Abstract
Despite the numerous studies which have been conducted during the past decade on species ranges and their relationship to the environment, our understanding of how environmental conditions shape species distribution is still far from complete. Yet, some process-based species distribution models have been able to simulate plants and insects distribution at a global scale. These models strongly rely on the completion of the annual cycle of the species and therefore on their accomplished phenology. In particular, they have shown that the northern limit of species' ranges appears to be caused mainly by the inability to undergo full fruit maturation, while the southern limit appears to be caused by the inability to flower or unfold leaves owing to a lack of chilling temperatures that are necessary to break bud dormancy. I discuss here why phenology is a key adaptive trait in shaping species distribution using mostly examples from plant species, which have been the most documented. After discussing how phenology is involved in fitness and why it is an adaptive trait susceptible to evolve quickly in changing climate conditions, I describe how phenology is related to fitness in species distribution process-based models and discuss the fate of species under climate change scenarios using model projections and experimental or field studies from the literature.
Keywords: fitness, species distribution, adaptation, niche
1. Introduction
More than ever an accurate understanding of how environmental conditions shape species distribution at the continental scale is necessary to predict future range shifts of species and their consequences on biodiversity. Which environmental factors determine the range boundaries of species and by which mechanisms are still challenging questions (Parmesan et al. 1999; Johnstone & Chapin 2003).
Species distribution, and more precisely species habitat, was linked to the concept of species niche for the first time by Grinnell (1917) who defined the species niche as all the sites where organisms of a species can live (where conditions are suitable for life). Ten years later, Elton (1927) proposed another definition of the niche based on the functions performed by the species in the community of which it is a member. The first definition has also been called the ‘address’ of the species and the second one its ‘profession’ (Odum 1959). Thirty years later, Hutchinson (1957) proposed a new formulation of Grinnell's definition of the niche as a region in a multi-dimensional space of environmental factors (n-dimensional hypervolume) that affect the welfare of a species. Hutchinson also proposed the concepts of fundamental niche and realized niche, the fundamental niche being the hypervolume of environmental variables within which individuals can survive and reproduce indefinitely, and the realized niche being the expression of the fundamental niche affected by species interactions. A species distribution is therefore often assimilated to the species realized niche. But it was only 60 years after Elton that the concept of species niche was linked to the concept of species traits by Rosenzweig (1987), who defined a species niche as the ensemble of traits that allow a species to survive in a particular environment. One needs to note that both definitions of the niche help in understanding species distributions but mainly at the global and regional scales, since at local scales species distribution is also highly dependent on stochastic events.
Historically, species distribution models have tried to describe the niche following Grinnell's or Hutchinson's definition. These models were initially called ‘envelope models’, the envelope referring to the hypervolume of Hutchinson. They have been renamed ‘niche-based models’ and more recently ‘habitat models’ (Guisan & Zimmermann 2000; Guisan & Thuiller 2005), which is from my point of view more adequate. Such models try to identify the environmental variables (e.g. climatic, pedologic, orographic, biotic) that describe a species distribution. They are calibrated on the observed species distribution and use several statistical models from simple linear models to neuronal networks and random forest classification methods (Thuiller 2003; Thuiller et al. 2003, 2005). Species distribution models that try to describe the niche following Rosenzweig's definition are more recent and rarer. Such models are based on the concept of fitness and hereafter will be called ‘fitness-based models’. They try to identify those traits which determine the fitness of individuals of species in particular environmental conditions, i.e. their annual survival and reproductive success (Régnière & You 1991; Régnière 1996; Chuine & Beaubien 2001; Régnière & Nealis 2002; Regnière & Logan 2003; Morin et al. 2007).
The two definitions of the niche that have been proposed are complementary and point out the crucial role of those traits affected by the environment in species distribution. Many traits of plant species are affected by the environment and since the trait-based definition of the niche, many studies have been devoted to identifying the limited set of traits that is responsible for a species distribution.
Phenology is a key feature of plant and poikilotherm species niche because it determines the ability to capture variable resources and it defines the season and duration of growth and reproduction (Schwartz 2003). In other animals, phenology is still essential to the reproductive success and survival of the individuals (Post 2003; Sparks et al. 2003). Several recent findings converge in identifying phenology as one and perhaps the most important trait-shaping species distribution. First, it is quite straightforward to understand that it is crucial for a species living in temperate or boreal environments to time its seasonal activities: when to begin developing in the spring, when to reproduce, when to enter dormancy or when to migrate, in order to exploit favourable climatic conditions and avoid unfavourable climatic conditions. Yet, it is only recently that species distribution models based on this idea have been developed. These models have proven that phenology is a keystone trait in the niche of perennial plant and insect species (Chuine & Beaubien 2001; Régnière & Nealis 2002; Regnière & Logan 2003; Morin et al. 2007). Second, several studies suggest that phenology plays an important role in fitness (Rathcke & Lacey 1985; Reeekie & Bazzaz 1987; Kozlowski 1992), and should therefore play an important role in species distribution. Third, phenology is one of the traits most affected by climatic conditions and this is probably the reason why it is the first trait which has been documented as being highly affected by climate change (Parmesan & Yohe 2003; Root et al. 2003; Walther et al. 2005; Menzel et al. 2006).
In this paper I intend to explain why phenology is one of the most important traits defining a species niche and therefore shaping its distribution. The vast majority of studies dealing with phenology have primarily concerned plant species and secondarily insect species. Almost all of them were conducted on temperate, boreal, alpine and Mediterranean species. I will thus limit my demonstration to these types of organisms and these regions. The importance of phenology in other types of organism such as birds (Visser et al. 2010) or fishes (Mooij et al. 2008) has been discussed elsewhere in the literature. In the following review, the term phenology stands for the occurrence of phenological events such as flowering or leaf unfolding.
2. Why is phenology an adaptive trait?
Despite the increasing number of investigations on species distributions and niches, little is known about what types of traits contribute to local adaptation across broad geographic gradients, i.e. to the niche according to Rosenzweig's definition. Several studies have indirectly pointed out the adaptive nature of phenology. First, many experimental and field studies have found clinal patterns in the phenology of plant species and these patterns are usually correlated to some environmental variables such as temperature, precipitation, latitude or altitude (Santamaria et al. 2003; Caicedo et al. 2004). For example, earlier reproduction is often found in northern populations and interpreted as an adaptation to cooler and shorter growing seasons and vice versa (e.g. Xanthium strumarium, Griffith & Watson 2005; Potamogeton pectinatus, Santamaria et al. 2003). Clinal pattern in leaf unfolding or bud set has also been widely documented in forest trees (Rehfeldt 1989; Rehfeldt et al. 1999, 2002; Chuine et al. 2001; Savolainen et al. 2004). Second, phylogenetic studies have shown that flowering time is a particularly conserved trait within temperate and tropical phylads (for review see Levin 2006), and explained the phylogenetic nature of extinction risk among plants experiencing rapid climate change (Willis et al. 2008). Davis et al. (2010) also identified similar phylogenetic patterns in long-term phenological response traits across geographically disjunct communities. Third, macroecological studies have shown that species niche position was correlated to time of flowering and that species growing in similar eco-regions had developed similar phenologies (Thuiller et al. 2004).
Along with this indirect evidence, other studies have shown experimentally that plant phenology, and more particularly reproductive phenology was an important determinant of fitness (Rathcke & Lacey 1985; Reeekie & Bazzaz 1987; Kozlowski 1992). The timing of flowering affects the success of fruit maturation and progeny quality (Pigott & Huntley 1981; Galloway 2002; Lacey et al. 2003) but also the success of pollination as well as the level of seed and fruit herbivory (for review see Levin 2006).
Finally, phenological traits also usually show a very high level of heritability and high level of genetic variability within and among populations in plants and insects (Etterson & Shaw 2001; Etterson 2004a,b; Franks et al. 2007; Volis 2007; van Asch et al. 2007). This shows that phenological traits have a strong potential to evolve rapidly pending strong enough selection gradients.
