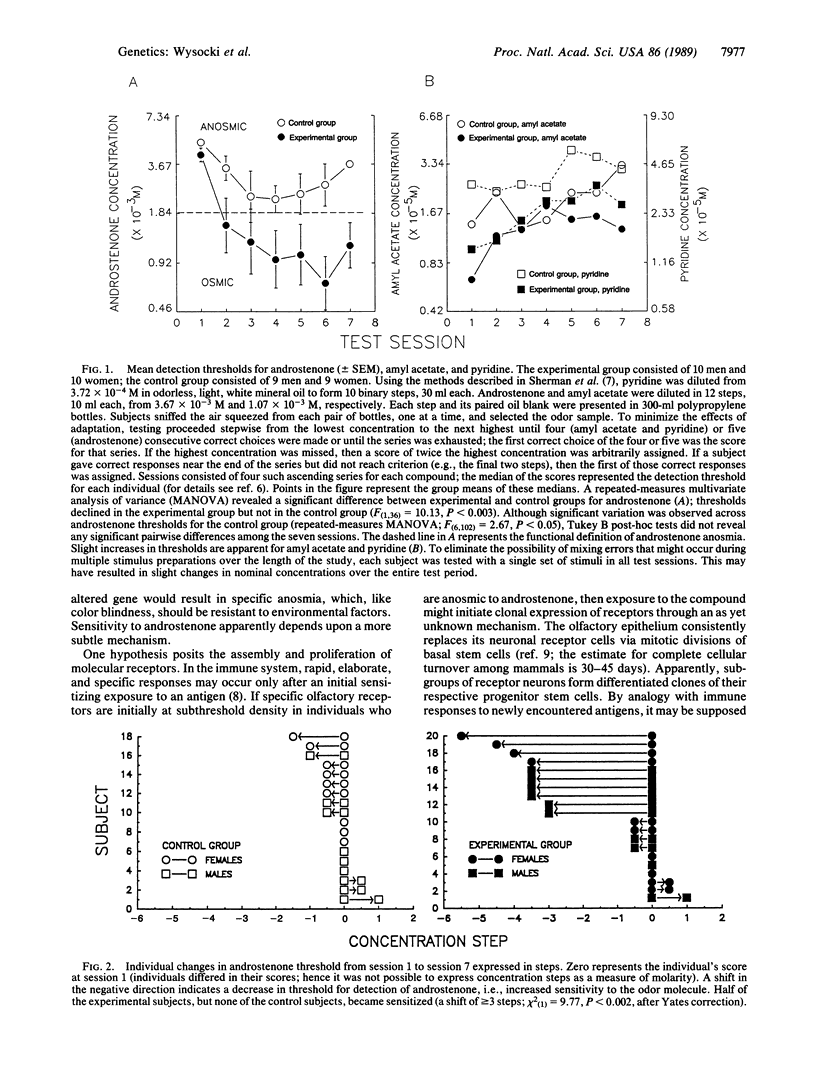
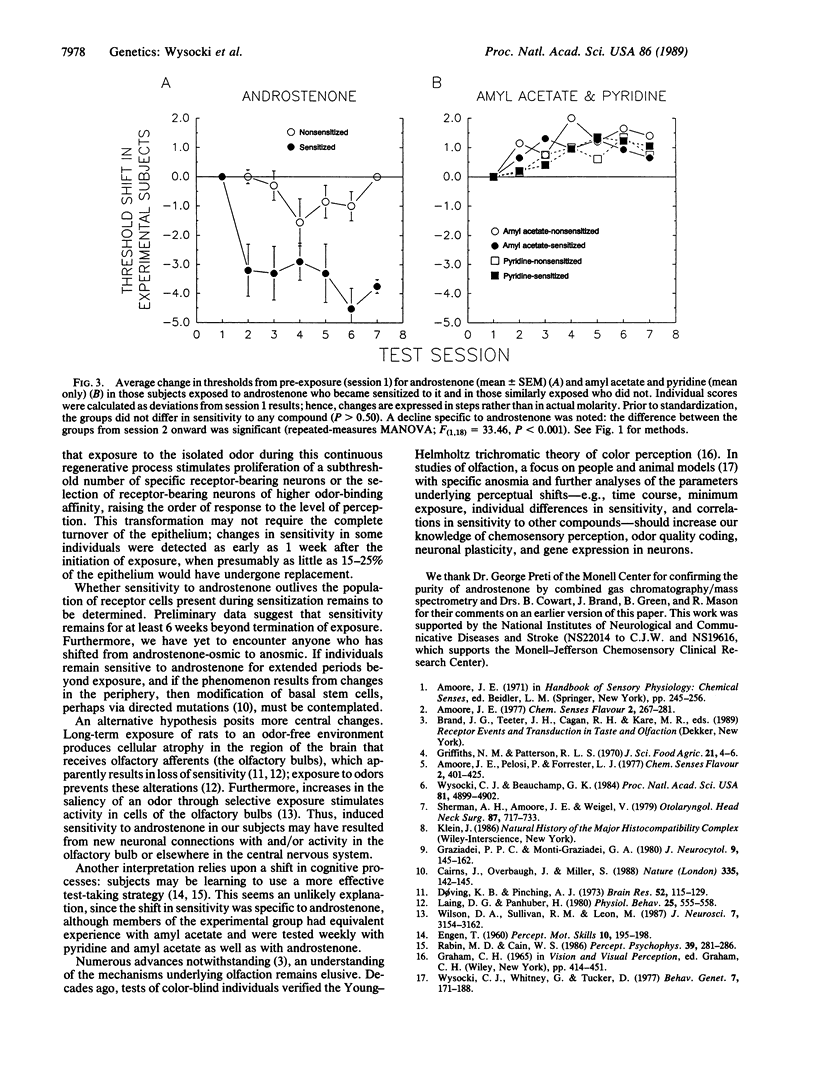
Abstract
Nearly half the adult human population does not perceive an odor when sniffing androstenone (5 alpha-androst-16-en-3-one), a volatile steroid found in human perspiration, boar saliva, some pork products (e.g., bacon), truffles, and celery. This variation in ability to perceive androstenone has a significant heritable component, suggesting that androstenone insensitivity is in part determined genetically. We now report that the ability to perceive androstenone was induced in 10 of 20 initially insensitive subjects who were systematically exposed to androstenone. Since olfactory neurons of the olfactory epithelium undergo periodic replacement from differentiating basal cells, and assuming the induction of sensitivity to be peripheral, we propose that a portion of the apparently anosmic human population does in fact possess olfactory neurons with specific receptors for androstenone. Such neurons may undergo clonal expansion, or selection of lineages with more receptors or receptors of higher affinity, in response to androstenone stimulation, much in the manner of lymphocytes responding to antigenic stimulation, thus raising odor stimulation to the level of conscious perception. As a guide to further study of the genetics and mechanism of variation of androstenone perception, we provisionally envisage three categories of human subjects, the truly anosmic, the inducible, and those subjects who either are constitutionally sensitive or have already experienced incidental induction.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cairns J., Overbaugh J., Miller S. The origin of mutants. Nature. 1988 Sep 8;335(6186):142–145. doi: 10.1038/335142a0. [DOI] [PubMed] [Google Scholar]
