Karentz D., Smayda T. J.1998Temporal patterns and variations in phytoplankton community organization and abundance in Narragansett Bay during 1959–1980. J. Plankton Res. 20, 145–168 (doi:10.1093/plankt/20.1.145) [Google Scholar]
- Keitt T. H., Fischer J.2006Detection of scale-specific community dynamics using wavelets. Ecology 87, 2895–2904 (doi:10.1890/0012-9658(2006)87[2895:DOSCDU]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Kromkamp J. C., Van Engeland T.2010Changes in phytoplankton biomass in the Western Scheldt Estuary during the period 1978–2006. Estuaries and Coasts 33, 270–285 (doi:10.1007/s12237-009-9215-3) [Google Scholar]
- Li W. K. W., Lewis M. W., Harrison W. G.2010Multiscalarity of the nutrient–chlorophyll relationship in coastal phytoplankton. Estuaries and Coasts 33, 440–447 (doi:10.1007/s12237-008-9119-7) [Google Scholar]
- Lieh H.1974Phenology and seasonality modelling. New York, NY: Springer [Google Scholar]
- Liu Y., Liang X. S., Weisberg R. H.2007Rectification of the bias in the wavelet power spectrum. J. Atmos. Oceanic Technol. 24, 2093–2102 (doi:10.1175/2007JTECHO511.1) [Google Scholar]
- Longhurst A.1995Seasonal cycles of pelagic production and consumption. Progr. Oceanogr. 36, 77–167 (doi:10.1016/0079-6611(95)00015-1) [Google Scholar]
- Lunetta R. S., Knight J. F., Paerl H. W., Streicher J. J., Peierls B. L., Gallo T., Lyon J. G., Mace T. H., Buzzelli C. P.2009Measurement of water colour using AVIRIS imagery to assess the potential for an operational monitoring capability in the Pamlico Sound Estuary, USA. Int. J. Remote Sensing 30, 3291–3314 (doi:10.1080/01431160802552801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarewicz J. C., Lewis T. W., Bertram P.1999Phytoplankton composition and biomass in the offshore waters of Lake Erie: pre- and post Dreissena introduction (1983–1993). J. Great Lakes Res. 25, 135–148 (doi:10.1016/S0380-1330(99)70722-7) [Google Scholar]
- McQuatters-Gollop A., Mee L. D., Raitsos D. E., Shapiro G. I.2008Non-linearities, regime shifts and recovery: the recent influence of climate on Black Sea chlorophyll. J. Mar. Systems 74, 649–658 (doi:10.1016/j.jmarsys.2008.06.002) [Google Scholar]
- Melack J. M.1979Temporal variability of phytoplankton in tropical lakes. Oecologia 44, 1–7 (doi:10.1007/BF00346388) [DOI] [PubMed] [Google Scholar]
- Menzel A., Fabian P.1999Growing season extended in Europe. Nature 397, 659–659 (doi:10.1038/17709) [Google Scholar]
- Myneni R. B., Keeling C. D., Tucker C. J., Asrar G., Nemani R. R.1997Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386, 698–702 (doi:10.1038/386698a0) [Google Scholar]
- Paerl H. W., Huisman J.2008Blooms like it hot. Science 320, 57–58 (doi:10.1126/science.1155398) [DOI] [PubMed] [Google Scholar]
- Paerl H. W., Karen L., Rossignol S., Hall N., Peierls B. L., Wetz M. S.2010Phytoplankton community indicators of short- and long-term ecological change in the anthropogenically and climatically impacted Neuse River Estuary, North Carolina, USA. Estuaries and Coasts 33, 485–497 (doi:10.1007/s12237-009-9137-0) [Google Scholar]
- Penuelas J., Filella I.2001Responses to a warming world. Science 294, 793–794 (doi:10.1126/science.1066860) [DOI] [PubMed] [Google Scholar]
- Percival D. B., Walden A. T.2000Wavelet methods for time series analysis. Cambridge, UK: Cambridge University Press [Google Scholar]
- Philippart C. J. M., van Iperen J. M., Cadée G. C., Zuur A. F.2010Long-term field observations on seasonality in chlorophyll-a concentrations in a shallow coastal marine ecosystem, the Wadden Sea. Estuaries and Coasts 33, 286–294 (doi:10.1007/s12237-009-9236-y) [Google Scholar]
- Platt T., Fuentes-Yaco C., Frank K. T.2003Spring algal bloom and larval fish survival. Nature 423, 398–399 (doi:10.1038/423398b) [DOI] [PubMed] [Google Scholar]
- Platt T., Sathyendranath S., White G. N., Fuentes-Yaco C., Zhai L., Devred E., Tang C.2010Diagnostic properties of phytoplankton time series from remote sensing. Estuaries and Coasts 33, 428–439 (doi:10.1007/s12237-009-9161-0) [Google Scholar]
- Pratt D. M.1959The phytoplankton of Narragansett Bay. Limnol. Oceanogr. 4, 425–440 (doi:10.4319/lo.1959.4.4.0425) [Google Scholar]
- R Development Core Team 2009R: Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Reynolds C. S.2006Ecology of phytoplankton. Cambridge, UK: Cambridge University Press [Google Scholar]