All this evidence shows directly and indirectly that phenological traits are a key component of fitness and are able to evolve relatively rapidly (for review see Levin 2006). Indeed, climate change is already inducing the genetic evolution of some adaptive traits in many species, such as dispersion traits in insect species (Hill et al. 1999; Thomas et al. 2001), and phenological traits in insects, mammals and plants (for review in animals see Bradshaw & Holzapfel 2008). The mosquito Wyeomyia smithii has, for example, shifted toward shorter, more southern daylengths as growing seasons have become longer. This shift was detectable over a time interval as short as 5 years (Bradshaw & Holzapfel 2001). Berteaux et al. (2004) showed that the parturition date in a population of North American red squirrels has advanced during the past 20 years and this change was the result of both plasticity (62%) and genetic evolution (13%). Franks et al. (2007) showed that the evolutionary response of the annual plant Brassica rapa to a recent climate fluctuation resulting in a multiyear drought led to the evolution of earlier onset of flowering. However, several studies indicate that the rate of evolution is unable to match predicted rate of warming (for review see Jump & Penuelas 2005).
Now a question arises: if phenological traits are adaptive and able to evolve rapidly, why do they not evolve to adapt to the environment surrounding the distributional ranges of species and expand those ranges? I see two major hypotheses that could explain this contradiction. First of all, like other adaptive traits involved in species niche, the evolution of phenological traits will depend on the balance between selection and migration. Theoretical work has demonstrated that evolutionary constraints on one or several adaptive traits such as asymmetric gene flow between margins and centre of the range, could limit the evolution of these traits and therefore of species range (Garcia-Ramos & Kirkpatrick 1997; Kirkpatrick & Barton 1997; Case & Taper 2000; Garcia-Ramos & Rodriguez 2002; but see Polechova et al. 2009). Second, the genetic evolution of phenological traits is constrained by genetic correlations among them (Ehrlen & Münzbergova 2009) but also with other adaptive traits such as drought or frost tolerance (for review see Levin 2006), or life-history traits such as seed size. Several studies converge in identifying seed size as a key trait shaping species niche. Seed size has been shown to be highly correlated to species range and to species northern range limits (Morin & Chuine 2006), with small-seeded species having larger ranges because of higher latitude limits. Although it has been shown in many species that seed size and mass was negatively correlated to seed number within a species (Fenner & Thompson 2005), seed size cannot vary too much within a species because if a critical mass is not reached, a seed's ability to germinate decreases drastically (Leadem 1985; Pitel & Wang 1989). As a key trait defining a species strategy (Fenner & Thompson 2005), the evolution of seed size is thus very much constrained and often associated to speciation events (Moles et al. 2005a,b). This most important life-history trait is thus likely to constrain the evolution of other key adaptive traits shaping species niche such as phenology. The time necessary to mature seeds indeed depends on seed size and on the environmental conditions, such as temperature, prevailing after flowering time (Moles & Westoby 2003). Flowering time should evolve in order to maximize seed maturation success, and thus occur at a time minimizing both frost and drought damage on the reproductive organs, and the length of the maturation period. Since fruit maturation timing is dependent on flowering timing, that is itself related to onset of growth timing in most species (I. Chuine 2010, unpublished results), seed size thus constrains the entire annual cycle of plants.
3. How is phenology involved in reproductive success, survival and growth?
As we shall see later on, a plant species phenology results from natural selection to optimize the period of activity and reproduction under given environmental conditions of temperature, light and water availability. This optimization has to cope with several trade-offs. For example, in the boreal and temperate zone, there is a trade-off between maximizing annual carbon assimilation with early leaf unfolding and late leaf senescence and reducing the risk of damage caused by frost on vegetative organs. Similarly, there is a trade-off between maximizing fruit set with early flowering and reducing the risk of damage by frost on reproductive organs. Plant species have adapted their phenology to their local environment using primarily temperature and photoperiod to detect such optimal conditions (Sarvas 1972, 1974; Hänninen 1990, 1991; Hänninen et al. 1993; Heide 1993a,b; Kramer 1994b, 1995; Chuine & Cour 1999; Chuine et al. 1999, 2001; Badeck et al. 2004).
Studies that have aimed at demonstrating the link between phenology and reproductive success are not numerous and have all concerned flowering phenology (Chidumayo 2006; Gimenez-Benavides et al. 2006; Volis 2007; Inouye 2008; Ehrlen & Münzbergova 2009). It is quite intuitive to imagine that flowering phenology will affect the reproductive success of a plant, particularly in temperate, alpine, boreal and Mediterranean regions. However, demonstrating this link experimentally is not always an easy task. If all these studies showed that flowering phenology is important in determining reproductive success, they do not converge on the relationship between the two. This relationship indeed depends on the climatic conditions that prevail in the region investigated and the life-history traits of the species. The high-mountain Mediterranean species Silene ciliata, for example, has a higher reproductive success when flowering earlier because it can take advantage of the snow melt for growing (Gimenez-Benavides et al. 2006). The perennial woody savanna species Lannea edulis also has a higher reproductive success when flowering earlier because of a higher germination rate of seeds produced earlier in the season (Chidumayo 2006). The authors explain this result by a selective pressure to flower earlier to escape dry season fires. On the contrary, the perennial herbaceous mountain species Delphinium barbeyi, Erigeron speciosus and Helianthella quinquenervis show a lower reproductive success when flowering earlier (Inouye 2008). This is explained by the fact that reproductive success of these species is correlated to snowpack. Greater snowpacks induce a later snowmelt and thereby a later beginning of growth and later flowering so that buds and flowers experience less frost damage.
The phenology of many insect species is also key for their reproductive success and survival. van Asch et al. (2007) have shown, for example, a severe fitness cost for the winter moth Operophtera brumata of a few days delay between egg-hatching and Quercus robur leaf unfolding (see also Singer & Parmesan 2010). A high fitness cost of asynchronization between reproductive timing of insect species and beginning of plant growth has been reported in many species (Regnière & Logan 2003; Mjaaseth et al. 2005; Parmesan 2006). Such asynchronization has been shown to severely affect bird populations feeding on caterpillars (Visser et al. 2004; Both & Visser 2005; Visser & Both 2005).
There is also much documented evidence that phenology is an important determinant of plant growth and survival. Leaf unfolding and leaf senescence timing have been shown to have a major control on spatial and temporal variation in biologically mediated sources and sinks of carbon in the Mediterranean, temperate and boreal latitudes at stand scale (e.g. White et al. 1999; Baldocchi & Wilson 2001; Barr et al. 2007), and at global scale (Keeling et al. 1996). Earlier leaf unfolding owing to global warming has been positively correlated to longer carbon uptake period, and to increased net annual CO2 flux and net primary productivity (Goulden et al. 1996; Churkina et al. 2005; Piao et al. 2007). Contrarily, leaf senescence tends to be later owing to global warming but is concomitant with less favourable conditions for photosynthesis than bud burst timing (Morecroft et al. 2003; Piao et al. 2007). Thus, warmer temperatures in spring increase carbon uptake because they enhance growth more than soil decomposition, but warmer temperatures in autumn reduce carbon uptake because they enhance growth less than soil decomposition. Piao et al. (2007) showed using net CO2 flux data and dynamic global vegetation models that the reduction in carbon uptake in autumn was more important than the increase in carbon uptake in spring, and as a consequence that temperate ecosystems were losing carbon with global warming.
In plant species, phenology, root distribution and leaf lifespan determine the ability to use soil resources and take up carbon. Mobile (water, nitrogen, silicon, magnesium, calcium) and immobile (phosphorus, potassium, ammonium) soil resource acquisition is thought to increase with longer growing season (Nord & Lynch 2009). Transpiration is indeed the major driver for the acquisition of mobile resources and is related to leaf lifespan, while root lifespan is the major driver of immobile resource acquisition. Phenology of leaves and roots is also very important to match the availability of resources which show a seasonal variability and acquisition capacity. Phenology also affects resource utilization, particularly for reproduction. For example, increased temperature tends to shorten the reproductive phase of annual plants by decreasing seed-filling duration, which is responsible for decreased yield (Egli 2004).