- Doving K. B., Pinching A. J. Selective degeneration of neurons in the olfactory bulb following prolonged odour exposure. Brain Res. 1973 Mar 30;52:115–129. doi: 10.1016/0006-8993(73)90653-7. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Monti Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria in rat. J Neurocytol. 1980 Apr;9(2):145–162. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- Griffiths N. M., Patterson R. L. Human olfactory responses to 5-alpha-androst-16-en-3-one--principal component of boar taint. J Sci Food Agric. 1970 Jan;21(1):4–6. doi: 10.1002/jsfa.2740210102. [DOI] [PubMed] [Google Scholar]
- Laing D. G., Panhuber H. Olfactory sensitivity of rats reared in an odorous or deodorized environment. Physiol Behav. 1980 Oct;25(4):555–558. doi: 10.1016/0031-9384(80)90121-3. [DOI] [PubMed] [Google Scholar]
- Rabin M. D., Cain W. S. Determinants of measured olfactory sensitivity. Percept Psychophys. 1986 Apr;39(4):281–286. doi: 10.3758/bf03204936. [DOI] [PubMed] [Google Scholar]
- Sherman A. H., Amoore J. E., Weigel V. The pyridine scale for clinical measurement of olfactory threshold: a quantitative reevaluation. Otolaryngol Head Neck Surg (1979) 1979 Nov-Dec;87(6):717–733. doi: 10.1177/019459987908700604. [DOI] [PubMed] [Google Scholar]
- Wilson D. A., Sullivan R. M., Leon M. Single-unit analysis of postnatal olfactory learning: modified olfactory bulb output response patterns to learned attractive odors. J Neurosci. 1987 Oct;7(10):3154–3162. doi: 10.1523/JNEUROSCI.07-10-03154.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki C. J., Beauchamp G. K. Ability to smell androstenone is genetically determined. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4899–4902. doi: 10.1073/pnas.81.15.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki C. J., Whitney G., Tucker D. Specific anosmia in the laboratory mouse. Behav Genet. 1977 Mar;7(2):171–188. doi: 10.1007/BF01066005. [DOI] [PubMed] [Google Scholar]