- Richardson A. D., et al. 2010Influence of spring and autumn phenological transitions on forest ecosystem productivity. Phil. Trans. R. Soc. B 365, 3227–3246 (doi:10.1098/rstb.2010.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer M.1991Should we expect strange attractors behind plankton dynamics—and if so, should we bother? J. Plankton Res. 13, 1291–1305 (doi:10.1093/plankt/13.6.1291) [Google Scholar]
- Schindler D. W.2001The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Canad. J. Fish. Aquatic Sci. 58, 18–29 (doi:10.1139/cjfas-58-1-18) [Google Scholar]
- Schindler D. W., et al. 1990Effects of climatic warming on Lakes of the Central Boreal Forest. Science 250, 967–970 (doi:10.1126/science.250.4983.967) [DOI] [PubMed] [Google Scholar]
- Siegel H., Ohde T., Gerth M., Lavik G., Leipe T.2007Identification of coccolithophore blooms in the SE Atlantic Ocean off Namibia by satellites and in-situ methods. Continental Shelf Res. 27, 258–274 (doi:10.1016/j.csr.2006.10.003) [Google Scholar]
- Smayda T. J.1997What is a bloom? A commentary. Limnol. Oceanogr. 42, 1132–1136 (doi:10.4319/lo.1997.42.5_part_2.1132) [Google Scholar]
- Smayda T. J.1998Patterns of variability characterizing marine phytoplankton, with examples from Narragansett Bay. ICES J. Mar. Sci. 55, 562–573 (doi:10.1006/jmsc.1998.0385) [Google Scholar]
- Smayda T. J., Borkman D., Beaugrand G., Belgrano A.2004Ecological effects of climate variation in the North Atlantic: phytoplankton. In Marine ecosystems and climate variation—the North Atlantic (eds Stenseth N. C., Ottersen G., Hurrell J. W., Belgrano A., Planque B.), pp. 49–58 Oxford, UK: Oxford University Press [Google Scholar]
- Smetacek V.1985The annual cycle of Kiel Bight plankton—a long-term analysis. Estuaries 8, 145–157 (doi:10.2307/1351864) [Google Scholar]
- Sommer U., Gaedke U., Schweizer A.1993The first decade of oligotrophication of Lake Constance II. The response of phytoplankton taxonomic composition. Oecologia 93, 276–284 (doi:10.1007/BF00317682) [DOI] [PubMed] [Google Scholar]
- Sommer U., Gliwicz Z. M., Lampert W., Duncan A.1986The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 106, 433–471 [Google Scholar]
- Straile D.2002North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc. R. Soc. Lond. B 269, 391–395 (doi:10.1098/rspb.2001.1907) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray S. J., Jones I. D., Maberly S. C.2008Long-term change in the phenology of spring phytoplankton: species-specific responses to nutrient enrichment and climatic change. J. Ecol. 96, 523–535 (doi:10.1111/j.1365-2745.2008.01355.x) [Google Scholar]
- Torrence C., Compo G. P.1998A practical guide to wavelet analysis. Bull. Am. Meteorol. Soc. 79, 61–78 (doi:10.1175/1520-0477(1998)079<0061:APGTWA>2.0.CO;2) [Google Scholar]
- Vasseur D. A., Fox J. W.2007Environmental fluctuations can stabilize food web dynamics by increasing synchrony. Ecol. Lett. 10, 1066–1074 (doi:10.1111/j.1461-0248.2007.01099.x) [DOI] [PubMed] [Google Scholar]
- Verity P. G., Borkman D. G.2010A decade of change in the Skidaway River Estuary. III. Plankton. Estuaries and Coasts 33, 513–540 (doi:10.1007/s12237-009-9208-2) [Google Scholar]
- White M. A., et al. 2009Intercomparison, interpretation, and assessment of spring phenology in North America estimated from remote sensing for 1982–2006. Global Change Biol. 15, 2335–2359 (doi:10.1111/j.1365-2486.2009.01910.x) [Google Scholar]
- Winder M., Schindler D. E.2004aClimate change uncouples trophic interactions in a lake ecosystem. Ecology 85, 2100–2106 (doi:10.1890/04-0151) [Google Scholar]
- Winder M., Schindler D. E.2004bClimatic effects on the phenology of lake processes. Global Change Biol. 10, 1844–1856 (doi:10.1111/j.1365-2486.2004.00849.x) [Google Scholar]
- Wittemyer G., Polansky L., Douglas-Hamilton I., Getz W. M.2008Disentangling the effects of forage, social rank, and risk on movement autocorrelation of elephants using Fourier and wavelet analyses. Proc. Natl Acad. Sci. USA 105, 19 108–19 113 (doi:10.1073/pnas.0801744105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L., Tucker C., Kaufmann R., Slayback D., Shabanov N., Myneni R.2001Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981 to 1999. J. Geophys. Res.—Atmos. 106, 20 069–20 083 [Google Scholar]