4. How is phenology involved in species distribution?
Northern range limits are often associated with decreased growing season length and mean temperature (Morin & Chuine 2006). Therefore, in the northern range limits, growth and reproduction must be completed in a shorter time period and, in addition, in less favourable conditions than in lower latitudes. Therefore, plants have to adapt the timing of their annual life cycle to the varying conditions they encounter throughout their range. This can be achieved by two means; firstly by a plastic response of their phenology to some environmental cues such as temperature or photoperiod; secondly by genetic differentiation of the populations for this response. We have seen earlier that genetic differentiation among populations of a plant species for its phenological traits was usually common, especially between northern range limit populations and more southern populations. However, phenological traits vary across a species range mainly because of a high level of plasticity in response to temperature and photoperiod.
The case of temperate tree species has particularly been studied and process-based phenological models for leaf unfolding, flowering, fruit maturation and more recently leaf senescence (Delpierre et al. 2009) have been developed during the past 30 years (for review see Chuine et al. 2003). Such models have also been developed for some insect species responsible for important damage in forest stands, such as gipsy moth, spruce budworm and mountain pine beetle (Régnière 1987, 1996; Logan et al. 1991, 2007; Régnière & You 1991; Régnière & Nealis 2002; Regnière & Logan 2003; for review see Regnière & Logan 2003). In both cases, the hypotheses formulated in the process-based phenological models derive from the results of experimental studies in controlled conditions, which aimed at identifying the environmental cues of the phenological events (for review see Chuine et al. 2003; Regnière & Logan 2003). These models have been used to develop fitness-based models. Fitness-based models aim at estimating the survival and reproductive success either of an average individual of a given population (for tree species) or a set of individuals of a population (for insect species) according to the environmental conditions which are primarily temperature, photoperiod and precipitation. Such models have been developed primarily to study the impact of phenology on fitness, but they rapidly revealed their ability to predict species distribution (Chuine & Beaubien 2001; Regnière & Logan 2003; Morin & Chuine 2005; Morin et al. 2008). In the following, I will describe the model PHENOFIT developed for temperate tree species and explain how phenology affects survival and reproductive success (Chuine & Beaubien 2001).
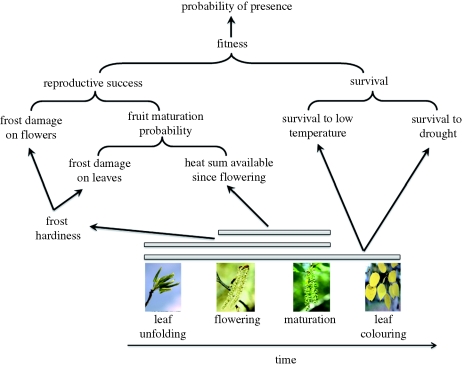
PHENOFIT outputs a probability of presence over several years for an individual of a particular species, estimated by the product of a probability to survive until the next reproductive season and the probability to produce viable seeds by the end of the annual cycle (reproductive success; figure 1). PHENOFIT is based on different process-based models: phenological models (Chuine 2000; Chuine et al. 2003), a frost-injury model (Leinonen 1996), a survival model and a reproductive success model. All parameters of these models, except that for survival in drought, for which very few data exist so far, are derived from observations of the different traits involved in the model rather than from present distributions of the species. These observations are primarily dates of leaf unfolding, of flowering, of fruit maturation and of leaf senescence. These can be obtained from natural population surveys. A second set of important information is the resistance of buds and leaf tissue to frost, which requires measurements in the laboratory using, for example, the method of electrolyte leakage following artificial frost. The third important information is the resistance of the species to drought, which is very difficult to assess and is currently under investigation in several research programmes. Such observations are unfortunately currently too rare to allow a rapid development and parameterization of such a model. Finally, although it is less crucial than for habitat models, which are fitted on species present distribution, information on the presence and absence of the species is important to validate the models. Yet, such information remains of poor quality, and efforts to compile species inventories at a fine spatial resolution, including land use history, would be necessary to yield good quality information. Given the extent of the task, this may require resort to citizen science programmes.
Figure 1.
Conceptual scheme of the process-based model PHENOFIT. The annual cycle is simulated using process-based phenological models calibrated on time series of each phenological event for several geographic provenances of the species.
The frost injury, survival and reproductive success models are based on the match between the simulated development and climate seasonality, e.g. survival is reduced if severe drought occurs between leafing and leaf colouring, reproductive success is reduced if frost occurs during flowering, etc. The reproductive success integrates the probability of completing fruit maturation by the end of the season and the proportion of fruits (between 0 and 1) that will reach this state, i.e. that will not be damaged by frost at either stage (from flower bud to mature fruit). The probability of completing fruit maturation primarily depends on the temperature conditions that prevail after flowering but is also affected by the proportion of leaves (between 0 and 1) that participate in carbon uptake, i.e. that have not been damaged by frost during their lifespan. PHENOFIT only uses climatic (daily temperatures and precipitation) and soil (water holding capacity) data as input variables.
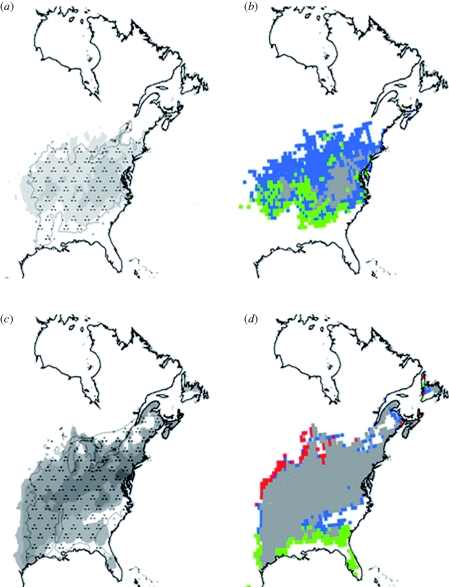
This model has been used to identify the climatic factors as well as the biological processes involved in determining tree species' ranges (Morin et al. 2007). The model showed that climatic constraints limit species' distributions primarily through their impact on phenological processes, and secondarily through their impact on drought and frost mortality. Contrary to what has been thought until recently, the northern limit of species' ranges appears to result mainly from the inability to undergo full fruit maturation and not from killing frost, while the southern limit appears to result from the inability to flower or unfold leaves because of a lack of chilling temperatures that are necessary to break bud dormancy (figure 2).
Figure 2.
Simulated present distributions of (a) Carya ovata and (c) Fraxinus americana using the process-based model PHENOFIT and climatic data CRU TS 2.0 (New et al. 2000). Presently observed distributions are dashed and simulated probabilities of presence according to the model are in grey. Limiting processes at the distribution margins of (b) Carya ovata and (d) Fraxinus americana: red (survival), green (flowering), blue (fruit maturation). Adapted from Morin et al. (2007).
Fitness-based models are very much complementary to habitat models. Although they are limited when explaining species distributions, especially in the sense of Rosenzweig, and suffer some methodological problems, habitat models are currently the only ones able rapidly to provide simulations of thousands of species distributions to assess the impact of global change on biodiversity for the following decades. Fitness-based models are, on the contrary, very time consuming to build up, but provide a more comprehensive understanding of species distribution, allowing precise identification of which processes or traits are responsible for species range limits in interaction with environmental factors. The limit of such models is clearly the quantity of information required to build and calibrate them, which makes their development much slower.
5. How will climate change affect species phenology and distribution?
There have been a large number of studies which have reported phenological changes during the past 60 years in various types of organisms (see for review Parmesan & Yohe 2003; Root et al. 2003; Walther et al. 2005; Menzel et al. 2006). Phenological shifts have been the first reported biological footprint of the impact of climate change. Along with these changes, shifts in distribution have also been reported in numerous species (Hughes 2000; Walther et al. 2001, 2002; Parmesan & Yohe 2003; Root et al. 2003; Walther 2003; Lenoir et al. 2008). Reported changes in phenology are mainly advancement of spring events such as leaf unfolding, flowering, emergence of adult forms of some insects, but delay of autumn events such as leaf senescence, diapause entrance. Reported changes in species distribution are mainly poleward and upward shifts and southern range local extinctions. According to the environmental determinisms of spring phenological events, advancements observed are explained by warmer springs that speed up cell growth once dormancy has been broken (Menzel et al. 2006). The late-autumn phases in plants are, however, less well understood as the environmental determinism of leaf senescence has not been elucidated so far (but see Delpierre et al. 2009).
Although there have been a tremendous number of studies which have investigated the environmental cues of species distributions (for review see Gaston 2003; Lomolino et al. 2005), very few studies have been devoted to identifying the traits or processes responsible for species distributions. These latter studies usually report potential growth limitation by low temperature and drought (Rehfeldt et al. 1999; Seynave et al. 2008) and reproduction limitation by low temperature (Pigott & Huntley 1981). Instead, most studies have investigated the relationship between fitness and adaptive traits, in particular phenology. Some of these studies concluded, for example, that although late-flowering alpine and subarctic plants may enhance their reproductive performance in warming scenarios because of earlier flowering triggering a better seed set (number and/or mass) (Molau 1993; Alatalo & Tøtland 1997), climate change will decrease reproductive success of high mountain Mediterranean species because of increased summer drought (Gimenez-Benavides et al. 2006).
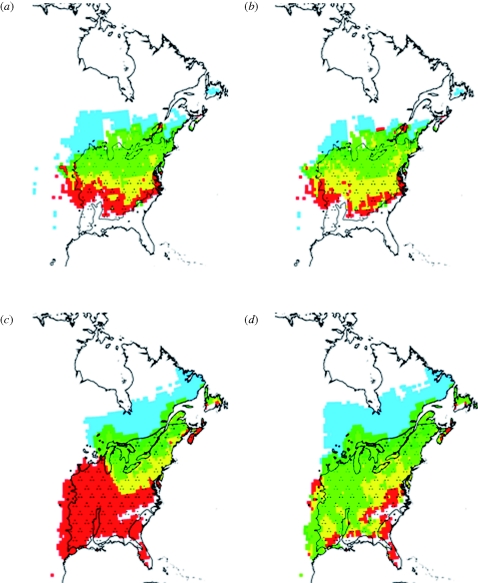
These observations confirm the hypotheses of fitness-based models that phenology and resistance to abiotic stresses such as frost and drought are key processes in determining species niche and thereby distribution. These models have been used with climate scenarios of the twenty-first century to study the evolution of species distribution and identify the key abiotic variables and biological processes which will be responsible for the forthcoming changes (Regnière & Logan 2003; Morin et al. 2008). In agreement with reported changes, projections show local extinctions in the south of species ranges, and colonization of new habitats in the north, though these are limited by dispersal ability for most species (figure 3; Morin et al. 2008). Although predicted distribution shifts appear very species-specific, the loss of habitats southward seems to be mostly due to increased drought mortality and decreased reproductive success in most species, while northward colonization seems to be primarily promoted by increased probability of fruit ripening and flower frost survival. However, projections show that different species will not face the same risks owing to climate change, because their responses to climate in terms of phenology and stress resistance differ as well as their dispersal rate. An important result of such projections is also that local extinction may proceed at a slower rate than forecasted so far by niche-based models, which tempers the alarming conclusions of these latter about biodiversity loss (Morin & Thuiller 2009). This important result is explained by the fact that fitness-based models take into account the local adaptation and the trait's plasticity to climate of the species, but also the nonlinear responses of the processes where temperature is involved (Morin et al. 2008; Morin & Thuiller 2009).
Figure 3.
Projected distributions of Carya ovata (a,b) and Fraxinus americana (c,d) in 2100 following IPCC scenario A2 (a,c) and B2 (b,d) and GCM HadCM3 using PHENOFIT: red (extinction), yellow (decreased fitness), green (increased fitness), dark green (colonized area), blue (unreached suitable area). Adapted from Morin et al. (2008).
6. How can we improve fitness-based species distribution models?
Although, fitness-based models are able, in their present state, to predict relatively accurate species distributions of trees and insects at a global scale, some improvements can be achieved to refine the simulations, especially for tree fitness-based models. Four types of improvement can be pursued. One of them concerns the phenological models specifically and the three others concern the species distribution models. First of all, and most importantly, an important question for temperate and boreal tree species is whether bud-burst will occur earlier or later in a warmer climate (Hänninen 1991; Kramer 1994a; Heide 2003). This depends on the response of the species phenology to chilling temperature and on the temperature that will prevail in autumn and winter. Morin et al. (2009) have shown that lack of sufficient chilling temperatures to break bud dormancy should decrease the rate of advancement in leaf unfolding date during the twenty-first century for many North American tree species, and that some species may even experience abnormal budburst owing to insufficient chilling. The study also showed that insufficient chilling exposure and abnormal budburst should appear predominantly in the southern range of the species. These predictions are based on an important assumption of process-based phenological models that chilling temperatures are required to break bud dormancy, which is necessary for subsequent cell growth leading to bud-burst. Yet, parameters of these models are always fitted on leaf unfolding or flowering dates only because no information on the date of dormancy break exists. Therefore, parameter estimates of the dormancy phase cannot be accurately evaluated. Yet, errors in these estimates could substantially change the predictions of leaf unfolding or flowering dates. There is thus an urgent need to measure dormancy break dates in temperate tree species in order to provide robust calibration of phenological process-based models.
Second, all species distribution models have to integrate or improve species dispersal and migration processes to be able to provide accurate projections of future distributions (Midgley et al. 2007; Morin & Lechowicz 2008; Thuiller et al. 2008). Three types of dispersion model have been developed so far that could be integrated with species distribution models: purely mechanistic dispersion models (Nathan et al. 2001; Nathan & Casagrandi 2004; Soons et al. 2004; Kuparinen 2006), kernel-based models (Clark 1996, 1998; Clark et al. 1998, 2001) and Gibbs-based models (Saltré et al. 2009).
Third, fitness-based tree distribution models, contrary to dynamic global vegetation models, at present do not integrate growth processes such as photosynthesis, respiration and carbon allocation. At the species level, such processes do not seem to be essential to provide an accurate representation of the distribution at a global scale. Thus, it seems unnecessary to have an exhaustive representation of the niche to be able to simulate a species distribution. However, an accurate modelling of growth processes and allocation processes could improve the simulation of some of the survival components of the fitness-based models. Species distribution models should also integrate biotic constraints, notably interspecific competition, to provide an accurate representation of species distributions at a regional scale.
Last but not least, at present none of the existing species distribution models integrates genetic evolution of the traits they simulate in response to climate change. Yet, we have seen previously that ongoing genetic evolution is underway in several species (see also Springer et al. 2008), and will affect their fate. In this respect, fitness-based distribution models seem to be the most adequate to integrate a genetic component and efforts should be put into integrating the genetic evolution of phenological traits and stress-resistance traits into such models.
Acknowledgements
I am grateful to Xavier Morin for agreeing to the reproduction of simulation results from the PHENOFIT model. This research was supported by an ANR research grant ANR-05-BDIV-009-01.
Footnotes
One contribution of 11 to a Theme Issue ‘The role of phenology in ecology and evolution’.
References
- Alatalo J. M., Tøtland O.1997Response to simulated climatic change in an alpine and subartic pollen risk strategist, Silene acaulis. Glob. Change Biol. 3, 74–79 (doi:10.1111/j.1365-2486.1997.gcb133.x) [Google Scholar]
- Badeck F. W., Bondeau A., Bottcher K., Doktor D., Lucht W., Schaber J., Sitch S.2004Responses of spring phenology to climate change. New Phytol. 162, 295–309 (doi:10.1111/j.1469-8137.2004.01059.x) [Google Scholar]
- Baldocchi D. D., Wilson K. B.2001Modeling CO2 and water vapor exchange of a temperate broadleaved forest across hourly to decadal time scales. Ecol. Mod. 142, 155–184 (doi:10.1016/S0304-3800(01)00287-3) [Google Scholar]
- Barr A. G., Black T. A., Hogg E. H., Griffis T. J., Theede A., Kljun N., Morgenstern K., Nesic Z.2007Climatic controls on the carbon and water balances of a boreal aspen forest, 1994–2003. Glob. Change Biol. 13, 561–576 (doi:10.1111/j.1365-2486.2006.01220.x) [Google Scholar]
- Berteaux D., Réale D., McAdam A. G., Boutin S.2004Keeping pace with fast climate change: can Arctic life count on evolution? Integr. Comp. Biol. 44, 140–151 (doi:10.1093/icb/44.2.140) [DOI] [PubMed] [Google Scholar]
- Both C., Visser M. E.2005The effect of climate change on the correlation between avian life-history traits. Glob. Change Biol. 11, 1606–1613 (doi:10.1111/j.1365-2486.2005.01038.x) [Google Scholar]
- Bradshaw W. E., Holzapfel C. M.2001Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA 98, 14 509–14 511 (doi:10.1073/pnas.241391498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw W. E., Holzapfel C. M.2008Genetic response to rapid climate change: it's seasonal timing that matters. Mol. Ecol. 17, 157–166 (doi:10.1111/j.1365-294X.2007.03509.x) [DOI] [PubMed] [Google Scholar]
- Caicedo A. L., Stinchcombe J. R., Olsen K. M., Schmit J., Purugganan M. D.2004Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proc. Natl Acad. Sci. USA 101, 15 670–15 675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case T. J., Taper M. L.2000Interspecific competition, environmental gradients, gene flow, and the coevolution of species' borders. Am. Nat. 155, 583–605 (doi:10.1086/303351) [DOI] [PubMed] [Google Scholar]
- Chidumayo E. N.2006Fitness implications of late bud break and time of burning in Lannea edulis (Sond.) Engl. (Anacardiaceae). Flora 201, 588–594 [Google Scholar]
- Chuine I.2000A unified model for tree phenology. J. Theor. Biol. 207, 337–347 [DOI] [PubMed] [Google Scholar]
- Chuine I., Beaubien E.2001Phenology is a major determinant of temperate tree range. Ecol. Lett. 4, 500–510 (doi:10.1046/j.1461-0248.2001.00261.x) [Google Scholar]
- Chuine I., Cour P.1999Climatic determinants of budburst seasonality in four temperate-zone tree species. New Phytol. 143, 339–349 [Google Scholar]
- Chuine I., Cour P., Rousseau D. D.1999Selecting models to predict the timing of flowering of temperate trees: implication for tree phenology modelling. Plant Cell Environ. 22, 1–13 (doi:10.1046/j.1365-3040.1999.00395.x) [Google Scholar]
- Chuine I., Aitken S. N., Ying C. C.2001Temperature thresholds of shoot elongation in provenances of Pinus contorta. Can. J. For. Res. 31, 1444–1455 (doi:10.1139/cjfr-31-8-1444) [Google Scholar]
- Chuine I., Kramer K., Hänninen H.2003Plant development models. In Phenology: an integrative environmental science (ed. Schwarz M. D.), pp. 217–235 The Netherlands: Kluwer [Google Scholar]
- Churkina G. D., Schimel B. H., Braswell X. M., Xiao G. J.2005Spatial analysis of growing season length control over net ecosystem exchange. Glob. Change Biol. 11, 1777–1787 (doi:10.1111/j.1365-2486.2005.001012.x) [Google Scholar]
- Clark J. S.1996Testing disturbance theory with long-term data: alternative life-history solutions to the distribution of events. Am. Nat. 148, 976–996 (doi:10.1086/285967) [Google Scholar]
- Clark J. S.1998Why trees migrate so fast: confronting theory with dispersal biology and the paleorecord. Am. Nat. 152, 204–224 (doi:10.1086/286162) [DOI] [PubMed] [Google Scholar]
- Clark J. S., et al. 1998Reid's paradox of rapid plant migration–dispersal theory and interpretation of paleoecological records. Bioscience 48, 13–24 (doi:10.2307/1313224) [Google Scholar]
- Clark J. S., Lewis M., Horvath L.2001Invasion by extremes: population spread with variation in dispersal and reproduction. Am. Nat. 157, 537–554 (doi:10.1086/319934) [DOI] [PubMed] [Google Scholar]
- Davis C. C., Willis C. G., Primack R. B., Miller-Rushing A. J.2010The importance of phylogeny to the study of phenological response to global climate change. Phil. Trans. R. Soc. B 365, 3201–3213 (doi:10.1098/rstb.2010.0130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpierre N., Dufrêne E., Soudani K., Ulrich E., Cecchini S., Boé J., François C.2009Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agr. For. Meteorol. 149, 938–948 (doi:10.1016/j.agrformet.2008.11.014) [Google Scholar]
- Egli D. B.2004Seed-fill duration and yield of grain crops. Adv. Agronomy 83, 243–279 (doi:10.1016/S0065-2113(04)83005-0) [Google Scholar]
- Ehrlen J., Münzbergova Z.2009Timing of flowering: opposed selection on different fitness components and trait covariation. Am. Nat. 173, 819–830 (doi:10.1086/598492) [DOI] [PubMed] [Google Scholar]
- Elton C. S.1927Animal ecology. London, UK: Sidgwick & Jackson [Google Scholar]
- Etterson J. R.2004aEvolutionary potential of Chamaecrista fasciculata in relation to climate change. 1. Clinal patterns of selection along an environmental gradient in the great plains. Evolution 58, 1446–1458 [DOI] [PubMed] [Google Scholar]
- Etterson J. R.2004bEvolutionary potential of Chamaecrista fasciculata in relation to climate change. II. Genetic architecture of three populations reciprocally planted along an environmental gradient in the great plains. Evolution 58, 1459–1471 [DOI] [PubMed] [Google Scholar]
- Etterson J. R., Shaw R. G.2001Constraint to adaptive evolution in response to global warming. Science 294, 151–154 (doi:10.1126/science.1063656) [DOI] [PubMed] [Google Scholar]
- Fenner M., Thompson K.2005The ecology of seeds. Cambridge, UK: Cambridge University Press [Google Scholar]
- Franks S. J., Sim S., Weis A. E.2007Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc. Natl Acad. Sci. USA 104, 1278–1282 (doi:10.1073/pnas.0608379104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway L. F.2002The effect of maternal phenology on offspring characters in the herbaceous plant Campanula americana. J. Ecol. 90, 851–858 (doi:10.1046/j.1365-2745.2002.00714.x) [Google Scholar]
- Garcia-Ramos G., Kirkpatrick M.1997Genetic models of adaptation and gene flow in peripheral populations. Evolution 5, 21–28 [DOI] [PubMed] [Google Scholar]
- Garcia-Ramos G., Rodriguez D.2002Evolutionary speed of species invasions. Evolution 56, 661–668 (doi:10.1554/0014-3820(2002)056[0661:ESOSI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Gaston K. J.2003The structure and dynamics of geographic ranges. New York, NY: Oxford University Press [Google Scholar]
- Gimenez-Benavides L., Escudero A., Iriondo J. M.2006Reproductive limits of a late-flowering high-mountain Mediterranean plant along an elevationnal climate gradient. New Phytol. 173, 367–382 (doi:10.1111/j.1469-8137.2006.01932.x) [DOI] [PubMed] [Google Scholar]
- Goulden M. L., Munger J. W., Fan S. M., Daube B. C., Wofsy S. C.1996Exchange of carbon dioxide by a deciduous forest: response to interannual climate variability. Science 271, 1576–1578 (doi:10.1126/science.271.5255.1576) [Google Scholar]
- Griffith T. M., Watson M. A.2005Stress avoidance in a common annual: reproductive timing is important for local adaptation and geographic distribution. J. Evol. Biol. 18, 1601–1612 (doi:10.1111/j.1420-9101.2005.01021.x) [DOI] [PubMed] [Google Scholar]
- Grinnell J.1917Field tests of theories concerning distributional control. Am. Nat. 51, 115–128 (doi:10.1086/279591) [Google Scholar]
- Guisan A., Thuiller W.2005Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- Guisan A., Zimmermann N. E.2000Predictive habitat distribution models in ecology. Ecol. Mod. 135, 147–186 (doi:10.1016/S0304-3800(00)00354-9) [Google Scholar]
- Hänninen H.1990Modelling bud dormancy release in trees from cool and temperate regions. Acta For. Fenn. 213, 1–47 [Google Scholar]
- Hänninen H.1991Does climatic warming increase the risk of frost damage in northern trees? Plant Cell Environ. 14, 449–454 (doi:10.1111/j.1365-3040.1991.tb01514.x) [Google Scholar]
- Hänninen H., Kellomäki S., Laitinen K., Pajari B., Repo T.1993Effect of increased winter temperature on the onset of height growth of Scots pine: a field test of a phenological model. Silva Fenn. 27, 251–257 [Google Scholar]
- Heide O. M.1993aDaylength and thermal time responses of budburst during dormancy release in some northern deciduous trees. Physiol. Plant. 88, 531–540 (doi:10.1111/j.1399-3054.1993.tb01368.x) [DOI] [PubMed] [Google Scholar]
- Heide O. M.1993bDormancy release in beech buds (Fagus sylvatica) requires both chilling and long days. Physiol. Plant. 89, 187–191 (doi:10.1111/j.1399-3054.1993.tb01804.x) [Google Scholar]
- Heide O. M.2003High autumn temperature delays spring bud burst in boreal trees, counterbalancing the effect of climatic warming. Tree Physiol. 23, 931–936 [DOI] [PubMed] [Google Scholar]
- Hill J. K., Thomas C. D., Blakeley D. S.1999Evolution of flight morphology in a butterfly that has recently expanded its geographic range. Oecologia 121, 165–170 (doi:10.1007/s004420050918) [DOI] [PubMed] [Google Scholar]
- Hughes L.2000Biological consequences of global warming: is the signal already apparent. Trends Ecol. Evol. 15, 56–61 (doi:10.1016/S0169-5347(99)01764-4) [DOI] [PubMed] [Google Scholar]
- Hutchinson M. F.1957Concluding remarks. Cold Spring Harb. Symp. Quant. Biol. 22, 415–427 [Google Scholar]
- Inouye D. W.2008Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology 89, 353–362 (doi:10.1890/06-2128.1) [DOI] [PubMed] [Google Scholar]
- Johnstone J. F., Chapin F. S.2003Non-equilibrium succession dynamics indicate continued northern migration of lodgepole pine. Glob. Change Biol. 9, 1401–1409 (doi:10.1046/j.1365-2486.2003.00661.x) [Google Scholar]
- Jump A. S., Penuelas J.2005Running to stand still: adaptation and the response of plants to rapid climate change. Ecol. Lett. 8, 1010–1020 (doi:10.1111/j.1461-0248.2005.00796.x) [DOI] [PubMed] [Google Scholar]
- Keeling C. D., Chin J. F. S., Whorf T. P.1996Increased activity of northern vegetation inferred from atmospheric CO2 measurements. Nature 382, 146–149 (doi:10.1038/382146a0) [Google Scholar]
- Kirkpatrick M., Barton N. H.1997Evolution of a species' range. Am. Nat. 150, 1–23 (doi:10.1086/286054) [DOI] [PubMed] [Google Scholar]
- Kozlowski J.1992Optimal allocation of resources to growth and reproduction: implications for age and size at maturity. Trends Ecol. Evol. 7, 15–18 [DOI] [PubMed] [Google Scholar]
- Kramer K.1994aA modelling analysis of the effects of climatic warming on the probability of spring frost damage to tree species in The Netherlands and Germany. Plant Cell Environ. 17, 367–377 (doi:10.1111/j.1365-3040.1994.tb00305.x) [Google Scholar]
- Kramer K.1994bSelecting a model to predict the onset of growth of Fagus sylvatica. J. Appl. Ecol. 31, 172–181 [Google Scholar]
- Kramer K.1995Phenotypic plasticity of the phenology of seven European tree species in relation to climatic warming. Plant Cell Environ. 18, 93–104 (doi:10.1111/j.1365-3040.1995.tb00356.x) [Google Scholar]
- Kuparinen A.2006Mechanistic models for wind dispersal. Trends Plant Sci. 11, 296–301 (doi:10.1016/j.tplants.2006.04.006) [DOI] [PubMed] [Google Scholar]
- Lacey E. P., Roach D. A., Herr D., Kincaid S., Perrot R.2003Multigenerational effects of flowering and fruiting phenology in Plantago lanceolata. Ecology 84, 2462–2475 (doi:10.1890/02-0101) [Google Scholar]
- Leadem C. L.1985Seed dormancy in three Pinus species of the Inland Mountain West. In Conifer tree seed in the Inland Mountain West Symposium, Missoula, MT, 5–6 August 1985. Gen. Tech. Rep. INT-203, pp. 117–124 Ogden, UT: USDA Forest Service [Google Scholar]
- Leinonen I.1996A simulation model for the annual frost hardiness and freeze damage of Scots pine. Ann. Bot. 78, 687–693 (doi:10.1006/anbo.1996.0178) [Google Scholar]
- Lenoir J., Gégout J. C., Marquet P. A., Ruffray P. D., Brisse H.2008A significant upward shift in plant species optimum elevation during the 20th century. Science 320, 1768–1771 (doi:10.1126/science.1156831) [DOI] [PubMed] [Google Scholar]
- Levin D. A.2006Flowering phenology in relation to adaptive radiation. Syst. Bot. 31, 239–246 (doi:10.1600/036364406777585928) [Google Scholar]
- Logan J. A., Casagrande R. A., Liebhold A. M.1991Modeling environment for simulation of gypsy-moth (Lepidoptera, Lymantriidae) larval phenology. Environ. Entomol. 20, 1516–1525 [Google Scholar]
- Logan J. A., Regniere J., Gray D. R., Munson A. S.2007Risk assessment in the face of a changing environment: gypsy moth and climate change in Utah. Ecol. Appl. 17, 101–117 (doi:10.1890/1051-0761(2007)017[0101:RAITFO]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- Lomolino M. V., Riddle B. R., Brown J. H. (eds)2005Biogeography. Sunderland, MA: Sinauer Associates [Google Scholar]
- Menzel A., et al. 2006European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976 (doi:10.1111/j.1365-2486.2006.01193.x) [Google Scholar]
- Midgley G. F., Thuiller W., Higgins S. I.2007Plant species migration as a key uncertainty in predicting future impacts of climate change on ecosystems: progress and challenges. In Terrestrial ecosystems in a changing world (eds Canadell D. G. J., Pataki D. E., Pitelka L. F.), pp. 129–137 Berlin, Germany: Springer [Google Scholar]
- Mjaaseth R. R., Hagen S. B., Yoccoz N. G., Ims R. A.2005Phenology and abundance in relation to climatic variation in a sub-Arctic insect herbivore–mountain birch system. Oecologia 145, 53–65 (doi:10.1007/s00442-005-0089-1) [DOI] [PubMed] [Google Scholar]
- Molau U.1993Relationships between flowering phenology and life history strategies in tundra plants. Arctic Alp. Res. 25, 391–402 (doi:10.2307/1551922) [Google Scholar]
- Moles A., Westoby M.2003Latitude, seed predation and seed mass. J. Biogeogr. 30, 105–128 (doi:10.1046/j.1365-2699.2003.00781.x) [Google Scholar]
- Moles A. T., Ackerly D. D., Webb C. O., Tweddle J. C., Dickie J. B., Pitman A. J., Westoby M.2005aFactors that shape seed mass evolution. Proc. Natl Acad. Sci. USA 102, 10 540–10 544 (doi:10.1073/pnas.0501473102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles A. T., Ackerly D. D., Webb C. O., Tweddle J. C., Dickie J. B., Westoby M.2005bA brief history of seed size. Science 307, 576–580 (doi:10.1126/science.1104863) [DOI] [PubMed] [Google Scholar]
- Mooij W. M., Domis L. N. D. S., Hülsmann S.2008The impact of climate warming on water temperature, timing of hatching and young-of-the-year growth of fish in shallow lakes in The Netherlands. J. Sea Res. 60, 32–43 (doi:10.1016/j.seares.2008.03.002) [Google Scholar]
- Morecroft M. D., Stokes V. J., Morison J. I. L.2003Seasonal changes in the photosynthetic capacity of canopy oak (Quercus robur) leaves: the impact of slow development on annual carbon uptake. Int. J. Biometeorol. 47, 221–226 (doi:10.1007/s00484-003-0173-3) [DOI] [PubMed] [Google Scholar]
- Morin X., Chuine I.2005Sensitivity analysis of the tree distribution model PHENOFIT to climatic input characteristics: implications for climate impact assessment. Glob. Change Biol. 11, 1493–1503 (doi:10.1111/j.1365-2486.2005.00996.x) [Google Scholar]
- Morin X., Chuine I.2006Niche breadth, competitive strength and range size of tree species: a trade-off based framework to understand species distribution. Ecol. Lett. 9, 185–195 (doi:10.1111/j.1461-0248.2005.00864.x) [DOI] [PubMed] [Google Scholar]
- Morin X., Lechowicz M.2008Contemporary perspectives on the niche that can improve models of species range shifts under climate change. Biol. Lett. 4, 573–576 (doi:10.1098/rsbl.2008.0181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin X., Thuiller W.2009Comparing niche- and process-based models to reduce prediction uncertainty in species range shifts under climate change. Ecology 90, 1301–1313 (doi:10.1890/08-0134.1) [DOI] [PubMed] [Google Scholar]
- Morin X., Augspurger C., Chuine I.2007Process-based modeling of tree species’ distributions: what limits temperate tree species’ range boundaries? Ecology 88, 2280–2291 (doi:10.1890/06-1591.1) [DOI] [PubMed] [Google Scholar]
- Morin X., Viner D., Chuine I.2008Tree species range shifts at a continental scale: new predictive insights from a process-based model. J. Ecol. 96, 784–794 (doi:10.1111/j.1365-2745.2008.01369.x) [Google Scholar]
- Morin X., Lechowicz M. J., Augspurger C., Keef J. O., Viner D., Chuine I.2009Leaf phenology in 22 North American tree species during the 21st century. Glob. Change Biol. 15, 961–975 (doi:10.1111/j.1365-2486.2008.01735.x) [Google Scholar]
- Nathan R., Casagrandi R.2004A simple mechanistic model of seed dispersal, predation and plant establishment: Janzen-Connell and beyond. J. Ecol. 92, 733–746 (doi:10.1111/j.0022-0477.2004.00914.x) [Google Scholar]
- Nathan R., Safriel U. F., Noy-Meir I.2001Field validation and sensitivity analysis of a mechanistic model for tree seed dispersal by wind. Ecology 82, 374–388 (doi:10.1890/0012-9658(2001)082[0374:FVASAO]2.0.CO;2) [Google Scholar]
- New M., Hulme M., Jones P.2000Representing twentieth century space–time climate variability. Part II: development of a 1901–1996 monthly grids of terrestrial surface climate. J. Clim. 13, 2217–2238 (doi:10.1175/1520-0442(2000)013<2217:RTCSTC>2.0.CO;2) [Google Scholar]
- Nord E. A., Lynch J. P.2009Plant phenology: a critical controller of soil resource acquisition. J. Exp. Bot. 60, 1927–1037 (doi:10.1093/jxb/erp018) [DOI] [PubMed] [Google Scholar]
- Odum E. P.1959Foundations of ecology. Philadelphia, PA: Saunders [Google Scholar]
- Parmesan C.2006Ecological and evolutionary responses to recent climate change. Ann. Rev. Ecol. Evol. Syst. 37, 637–639 (doi:10.1146/annurev.ecolsys.37.091305.110100) [Google Scholar]
- Parmesan C., Yohe G.2003A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (doi:10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- Parmesan C., et al. 1999Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature 399, 579–583 (doi:10.1038/21181) [Google Scholar]
- Piao S. L., et al. 2007Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 451, 49–52 (doi:10.1038/nature06444) [DOI] [PubMed] [Google Scholar]
- Pigott C. D., Huntley J. P.1981Factors controlling the distribution of Tilia cordata at the northern limits of its geographical range. III Nature and cause of seed sterility. New Phytol. 87, 817–839 [Google Scholar]
- Pitel J. A., Wang B. S. P.1989Physiological and chemical treatments to improve germination of whitebark pine seeds. In Whitebark pine ecosystem symposium: ecology and management of a high-mountain resource, Bozeman, MT, 29–31 March 1989. Gen. Tech. Rep. INT-270, pp. 130–133 Ogden, UT: USDA Forest Service [Google Scholar]
- Polechova J., Barton N., Marion G.2009Species' range: adaptation in space and time. Am. Nat. 174, 186–204 [DOI] [PubMed] [Google Scholar]
- Post E.2003Timing of reproduction in large mammals. In Phenology: an integrative environmental science (ed. Schwartz M. D.), pp. 437–450 London, UK: Kluwer Academic [Google Scholar]
- Rathcke B., Lacey E. P.1985Phenological patterns of terrestrial plants. Annu. Rev. Ecol. Syst. 16, 179–214 (doi:10.1146/annurev.es.16.110185.001143) [Google Scholar]
- Reeekie E. G., Bazzaz F. A.1987Reproductive efforts in plants. Am. Nat. 129, 876–919 (doi:10.1086/284681) [Google Scholar]
- Régnière J.1987Temperature-dependent development of eggs and larvae of Choristoneura fumiferana (Clem.) (Lepidoptera: Tortricidae) and simulation of its seasonal history. Can. Entomol. 119, 717–728 (doi:10.4039/Ent119717-7) [Google Scholar]
- Régnière J.1996A generalized approach to landscape-wide seasonal forecasting with temperature-driven simulation models. Environ. Entomol. 25, 869–881 [Google Scholar]
- Regnière J., Logan J. A.2003Animal life cycle models. In Phenology: an integrative environmental science (ed. Schwartz M. D.), pp. 237–254 London, UK: Kluwer Academic [Google Scholar]
- Régnière J., Nealis V.2002Modelling seasonality of gypsy moth, Lymantria dispar (Lepidoptera: Lymantriidae), to evaluate probability of its persistence in novel environments. Can. Entomol. 134, 805–824 (doi:10.4039/Ent134805-6) [Google Scholar]
- Régnière J., You M.1991A simulation model of spruce budworm (Lepidoptera: Tortricidae) feeding on balsam fir and white spruce. Ecol. Modell. 54, 277–297 (doi:10.1016/0304-3800(91)90080-K) [Google Scholar]
- Rehfeldt G. E.1989Ecological adaptations in Douglas-fir (Pseudostuga menziesii var. glauca): a synthesis. Forest. Ecol. Manage. 28, 203–215 (doi:10.1016/0378-1127(89)90004-2) [Google Scholar]
- Rehfeldt G. E., Ying C. C., Spittlehouse D. L., Hamilton D. A.1999Genetic responses to climate for Pinus contorta in British Columbia: niche breadth, climate change and reforestation. Ecol. Monogr. 69, 375–407 [Google Scholar]
- Rehfeldt G. E., Tchebakova N. M., Parfenova Y. I., Wykoff W. R., Kuzmina N. A., Milyutin L. I.2002Intraspecific responses to climate in Pinus sylvestris. Glob. Change Biol. 8, 912–929 (doi:10.1046/j.1365-2486.2002.00516.x) [Google Scholar]
- Root T. L., Price J. T., Hall K. R., Schneider S. H., Rosenzweig C., Pounds J. A.2003Fingerprints of global warming on wild animals and plants. Nature 421, 57–60 (doi:10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- Rosenzweig M. L.1987Habitat selection as a resource of biological diversity. Evol. Ecol. 1, 315–330 (doi:10.1007/BF02071556) [Google Scholar]
- Saltré F., Chuine I., Brewer S., Gaucherel C.2010A phenomenological model without dispersal kernel to model species migration. Ecol. Modell. 220, 3546–3554 (doi:10.1016/j.ecolmodel.2009.06.026) [Google Scholar]
- Santamaria L., Figuerola J., Pilon J. J., Mjelde M., Green A. J., De Boer T., King R. A., Gornall R. J.2003Plant performance across latitude: the role of plasticity and local adaptation in an aquatic plant. Ecology 84, 2454–2461 [Google Scholar]
- Sarvas R.1972Investigations on the annual cycle of development on forest trees Active period. Commun. Inst. For. Fenn. 76, 1– 110 [Google Scholar]
- Sarvas R.1974Investigations on the annual cycle of development of forest trees. Autumn dormancy and winter dormancy. Commun. Inst. For. Fenn. 84, 1–101 [Google Scholar]
- Savolainen O., Bokma F., Garcia-Gil R., Komulainen P., Repo T.2004Genetic variation in cessation of growth and frost hardiness and consequences for adaptation of Pinus sylvestris to climatic changes. Forest. Ecol. Manage. 197, 79–90 (doi:10.1016/j.foreco.2004.05.006) [Google Scholar]
- Schwartz M. D.2003Phenology: an integrative environmental science. Dordrecht, The Netherlands: Kluwer Academic [Google Scholar]
- Seynave I., Gegout J. C., Herve J. C., Dhote J. F.2008Is the spatial distribution of European beech (Fagus sylvatica L.) limited by its potential height growth? J. Biogeogr. 35, 1851–1862 (doi:10.1111/j.1365-2699.2008.01930.x) [Google Scholar]
- Singer M. C., Parmesan C.2010Phenological asynchrony between herbivorous insects and their hosts: signal for climate change or pre-existing adaptive strategy? Phil. Trans. R. Soc. B 365, 3161–3176 (doi:10.1098/rstb.2010.0144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soons M. B., Heil G. W., Nathan R., Katul G. G.2004Determinants of long-distance seed dispersal by wind in grasslands. Ecology 85, 3056–3068 (doi:10.1890/03-0522) [Google Scholar]
- Sparks T., Crick H. Q. P., Dunn P. O., Sokolov L. V.2003Phenology of selected lifeforms: birds. In Phenology: an integrative environmental science (ed. Schwartz M. D.), pp. 421–436 London, UK: Kluwer Academic [Google Scholar]
- Springer C. J., Orozco R. A., Kelly J. K., Ward J. K.2008Elevated CO2 influences the expression of floral-initiation genes in Arabidopsis thaliana. New Phytol. 178, 63–67 (doi:10.1111/j.1469-8137.2008.02387.x) [DOI] [PubMed] [Google Scholar]
- Thomas C. D., Bodsworth E. J., Wilson R. J., Simmons A. D., Davies Z. G., Musche M., Conradt L.2001Ecological and evolutionary processes at expending range margins. Nature 411, 577–581 (doi:10.1038/35079066) [DOI] [PubMed] [Google Scholar]
- Thuiller W.2003BIOMOD—optimizing predictions of species distributions and projecting potential future shifts under global change. Glob. Change Biol. 9, 1353–1362 (doi:10.1046/j.1365-2486.2003.00666.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuiller W., Araujo M. B., Lavorel S.2003Generalized models vs. classification tree analysis: predicting spatial distributions of plant species at different scales. J. Veg. Sci. 14, 669–680 (doi:10.1111/j.1654-1103.2003.tb02199.x) [Google Scholar]
- Thuiller W., Lavorel S., Midgley G., Lavergne S., Rebelo T.2004Relating plant traits and species distributions along bioclimatic gradients for 88 Leucadendron taxa. Ecology 85, 1688–1699 (doi:10.1890/03-0148) [Google Scholar]
- Thuiller W., Richardson D. M., Pysek P., Midgley G. F., Hughes G. O., Rouget M.2005Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob. Change Biol. 11, 2234–2250 (doi:10.1111/j.1365-2486.2005.001018.x) [DOI] [PubMed] [Google Scholar]
- Thuiller W., et al. 2008Predicting global change impacts on plant species distributions: future challenges. Perspect. Plant Ecol. Evol. Syst. 9, 137–152 (doi:10.1016/j.ppees.2007.09.004) [Google Scholar]
- van Asch M., Tienderen P. H. v., Holleman L. J. M., Visser M. E.2007Predicting adaptation of phenology in response to climate change, an insect herbivore example. Glob. Change Biol. 13, 1596–1604 (doi:10.1111/j.1365-2486.2007.01400.x) [Google Scholar]
- Visser M. E., Both C.2005Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B 272, 2561–2569 (doi:10.1098/rspb.2005.3356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M. E., Both C., Lambrechts M. M.2004Global climate change leads to mistimed avian reproduction. In Birds and climate change (eds Moller A. P., Fiedler W.), pp. 89–110 London, UK: Elsevier [Google Scholar]
- Visser M. E., Caro S. P., van Oers K., Schaper S. V., Helm B.2010Phenology, seasonal timing and circannual rhythms: towards a unified framework. Phil. Trans. R. Soc. B 365, 3113–3127 (doi:10.1098/rstb.2010.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volis S.2007Correlated patterns of variation in phenology and seed production in populations of two annual grasses along an aridity gradient. Evol. Ecol. 21, 381–393 (doi:10.1007/s10682-006-9108-x) [Google Scholar]
- Walther G. R.2003Plants in a warmer world. Perspect. Plant Ecol. Evol. Syst. 6, 169–185 (doi:10.1078/1433-8319-00076) [Google Scholar]
- Walther G. R., Burga C. A., Edwards P. J.2001Fingerprints of climate change—adapted behaviour and shifting species ranges. New York/London: Kluwer Academic/Plenum [Google Scholar]
- Walther G.-R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J.-M., Hoegh-Guldberg O., Bairlein F.2002Ecological responses to recent climate change. Nature 416, 389–395 (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- Walther G. R., Berger S., Sykes M. T.2005An ecological ‘footprint’ of climate change. Proc. R. Soc. B 272, 1427–1432 (doi:10.1098/rspb.2005.3119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M. A., Running S. W., Thornton P. E.1999The impact of growing-season length variability on carbon assimilation and evapotranspiration over 88 years in the eastern US deciduous forest. Int. J. Biometeorol. 42, 139–145 (doi:10.1007/s004840050097) [DOI] [PubMed] [Google Scholar]
- Willis C. G., Ruhfel B., Primack A. J. M.-R. R. B., Davis C. C.2008Phylogenetic patterns of species loss in Thoreau's woods are driven by climate change. Proc. Natl Acad. Sci. USA 105, 17 029–17 033 (doi:10.1073/pnas.0806446105) [DOI] [PMC free article] [PubMed] [Google Scholar]